Abstract
In the context of the SARS-CoV-2 pandemic, the reuse of personal protective equipment, specifically face coverings, has been recommended. Reuse of such items necessitates procedures to inactivate contaminating human respiratory and gastrointestinal pathogens. We previously demonstrated decontamination of face coverings contaminated with either infectious SARS-CoV-2 and animal coronaviruses or a highly resistant, non-enveloped norovirus via a novel photochemical treatment. Contaminated materials were coated with photosensitive methylene blue dye and were subsequently exposed to a visible bright light source (LED-equipped light boxes) to trigger the generation of virucidal singlet oxygen. A possible factor restricting the widespread use of such photochemical decontamination is its reliance on the availability of electricity to power light sources. Here, we show that natural sunlight can be used in lieu of artificial light. We demonstrate efficient inactivation of a SARS-CoV-2 surrogate, porcine respiratory coronavirus, via 10 µM dye coating in conjunction with short outdoor exposures of 5–30 min (blue sky to cloudy day; mean 46,578 lx). A tenacious human norovirus surrogate, murine norovirus, is inactivated via methylene blue solar decontamination involving 100 µM dye concentrations and 30 min of high-illuminance sunlight (blue sky; mean 93,445 lx) or 2 h of mid- to low-illuminance (cloudy day; mean 28,558 lx). The protocol developed here thus solidifies the position of methylene blue solar decontamination as an important equitable tool in the package of practical pandemic preparedness.
1. Introduction
As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic accelerated, it highlighted not only the general global unpreparedness in the face of a pandemic, but also unmasked the worldwide inequity of available resources [1]. The global demand for PPE in general, and for disposable surgical face masks (SMs) in particular, exceeded manufacturing capacities [2,3,4,5]. Following initial shortages, a surge of discarded single-use SMs has since translated to pollution in both terrestrial and aquatic environments [6,7]; meanwhile, PPE availability remains problematic in low-income countries.
To combat critical shortages, and in a departure from the prevailing culture of throwaway living [8,9], reuse of typically single-use only PPE has been recommended [2,3,10,11]. Prior sanitisation is paramount to safe reuse and must guarantee the complete inactivation of SARS-CoV-2 as well as that of other, hardier respiratory or gastrointestinal human pathogens (the US FDA recommends a robust proof of infectious bioburden reduction of three orders of magnitude for viral pathogens) [12]. This is relevant as contamination with pathogens other than SARS-CoV-2 might easily occur in the current context of widespread and/or inexpert mask use, but also plays an important role in positioning the world for future pandemics [13].
Human respiratory pathogens include enveloped corona-, pneumo-, metapneumo-, paramyxo- and orthomyxoviruses, as well as non-enveloped coxsackie- and rhinoviruses; gastrointestinal pathogens include boca-, astro-, picorna-, rota- and noroviruses (all non-enveloped) [14]. Lipid-enveloped viruses are susceptible to inactivating treatments, whereas non-enveloped viruses are known to be significantly more resistant.
While highly efficient against both enveloped and non-enveloped viruses [15,16,17], many established decontamination technologies are not equitable, depending heavily on the availability of large facilities, expensive machinery and/or electricity. Accessible and sustainable alternative decontamination methods are thus necessary in restricted surroundings. We recently demonstrated the efficient and cost-effective decontamination of SMs and respirators contaminated with infectious SARS-CoV-2 and several BSL2 animal coronaviruses, including porcine respiratory coronavirus (PRCV), via a novel photochemical treatment (DeMaND study) [18]. Photosensitive methylene blue dye, in combination with visible light, generates virucidal singlet oxygen from oxygen in the air and inactivates viruses by damaging viral membranes, proteins and nucleic acids [19,20,21,22]. For its application to PPE decontamination, contaminated materials were spray-coated with methylene blue and were subsequently exposed to a visible bright light source (custom-built LED light boxes with an output of either 12,500 lux of red light or 50,000 lux of white light) for 30 min. We subsequently adapted this protocol to the decontamination of a highly resistant, small, non-enveloped norovirus, utilising the murine norovirus (MNV) surrogate to model the inactivation of notoriously tenacious [23,24] human noroviruses [25].
A possible factor yet restricting the widespread use of photochemical decontamination is its reliance on the availability of electricity to power LED light sources. Electricity remains unavailable to nearly 16% of the world population, a situation further exacerbated by pandemic-associated slowing of both new grid and off-grid connection rates and disruptions associated with extreme weather events [26]. Vos et al. recently investigated whether sunlight can be used in lieu of artificial light for the photochemical treatment of coronavirus-contaminated PPE [27]. Total inactivation of a SARS-CoV-2 surrogate, murine hepatitis virus, was achieved within 5 min of simulated sunlight irradiation (corresponding to a solar irradiation yearly average at mid-latitude: air mass AM1.5 solar spectrum and integrated power of 1000 W/m2) in methylene blue solution, while simulated sunlight alone displayed antiviral activity only after several hours. Importantly, treatment with 10 µM methylene blue resulted in the complete viral inactivation on SMs after only 5 min of simulated sunlight exposure [27].
Here, we show that natural sunlight can be used in lieu of artificial light for the inactivation of both susceptible coronaviruses and hardier non-enveloped viruses. We demonstrate the efficient inactivation of PRCV via 10 µM dye coating in conjunction with short outdoor exposures of 5–30 min (blue sky to cloudy day; mean 46,578 lx). The hardier MNV is shown to be inactivated via methylene blue solar decontamination involving 100 µM dye concentrations and 30 min of high-illuminance sunlight (blue sky; mean 93,445 lux) or 2 h of mid- to low-illuminance (cloudy day; mean 28,558 lx). The inactivation of a norovirus, the most resistant of the respiratory and gastrointestinal human viruses, can predict the inactivation of any less resistant viral mask contaminant. The protocol developed here thus solidifies the position of methylene blue solar decontamination as an important equitable tool in the package of practical pandemic preparedness.
2. Materials and Methods
2.1. Viruses and Cells
The continuous swine testicle (ST) cell line, grown from testicular foetal swine tissues as described by McClurkin and Norman (1966) [28], was maintained in MEM (Sigma, St. Louis, MO, USA), supplemented with 10% heat inactivated foetal calf serum (FBS Supreme, Pan Biotec, Aidenbach, Germany), 1% sodium pyruvate 100× (Gibco, New York, NY, USA), antibiotics (200 U/mL penicillin and 0.2 mg/mL streptomycin (Sigma)) and 1% non-essential amino acids (Sigma). Stocks of PRCV strain 91V44 [29] were produced by the infection of ST cells, as previously described [15,17]. Titres were determined via the median tissue culture infective dose (TCID50) method, a well-established and reliable single measure for virus titration; ST cells were seeded in 96-well plates, infected with ten-fold serial dilutions of PRCV and incubated for three days at 37 °C with 5% CO2. Three days after inoculation, cell monolayers were analysed for the presence of cytopathic effects following crystal violet staining (0.2% crystal violet, 30 min). Titres, expressed as TCID50/mL, were calculated according to the Reed and Muench transformation [30]. A PRCV stock with a titre of 7.47 log10 TCID50/mL was used in subsequent steps.
The murine macrophage cell line RAW264.7 (ATCC TIB-71) was maintained in Dulbecco’s modified Eagle’s medium (Sigma) complemented with 10% heat inactivated foetal calf serum (FBS Supreme, Pan Biotec), 2% of an association of penicillin and streptomycin (200 U/mL penicillin and 0.2 mg/mL streptomycin (Sigma)) and 1% 1 M HEPES buffer (pH 7.6) (Gibco) (DMEMc) at 37 °C with 5% CO2. Stocks of MNV isolate MNV-1.CW1 were produced by infection of RAW264.7 cells as previously described [16,17]. Titres were determined via the TCID50 method; RAW 264.7 cells were seeded in 96-well plates, infected with ten-fold serial dilutions of MNV, incubated for three days at 37 °C with 5% CO2 and finally stained with 0.2% crystal violet for 30 min. Titres, expressed as TCID50/mL, were calculated according to the Reed and Muench transformation [30]. A MNV stock with a titre of 7.38 log10 TCID50/mL was used in the subsequent steps.
2.2. Surgical Masks
Type IIR BFE ≥ 99% three-layer medical masks manufactured by Xiantao Yongli Medical Products Co., Ltd. (Xiantao, China) (LOT 20200512; REF YLEN104) were utilised in all assays. All SMs, verified to be from the same manufacturing lot, were supplied by the ULiège Service for Hygiene and Health Protection at Work (SUPHT).
2.3. Light Metre
Sunlight intensity was measured throughout the duration of all assays using a handheld climate measuring instrument (testo 440; reference 0560 4401) equipped with a cabled lux probe (range of 0 to 100,000 lux (lx)) (reference 0635 0551). Illuminance datapoints were logged every second.
2.4. Porcine Respiratory Coronavirus and Murine Norovirus Inoculation onto Surgical Masks
The workflow followed previously described protocols for SM inoculation with PRCV and MNV [15,16,17,18,25]. Per SM, 100 µL of undiluted viral suspension was injected under the first outer layer at the centre of each of the three square coupons (34 mm × 34 mm). Inoculated SMs were allowed to dry for 20 min (under a laminar flow hood) at room temperature before further treatment steps.
2.5. Methylene Blue Photochemical Treatment of Surgical Masks
Virus-inoculated SMs were placed horizontally and were sprayed with a total volume of 7–8 mL of either a 10 µM (for assays involving PRCV) or 100 µM (for assays involving MNV) methylene blue solution (Sigma-Aldrich (M9140); solution prepared in deionised water; final volume determined based on six repetitive sprays into a graduated cylinder). The choice of a ten-fold higher methylene blue concentration for the treatment of norovirus-contaminated SMs was based on previously published experimental protocols describing the adaptation of the original DeMaND protocol (implementing 10 µM methylene blue for coronavirus inactivation) to the decontamination of small non-enveloped viruses [25]. In total, each SM was sprayed four times on the outer side and twice on the inner side (facing the wearer). Surgical masks were allowed to dry for 30 min and were then mounted on a horizonal rack and transferred to an open-top plastic box which was placed in direct sunlight for various exposure times (ranging from 5 min to 120 min). Virus-inoculated, but methylene-blue untreated SMs (sun-only) were simultaneously exposed to sunlight in parallel to all but one assay. Virus-only positive controls (inoculated but untreated SMs) accompanied each assay. The light metre was placed directly next to the SMs and was deployed during all sunlight exposures.
Sun-exposure assays were performed close to the summer solstice (21 June 2022) between 9 May 2022 and 19 July 2022 at 50°34′35.6′′ N 5°34′49.7′′ E longitude and latitude (Sart Tilman, Liège, Belgium, temperate zone, Northern Hemisphere). All assays were performed around midday/solar noon (earliest logged datapoint: 11:30 h; latest logged datapoint: 14:30 h).
Unless explicitly stated otherwise, all methylene blue-sun (MBS) and sun-only assays were performed as biological triplicates (n = 3), wherein three individual SMs were virus-inoculated, remained untreated or were sprayed with methylene blue, and were then simultaneously exposed to solar irradiance. Three square coupons per masks constituted technical triplicates of the assays (n = 3).
2.6. Virus Elution from Surgical Masks
Upon completion of the decontamination protocol, downstream SM coupon excision and virus elution followed previously described protocols [15,16]. Briefly, PRCV or MNV were eluted from three excised coupons per SM into 4 mL MEM (PRCV) or DMEM (MNV) via a 1 min vortex at maximum speed (2500 rounds per minute; VWR VX-2500 Multi-Tube Vortexer).
2.7. Titration of Eluted Virus (Validation of Decontamination Efficacy)
Titres of infectious virus recovered from individual coupons were determined via TCID50 assay (see above). Back titrations of inoculum stocks were performed in parallel to each series of decontamination experiments.
2.8. Evaluation of the Efficacy of Methylene Blue Solar Treatment for Coronavirus Inactivation
To validate previously published coronavirus-inactivating photochemical treatment conditions (10 µM methylene blue and 30 min of LED exposure) in conjunction with natural sunlight, we first replicated this protocol precisely, only replacing the artificial light source with direct bright sunlight on a blue-sky cloudless day.
2.9. Evaluation of Time-Dependent Virucidal Kinetics of Methylene Blue Solar Treatment and Sunlight Exposure on Porcine Respiratory Coronavirus
Having set a baseline for further assays via prior high-illuminance 30 min sunlight exposures, we then tested both shorter sunlight exposure times (5–15 min) and chose to investigate changeable (intermittent cloud coverage) or entirely overcast days with low sunlight irradiance for their suitability in a PRCV-inactivating MBS protocol.
2.10. Evaluation of Time- and Sunlight-Intensity Dependent Virucidal Kinetics of Methylene Blue Solar Treatment and Sunlight Exposure on Murine Norovirus
As small, non-enveloped viruses, noroviruses are typically harder to inactivate than large, enveloped coronaviruses. To find an optimal MBS treatment protocol, we thus initially tested 100 µM methylene blue concentrations in conjunction with a range of different exposure times (30 to 90 min) and irradiance values (blue-sky cloudless to overcast days) on MNV-inoculated SMs.
2.11. Validation of the Efficacy of Two Methylene Blue Solar Photochemical Decontamination Protocols for Norovirus Inactivation
Based on these preliminary assays, we chose to validate two different MBS protocols for MNV inactivation. MNV-inoculated SMs were sprayed with 100 µM methylene blue and were either exposed for 30 min to direct bright sunlight or for 120 min to lower-illuminance sunlight on a cloudy day.
3. Data Analysis
Differences in infectious viral titres were computed and all graphs created using GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, USA). Statistical analyses of differences in infectious viral titres were performed using GraphPad Prism version 7.0 (GraphPad Software), and p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 and ns is p ≥ 0.05.
4. Results and Discussion
A 30 min methylene blue high-illuminance solar treatment effectively decontaminates coronavirus-contaminated surgical masks.
The photochemical treatment of coronavirus-contaminated SMs, involving the application of a 10 μM methylene blue solution and a 30 min exposure to high-illuminance sunlight (mean light intensity: 90,145 lux (lx)), reduced infectious PRCV titres to below the assay detection limit (LOD) of 0.8 log10 TCID50/mL (≥3.19 (±0.19) log10 TCID50/mL reduction) (Figure 1). This reduction in infectious PRCV titres both closely mirrors coronavirus decontamination efficacies previously achieved by the DeMaND study teams using artificial LED light sources for the 30 min light exposure step [18], and is in line with FDA policy recommendations regarding SM reuse [12].
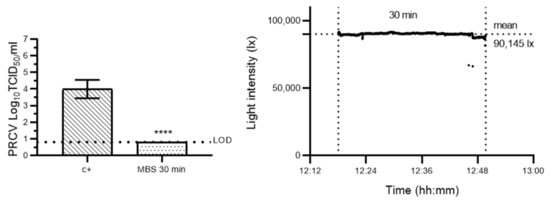
Figure 1.
Evaluation of the efficacy of an adapted methylene blue photochemical treatment protocol for coronavirus inactivation, wherein irradiation in a custom-built LED light box is replaced by natural sunlight exposure. Left panel: porcine respiratory coronavirus (PRCV)-inoculated SM coupons remained untreated (c+) or were pre-treated with a 10 µM methylene blue solution and then exposed to direct natural sunlight (MBS) (mean light intensity: 90,145 lx) for 30 min. The infectivity of PRCV subsequently recovered from surgical mask coupons (n = 9) was analysed in swine testicular cells. The cell culture limit of detection (LOD) was 0.80 log10 TCID50/mL for all analyses. Means log10 TCID50/mL and standard deviations are represented. p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001. Right panel: sunlight intensity was measured throughout the duration of each assay using a handheld light metre (testo 440) in which illuminance datapoints were logged every second (up to 100,000 lux (lx)).
A 10 µM methylene blue solar treatment involving short exposures to natural sunlight of varying illuminance is sufficient for porcine respiratory coronavirus inactivation on surgical masks; sunlight alone is insufficient to reliably sanitise coronavirus-inoculated surgical masks.
In high-throughput environments that necessitate ready PPE availability (such as hospitals, nursing homes), decontamination speed may be particularly important. Further, high light intensities of over 90,000 lux are not always a given, e.g., illuminance varies between 10,000 lx on overcast autumn days and 100,000 lux on cloudless summer days in temperate regions of the Northern Hemisphere [31]. We thus tested MBS protocols involving both shorter sunlight exposure times (5–15 min) and changeable or entirely overcast days with low sunlight irradiance for their efficacy in sanitising PRCV-contaminated SMs. In parallel, we investigated whether sunlight exposure alone may inactivate PRCV. Preliminary assays performed on a smaller subset of samples (1 SM; 3 coupons per SM per condition) showed that 5, 10 and 15 min sunlight exposures to mid- to high-illuminance sunlight (mean light intensities: 84,738 lx, 82,771 lx and 54,072 lx, respectively) reduced infectious PRCV titres to below the assay LODs, resulting in infectious titre losses of ≥5.00 (±0.14), 4.25 (±0.29) and 4.75 (±0.14) log10 TCID50/mL, respectively (Figure 2). Sunlight alone achieved a maximum titre reduction of only 1.41 (±0.26) log10 TCID50/mL after 15 min of exposure.
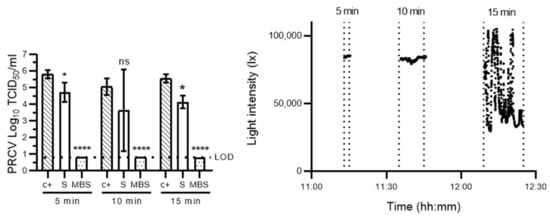
Figure 2.
Evaluation of time-dependent virucidal kinetics of methylene blue solar treatment and sunlight exposure on porcine respiratory coronavirus. Left panel: porcine respiratory coronavirus (PRCV)-inoculated surgical mask coupons remained untreated (c+), were exposed to direct natural sunlight (S) or were pre-treated with a 10 µM MB solution and then exposed to sunlight (MBS) for 5 (mean light intensity: 84,738 lx), 10 (mean light intensity: 82,771 lx) or 15 (mean light intensity: 54,072 lx) minutes. The infectivity of PRCV subsequently recovered from surgical mask coupons (n = 3) was analysed in swine testicular cells. The cell culture limit of detection (LOD) was 0.80 log10 TCID50/mL for all analyses. Means log10 TCID50/mL and standard deviations are represented. p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001, * p < 0.05 and ns is p ≥ 0.05. Right panel: sunlight intensity was measured throughout the duration of each assay using a handheld light metre (testo 440) in which illuminance datapoints were logged every second (up to 100,000 lux (lx)).
To then validate a short-exposure MBS protocol for coronavirus inactivation, 10 µM methylene blue pre-treatment in combination with a 5 min exposure was tested on a cloudy day (mean light intensity: 46,578 lx). Following this protocol, PRCV titres dropped by >4.67 (±0.16) log10 TCID50/mL (Figure 3), and this was in excess of the three orders of magnitude reduction outlined by the FDA [12]. Importantly, these results correspond with those recently achieved by Vos et al. where treatment with 10 µM methylene blue resulted in total murine hepatitis virus inactivation on SMs after 5 min of simulated sunlight exposure corresponding to a solar irradiation yearly average at mid-latitude [27].
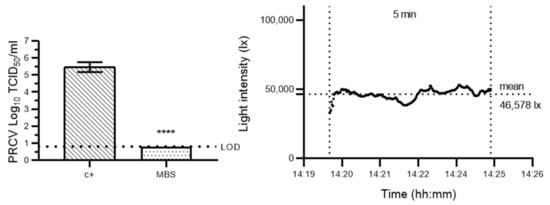
Figure 3.
Validation of a short-exposure methylene blue solar treatment protocol for porcine respiratory coronavirus inactivation on surgical masks. Left panel: porcine respiratory coronavirus (PRCV)-inoculated surgical mask coupons remained untreated (c+) or were pre-treated with a 10 µM methylene blue solution and then exposed to sunlight (MBS) (mean light intensity: 46,578 lx) for 5 min. The infectivity of PRCV subsequently recovered from surgical mask coupons (n = 3) was analysed in swine testicular cells. The cell culture limit of detection (LOD) was 0.80 log10 TCID50/mL for all analyses. Means log10 TCID50/mL and standard deviations are represented. p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001. Right panel: sunlight intensity was measured throughout the duration of each assay using a handheld light metre (testo 440) in which illuminance datapoints were logged every second (up to 100,000 lux (lx)).
While a 100 µM methylene blue solar treatment with 30 min of high-intensity natural sunlight sanitises murine norovirus-contaminated surgical masks, methylene blue solar treatment with lower or variable irradiance and 30–90 min sunlight exposures yields inconsistent results. Sunlight alone is insufficient to reliably sanitise norovirus-inoculated surgical masks even after long exposures.
As small, non-enveloped viruses, noroviruses are typically harder to inactivate than large, enveloped coronaviruses. Noroviruses are known both for their resistance in the face of decontamination and for their environmental tenacity [16,23,32]. While sunlight alone has been reported to inactivate SARS-CoV-2 and its surrogates within several minutes to several hours [27,31,33,34], noroviruses have been shown to persist in exposed outdoor environments for extended time periods [35]. To find an optimal MBS treatment protocol for hardier viruses, we thus initially tested 100 µM methylene blue concentrations in conjunction with a range of exposure durations and irradiances on MNV-inoculated SMs (1 SM; 3 coupons per SM per assay). Sunlight-only assays showed that, indeed, sunlight alone was insufficient to consistently inactivate MNV (Figure 4). However, high-irradiance MBS of circa 100,000 lx led to near-complete viral inactivation after 30, 60 and 90 min of exposure (loss of 4.33 (±0.30) log10 TCID50/mL after 30 min) (Figure 4A). In contrast, lower-irradiance MBS treatments with mean light intensities of 86,760 lx (Figure 4B) or 70,674 lx approximately (some datapoints outside of probe range; Figure 4C) were unable to consistently decontaminate norovirus-inoculated SMs. Here, while MNV was completely inactivated on several SM coupons, others retained not-inconsiderable amounts of infectious virus, leading to high intra-assay variabilities.
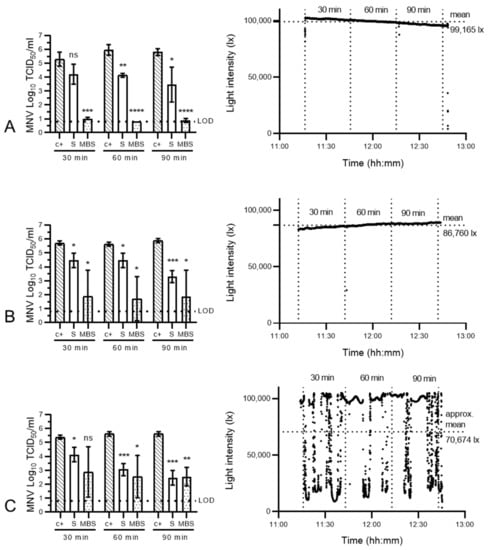
Figure 4.
Evaluation of time- and light-exposure-dependent virucidal kinetics of methylene blue solar treatment and sunlight exposure on murine norovirus. Left panels: murine norovirus (MNV)-inoculated surgical masks remained untreated (c+), were exposed to direct natural sunlight (S) or were pre-treated with a 100 µM MB solution and then exposed to sunlight (MBS) at varying intensities (mean light intensities: 99,165 lx (A), 86,760 lx (B) or >70,674 lx (C) (the latter value is an approximation as datapoints were outside of the probe range)) for 30, 60 or 90 min. The infectivity of MNV subsequently recovered from surgical mask coupons (n = 3) was analysed in RAW 264.7 cells. The cell culture limit of detection (LOD) was 0.80 log10 TCID50/mL for all analyses. Means log10 TCID50/mL and standard deviations are represented. p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 and ns is p ≥ 0.05. Right panels: sunlight intensity was measured throughout the duration of each assay using a handheld light metre (testo 440) in which illuminance datapoints were logged every second (up to 100,000 lux (lx)).
Both 30 min high-illuminance and 2 h mid-illuminance methylene blue solar treatments effectively decontaminate norovirus-contaminated surgical masks.
To validate reliable MBS decontamination for small, non-enveloped viruses, two treatment protocols were subsequently developed. Indeed, 100 µM methylene blue pre-treatments in combination with either a high-illuminance 30 min sunlight exposure (Figure 5A) or lower illuminance 2 h exposure (Figure 5B) were tested. These assays confirmed that MNV was highly resistant to sunlight alone but was sensitised by methylene blue pre-treatment. Infectious MNV titres dropped by 1.24 (±0.13) log10 TCID50/mL after 30 min of high-intensity sunlight exposure (mean light intensity: 93,445 lx) (Figure 5A) and by 1.14 (±0.17) log10 TCID50/mL following 2 h of low-intensity exposure (mean light intensity: 28,558 lx) (Figure 5B). Meanwhile, methylene blue treatment prior to exposure resulted in consistent and superior decontamination, achieving a titre reduction of 4.36 (±0.11) log10 TCID50/mL for the former, and 4.53 (±0.14) log10 TCID50/mL for the latter condition. These infectious MNV titre reductions mirrored norovirus decontamination efficacies previously achieved by our team leveraging an artificial LED light source (30 min of red light exposure) [25]. While the dependence of the 30 min exposure protocol on high-illuminance sunlight is a clear constraint in the mid-latitudes (circa 45 °C latitude), as well as the Arctic and Antarctic circles, it is likely to be a lesser concern in equatorial and tropical zones of the globe (0 to 23.5° latitude) where sunlight intensity is roughly greater by 20–30% [36].
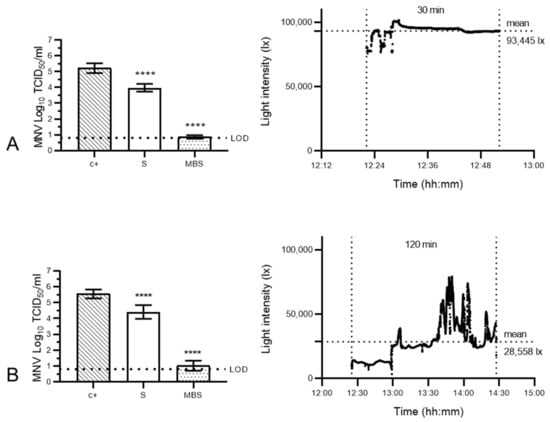
Figure 5.
Validation of two methylene blue solar treatment protocols for murine norovirus inactivation on surgical masks. Murine norovirus (MNV)- inoculated surgical masks remained untreated (c+), were exposed to direct natural sunlight (S) or were pre-treated with a 100 µM MB solution and then exposed to sunlight (MBS) at high intensity (mean light intensity: 93,445 lx) for 30 min (A) or lower intensity (mean light intensity: 28,558 lx) for 120 min (B). The infectivity of MNV subsequently recovered from surgical mask coupons (n = 9) was analysed in RAW 264.7 cells. The cell culture limit of detection (LOD) was 0.80 log10 TCID50/mL for all analyses. Means log10 TCID50/mL and standard deviations are represented. p-values were computed by using a two-sided independent sample t-test, where **** p < 0.0001. Right panel: sunlight intensity was measured throughout the duration of each assay using a handheld light metre (testo 440) in which illuminance datapoints were logged every second (up to 100,000 lux (lx)).
Here, we showed that MBS involving a 10 µM methylene blue pre-treatment in combination with a 5 min outdoor exposure on a cloudy day proved sufficient for inactivation of the enveloped PRCV. These results are in line with similar results published by other teams [27,37]. Scholte et al. recently demonstrated the inactivation of a series of enveloped high-consequence pathogens via a 10–100 µM methylene blue combined with 700 lx artificial ambient light in an indoor setting [37]. However, while they showed that Ebola-, Lassa- and coronaviruses were readily inactivated, Nipah virus appeared more resistant to inactivation under ambient conditions. While lipid-enveloped viruses are typically susceptible to inactivating treatments (this, as exemplified by Scholte et al. in varying measures), non-enveloped viruses are known to be significantly more resistant. The inactivation of non-enveloped viruses must constitute an integral part of comprehensive MBS treatment. We demonstrated that an MBS protocol involving a 100 µM methylene blue pre-treatment followed by either 30 min of high-illuminance or 2 h of mid to low-illuminance sun exposure effectively sanitised MNV-contaminated SMs. In the context of a holistic approach targeting a broad range of pathogens, SMs should thus be decontaminated following one of the two protocols validated for MNV.
The decontamination of a small non-enveloped virus such as MNV can be considered a reliable indicator for inactivation of any pathogen ranking lower in the hierarchy of pathogen resistance. A limitation of this work pertains to the fact that the inactivation of other tenacious agents (prions, bacterial spores, protozoal oocysts, helminth eggs and mycobacteria) was not investigated. In healthcare settings likely to be contaminated with a variety of nosocomial pathogens, MBS should ideally be combined with other protective measures until future studies can confirm its efficacy for “wide-spectrum” inactivation.
There is considerable clinical experience to support that methylene blue exhibits good safety. Methylene blue is routinely applied directly to mucous membranes in the treatment of maxillary sinusitis [22] and has further been used as a treatment of infected wounds both in human [38] and veterinary medicine [39]. In addition, ultraviolet spectroscopy analyses testing the amount of methylene blue that may leach off surgical masks and be inhaled by a wearer during the course of a ten-hour healthcare provider work shift, recently demonstrated that methylene blue does not leach off surgical mask (or other PPE) materials even at high concentrations; following the application of 50-fold the amount of methylene blue used in our present study, no detectable methylene blue was observed, thus providing safety evidence for the use of MBS for SM decontamination [40]. Both SM integrity and fit have been shown to be maintained following multiple cycles of methylene blue-based decontamination [18], further supporting the safety of this method.
5. Conclusions
A possible factor restricting the widespread use of low-cost photochemical PPE decontamination is its reliance on the availability of electricity to power LED light sources. Here, we showed that natural sunlight can be used in lieu of artificial light for the inactivation of both susceptible coronaviruses and hardier non-enveloped viruses on SMs via MBS. We demonstrated the efficient inactivation of PRCV via 10 µM dye coating in conjunction with 5 min outdoor exposures. Hardier MNV was inactivated via methylene blue solar decontamination involving 100 µM dye concentrations and 30 min of high- or 2 h of mid- to low-illuminance sunlight. Inactivation of a norovirus, the most resistant of the respiratory and gastrointestinal human viruses, can predict the inactivation of any less-resistant viral mask contaminant. We described methylene blue solar decontamination not only in its unique application to the inactivation of a tenacious non-enveloped virus, but also in the context of a “real world” scenario: the energy-independent use of actual natural sunlight to inactivate highly tenacious noroviruses serves to validate methylene blue solar decontamination for use in field conditions and contributes in a meaningful manner to the growing amount of data on this valuable novel technique. The protocol thus solidifies the position of methylene blue solar decontamination as an important equitable tool that can be easily deployed in those areas of the world where electricity is not readily available.
Author Contributions
Conceptualisation, B.H., E.H., E.T. and L.F.L.-B.; Data curation, A.F. and L.F.L.-B.; Formal analysis, A.F. and L.F.L.-B.; Funding acquisition, E.T. and L.F.L.-B.; Investigation, A.F., L.D. and L.F.L.-B.; Methodology, L.D., C.W., E.T. and L.F.L.-B.; Project administration, E.T. and L.F.L.-B.; Supervision, E.T. and L.F.L.-B.; Validation, E.T. and L.F.L.-B.; Visualization, A.F. and L.F.L.-B.; Writing—original draft, A.F. and L.F.L.-B.; Writing—review and editing, L.D., B.H., E.T. and L.F.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by German Federal Ministry of Health (BMG) COVID-19 Research and Development Funding to the WHO and by two ULiège Fonds Spéciaux pour la Recherche grants (Crédits Sectoriels de Recherche en Sciences de la Santé—2021 and FSR Crédits Classiques—2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The MNV1-CW1 isolate was a kind gift from H. Virgin (Washington University, St. Louis, MO, USA).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- McMahon, D.E.; Peters, G.A.; Ivers, L.C.; Freeman, E.E. Global resource shortages during covid-19: Bad news for low-income countries. PLoS Negl. Trop. Dis. 2020, 14, e0008412. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020; pp. 1–7.
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed]
- WHO. Strengthening the Health System Response to COVID-19 Recommendations for the WHO European Region Policy Brief; WHO: Geneva, Switzerland, 2020.
- The Lancet. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Tesfaldet, Y.T.; Ndeh, N.T. Assessing face masks in the environment by means of the DPSIR framework. Sci. Total Environ. 2022, 814, 152859. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Pashalidis, I. Single-use surgical face masks, as a potential source of microplastics: Do they act as pollutant carriers? J. Mol. Liq. 2021, 326, 115247. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.J.; Schlich, T. A history of the medical mask and the rise of throwaway culture. Lancet 2020, 396, 19–20. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Mustapha, K.B.; Godsell, J.; Adamu, Z.; Babatunde, K.A.; Akintade, D.D.; Acquaye, A.; Fujii, H.; Ndiaye, M.M.; Yamoah, F.A.; et al. A critical analysis of the impacts of COVID-19 on the global economy and ecosystems and opportunities for circular economy strategies. Resour. Conserv. Recycl. 2021, 164, 105169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bong, C.; Lim, W.; Bae, P.K.; Abafogi, A.T.; Baek, S.H.; Shin, Y.-B.; Bak, M.S.; Park, S. Fast and easy disinfection of coronavirus-contaminated face masks using ozone gas produced by a dielectric barrier discharge plasma generator. Environ. Sci. Technol. Lett. 2020, 8, 339–344. [Google Scholar] [CrossRef]
- Dutch National Institute for Public Health and the Environment (RIVM). Reuse of FFP2 Masks; RIVM: Utrecht, The Netherlands, 2020; pp. 1–5. [Google Scholar]
- Center for Devices and Radiological Health. Enforcement Policy for Face Masks and Respirators during the Coronavirus Disease (COVID-19) Public Health Emergency (Revised) Guidance for Industry and Food and Drug Administration Staff; Center for Devices and Radiological Health: Silver Spring, MD, USA, 2020. [Google Scholar]
- Croke, L. Preparing for the next infectious disease pandemic. Aorn J. 2020, 112, P12–P14. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef]
- Wielick, C.; Ludwig-Begall, L.F.; Dams, L.; Razafimahefa, R.M.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Jolois, O.; Farnir, F.; Haubruge, E.; et al. The use of germicidal ultraviolet light, vaporised hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with an infectious norovirus. Infect. Prev. Pract. 2020, 3, 100111. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Wielick, C.; Jolois, O.; Dams, L.; Razafimahefa, R.M.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Thiry, E.; et al. “Don, doff, discard” to “don, doff, decontaminate”—FFR and mask integrity and inactivation of a SARS-CoV-2 surrogate and a norovirus following multiple vaporised hydrogen peroxide-, ultraviolet germicidal irradiation-, and dry heat decontaminations. PLoS ONE 2021, 16, e0251872. [Google Scholar] [CrossRef] [PubMed]
- Lendvay, T.S.; Chen, J.; Harcourt, B.H.; Scholte, F.E.M.; Lin, Y.L.; Kilinc-Balci, F.S.; Lamb, M.M.; Homdayjanakul, K.; Cui, Y.; Price, A.; et al. Addressing personal protective equipment (PPE) decontamination: Methylene blue and light inactivates SARS-COV-2 on N95 respirators and medical masks with maintenance of integrity and fit. Infect. Control Hosp. Epidemiol. 2021, 43, 876–885. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef]
- Seghatchian, J.; Walker, W.H.; Reichenberg, S. Updates on pathogen inactivation of plasma using Theraflex methylene blue system. Transfus. Apher. Sci. 2008, 38, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Genina, E.A.; Bashkatov, A.N.; Chikina, E.E.; Knyazev, A.B.; Mareev, O.V.; Tuchin, V.V. Methylene blue mediated laser therapy of maxillary sinusitis. Laser Phys. 2006, 16, 1128–1133. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef]
- Zonta, W.; Mauroy, A.; Farnir, F.; Thiry, E. Virucidal Efficacy of a Hydrogen Peroxide Nebulization Against Murine Norovirus and Feline Calicivirus, Two Surrogates of Human Norovirus. Food Environ. Virol. 2016, 8, 275–282. [Google Scholar] [CrossRef]
- Wielick, C.; Fries, A.; Dams, L.; Razafimahefa, R.M.; Heyne, B.; Harcourt, B.H.; Lendvay, T.S.; Willaert, J.F.; de Jaeger, S.; Haubruge, E.; et al. Of masks and methylene blue—The use of methylene blue photochemical treatment to decontaminate surgical masks contaminated with a tenacious small non-enveloped norovirus. Am. J. Infect. Control 2021, 50, 871–877. [Google Scholar] [CrossRef]
- Cozzi, L.; Gould, T.; Bouckart, S.; Crow, D.; Kim, T.Y.; Mcglade, C.; Olejarnik, P.; Wanner, B.; Wetzel, D. World Energy Outlook 2021; IEA: Paris, France, 2021. [Google Scholar]
- Vos, K.A.; Gordon, P.M.K.; Heyne, B. Methylene blue in combination with sunlight as a low cost and effective disinfection method for coronavirus-contaminated PPE. Am. J. Infect. Control 2022, 50, 906–908. [Google Scholar] [CrossRef] [PubMed]
- McClurkin, A.W.; Norman, J.O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can. J. Comp. Med. Vet. Sci. 1966, 30, 190–198. [Google Scholar] [PubMed]
- Cox, E.; Hooyberghs, J.; Pensaert, M.B. Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res. Vet. Sci. Sci. 1990, 48, 165–169. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Raiteux, J.; Eschlimann, M.; Marangon, A.; Rogée, S.; Dadvisard, M.; Taysse, L.; Larigauderie, G. Inactivation of SARS-CoV-2 by Simulated Sunlight on Contaminated Surfaces. Microbiol. Spectr. 2021, 9, e0033321. [Google Scholar] [CrossRef]
- Zonta, W.; Mauroy, A.; Farnir, F.; Thiry, E. Comparative Virucidal Efficacy of Seven Disinfectants Against Murine Norovirus and Feline Calicivirus, Surrogates of Human Norovirus. Food Environ. Virol. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Lytle, C.D. Estimated Inactivation of Coronaviruses by Solar Radiation With Special Reference to COVID-19. Photochem. Photobiol. 2020, 96, 731–737. [Google Scholar] [CrossRef]
- Schuit, M.; Ratnesar-Shumate, S.; Yolitz, J.; Williams, G.; Weaver, W.; Green, B.; Miller, D.; Krause, M.; Beck, K.; Wood, S.; et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020, 222, 564–571. [Google Scholar] [CrossRef]
- Bosch, A.; Pintó, R.M.; Abad, F.X. Survival and Transport of Enteric Viruses in the Environment. In Viruses in Foods; Springer: Boston, MA, USA, 2006; pp. 151–187. [Google Scholar]
- Levy, R. NASA Earth Observatory. Incoming Sunlight. 2022. Available online: https://earthobservatory.nasa.gov/features/EnergyBalance/page2.php (accessed on 8 November 2022).
- Scholte, F.E.; Kabra, K.B.; Tritsch, S.R.; Montgomery, J.M.; Spiropoulou, C.F.; Mores, C.N.; Harcourt, B.H. Exploring inactivation of SARS-CoV-2, MERS-CoV, Ebola, Lassa, and Nipah viruses on N95 and KN95 respirator material using photoactivated methylene blue to enable reuse. Am. J. Infect. Control 2022, 50, 863–870. [Google Scholar] [CrossRef]
- Shen, X.; Dong, L.; He, X.; Zhao, C.; Zhang, W.; Li, X.; Lu, Y. Treatment of infected wounds with methylene blue photodynamic therapy: An effective and safe treatment method. Photodiagnosis Photodyn. Ther. 2020, 32, 102051. [Google Scholar] [CrossRef] [PubMed]
- Sellera, F.P.; Barbosa, B.S.; Gargano, R.G.; Ríspoli, V.F.P.; Sabino, C.P.; Ollhoff, R.D.; Baptista, M.S.; Ribeiro, M.S.; de Sá, L.R.M.; Pogliani, F.C. Methylene blue-mediated antimicrobial photodynamic therapy can be a novel non-antibiotic platform for bovine digital dermatitis. Photodiagnosis Photodyn. Ther. 2021, 34, 102274. [Google Scholar] [CrossRef] [PubMed]
- Lendvay, T.S.; Xu, J.; Chen, J.; Clark, T.; Cui, Y. Methylene blue applied to N95 respirators and medical masks for SARS-CoV-2 decontamination: What is the likelihood of inhaling methylene blue? Am. J. Infect. Control 2022, 50, 857–862. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).