Inundation Depth Shape Phenotypic Variability of Phragmites australis in Liaohe Estuary Wetland, Northeast China
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Collection and Measurement of Samples
2.3. Data Processing and Statistics
3. Results
3.1. Comparison of P. australis Functional Traits
3.2. Variation Analysis of Functional Traits
3.3. Correlation between Traits and Inundation Depth
3.4. Variation Structure among P. australis Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirwan, M.L.; Megonigal, J.P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Reef, R.; Masqué, P. Vulnerability of an arid zone coastal wetland landscape to sea level rise and intense storms. Limnol. Oceanogr. 2021, 66, 3976–3989. [Google Scholar] [CrossRef]
- Mengel, M.; Nauels, A.; Rogelj, J.; Schleussner, C.F. Committed sea-level rise under the Paris Agreement and the legacy of delayed mitigation action. Nat. Commun. 2018, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Shirzaei, M.; Bürgmann, R. Global climate change and local land subsidence exacerbate inundation risk to the San Francisco Bay Area. Sci. Adv. 2018, 4, eaap9234. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.B.; Wigand, C.; Davey, E.W.; Andrews, H.M.; Bishop, J.; Raposa, K.B. Wetland loss patterns and inundation-productivity relationships prognosticate widespread salt for southern New England. Estuaries Coast 2017, 40, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.L.; Staudhammer, C.L.; Oberbauer, S.F.; Olivas, P.; Ryan, M.G.; Schedlbauer, J.L.; Loescher, H.W.; Starr, G. El Niño Southern Oscillation (ENSO) enhances CO2 exchange rates in freshwater Marsh ecosystems in the Florida everglades. PLoS ONE 2014, 9, e115058. [Google Scholar] [CrossRef]
- Webb, R.H.; Leake, S.A. Ground-water surface-water interactions and long-term change in riverine riparian vegetation in the southwestern United States. J. Hydrol. 2006, 320, 302–323. [Google Scholar] [CrossRef]
- Zhao, J.; Malone, S.L.; Oberbauer, S.F.; Olivas, P.C.; Schedlbauer, J.L.; Staudhammer, C.L.; Starr, G. Intensified inundation shifts a freshwater wetland from a CO(2) sink to a source. Glob. Chang. Biol. 2019, 25, 3319–3333. [Google Scholar] [CrossRef]
- Murren, C.J.; Auld, J.R.; Callahan, H.; Ghalambor, C.K.; Handelsman, C.A.; Heskel, M.A.; Kingsolver, J.G.; Maclean, H.J.; Masel, J.; Maughan, H.; et al. Constraints on the evolution of phenotypic plasticity: Limits and costs of phenotype and plasticity. Heredity 2015, 115, 293–301. [Google Scholar] [CrossRef]
- Maes, S.L.; Perring, M.P.; Depauw, L.; Bernhardt-Römermann, M.; Blondeel, H.; Brūmelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; Govaert, S.; et al. Plant functional trait response to environmental drivers across European temperate forest understorey communities. Plant Biol. 2020, 22, 410–424. [Google Scholar] [CrossRef]
- Bhattarai, G.P.; Meyerson, L.A.; Anderson, J.; Cummings, D.; Allen, W.J.; Cronin, J.T. Biogeography of a plant invasion: Genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecol. Monogr. 2016, 87, 57–75. [Google Scholar] [CrossRef]
- Moore, T.E.; Schlichting, C.D.; Aiello-Lammens, M.E.; Mocko, K.; Jones, C.S. Divergent trait and environment relationships among parallel radiations in Pelargonium (Geraniaceae): A role for evolutionary legacy? New Phytol. 2018, 219, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tomlinson, K.W.; Li, J. Strong intraspecific trait variation in a tropical dominant tree species along an elevational gradient. Plant Divers. 2020, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; de Kroon, H. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhao, W.; Xing, M.; Zhao, J.; Jiang, Z.; You, J.; Ni, B.; Ni, Y.; Liu, C.; Li, J.; et al. Resource allocation strategies among vegetative growth, sexual reproduction, asexual reproduction and defense during growing season of Aconitum kusnezoffii Reichb. Plant J. 2021, 105, 957–977. [Google Scholar] [CrossRef] [PubMed]
- Rubio de Casas, R.; Willis, C.G.; Pearse, W.D.; Baskin, C.C.; Baskin, J.M.; Cavender-Bares, J. Global biogeography of seed dormancy is determined by seasonality and seed size: A case study in the legumes. New Phytol. 2017, 214, 1527–1536. [Google Scholar] [CrossRef]
- Metz, J.; Lampei, C.; Bäumler, L.; Bocherens, H.; Dittberner, H.; Henneberg, L.; de Meaux, J.; Tielbörger, K. Rapid adaptive evolution to drought in a subset of plant traits in a large-scale climate change experiment. Ecol. Lett. 2020, 23, 1643–1653. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Shipley, B.; De Bello, F.; Cornelissen, J.H.; Laliberté, E.; Laughlin, D.C.; Reich, P.B. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 2016, 180, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 101, e02924. [Google Scholar] [CrossRef] [PubMed]
- Pither, J. Climate tolerance and interspecific variation in geographic range size. Proc. Biol. Sci. 2003, 270, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.A.; Morgan, P.A.; Tilburg, C.E.; Travis, S.E. Environmental variables, not Allee effects, drive patch vigor in exotic Phragmites australis stands invading the Saco River Estuary, Maine, USA. Aquat. Bot. 2017, 136, 220–229. [Google Scholar] [CrossRef]
- Ward, D.; Shrestha, M.K.; Golan-Goldhirsh, A. Evolution and ecology meet molecular genetics: Adaptive phenotypic plasticity in two isolated Negev desert populations of Acacia raddiana at either end of a rainfall gradient. Ann. Bot. 2012, 109, 247–255. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Yin, M.; Eller, F.; Liu, L.; Brix, H.; Guo, W. Transcriptome Analysis of Tetraploid and Octoploid Common Reed (Phragmites australis). Front. Plant Sci. 2021, 12, 653183. [Google Scholar] [CrossRef]
- Messier, J.; Lechowicz, M.J.; McGill, B.J.; Violle, C.; Enquist, B.J.; Cornelissen, H. Interspecific integration of trait dimensions at local scales: The plant phenotype as an integrated network. J. Ecol. 2017, 105, 1775–1790. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan Species As Models for Ecophysiological Responses to Global Change: The Common Reed Phragmites australis. Front. Plant Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Berthold, M.; Karstens, S.; Buczko, U.; Schumann, R. Potential export of soluble reactive phosphorus from a coastal wetland in a cold-temperate lagoon system: Buffer capacities of macrophytes and impact on phytoplankton. Sci. Total Environ. 2018, 616–617, 46–54. [Google Scholar] [CrossRef]
- Xue, L.; Li, X.; Yan, Z.; Zhang, Q.; Ding, W.; Huang, X.; Tian, B.; Ge, Z.; Yin, Q. Native and non-native halophytes resiliency against sea-level rise and saltwater intrusion. Hydrobiologia 2017, 806, 47–65. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Sardans, J.; Tong, C.; Jia, R.; Zeng, C.; Peñuelas, J. Flood regime affects soil stoichiometry and the distribution of the invasive plants in subtropical estuarine wetlands in China. Catena 2015, 128, 144–154. [Google Scholar] [CrossRef]
- Szura, K.; McKinney, R.; Wigand, C.; Oczkowski, A.; Hanson, A.; Gurak, J.; Gárate, M. Burrowing and foraging activity of marsh crabs under different inundation regimes. J. Exp. Mar. Biol. Ecol. 2017, 486, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, G. Salt tolerance of the African haplotype of Phragmites australis (Poaceae). Afr. J. Ecol. 2021, 59, 724–734. [Google Scholar] [CrossRef]
- Ma, X.; Yan, J.; Wang, F.; Qiu, D.; Jiang, X.; Liu, Z.; Sui, H.; Bai, J.; Cui, B. Trait and density responses of Spartina alterniflora to inundation in the Yellow River Delta, China. Mar. Pollut. Bull. 2019, 146, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Farrer, E.C.; Birnbaum, C.; Waryszak, P.; Halbrook, S.R.; Brady, M.V.; Bumby, C.R.; Candaele, H.; Kulick, N.K.; Lee, S.F.H.; Schroeder, C.S.; et al. Plant and microbial impacts of an invasive species vary across an environmental gradient. J. Ecol. 2021, 109, 2163–2176. [Google Scholar] [CrossRef]
- Cerri, M.; Ferranti, F.; Coppi, A.; Foggi, B.; Gigante, D.; Lastrucci, L.; Onofri, A.; Venanzoni, R.; Viciani, D.; Reale, L. Influence of die-back syndrome on reproductive strategies within Phragmites australis populations. Plant Biosyst. —Int. J. Deal. All Asp. Plant Biol. 2018, 153, 250–256. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.; Verhoeven, J.T.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Gao, L.; Tang, S.; Zhuge, L.; Nie, M.; Zhu, Z.; Li, B.; Yang, J. Spatial genetic structure in natural populations of Phragmites australis in a mosaic of saline habitats in the Yellow River Delta, China. PLoS ONE 2012, 7, e43334. [Google Scholar] [CrossRef]
- Zhou, D.; Ni, Y.; Yu, X.; Lin, K.; Du, N.; Liu, L.; Guo, X.; Guo, W. Trait-based adaptability of Phragmites australis to the effects of soil water and salinity in the Yellow River Delta. Ecol. Evol. 2021, 11, 11352–11361. [Google Scholar] [CrossRef]
- Clevering, O.A.; Brix, H.; Lukavská, J. Geographic variation in growth responses in Phragmites australis. Aquat. Bot. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Cronin, J.T.; Bhattarai, G.P.; Brix, H.; Lambertini, C.; Lučanová, M.; Rinehart, S.; Suda, J.; Pyšek, P. Do ploidy level and nuclear genome size and latitude of origin modify the expression of Phragmites australis traits and interactions with herbivores? Biol. Invasions 2016, 18, 2531–2549. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Riis, T.; Raven, B.M.; Hoffman, C.C.; Verhoeven, J.T.A.; Soons, M.B. Effects of increased flooding on riparian vegetation: Field experiments simulating climate change along five European lowland streams. Glob. Chang. Biol. 2017, 23, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.S.; Javaid, A.; Hameed, M.; Fatima, S.; Ahmad, F.; Ashraf, M.; Shah, S.M.R.; Ahmad, M.S.A.; Ahmad, I. Survival strategies in two high altitude Sorghum species from western Himalayas. Acta Physiol. Plant. 2022, 44, 60. [Google Scholar] [CrossRef]
- Striker, G.G.; Izaguirre, R.F.; Manzur, M.E.; Grimoldi, A.A. Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biol. 2012, 14, 50–55. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef]
- Akman, M.; Bhikharie, A.V.; McLean, E.H.; Boonman, A.; Visser, E.J.; Schranz, M.E.; van Tienderen, P.H. Wait or escape? Contrasting submergence tolerance strategies of Rorippa amphibia, Rorippa sylvestris and their hybrid. Ann. Bot. 2012, 109, 1263–1276. [Google Scholar] [CrossRef]
- Morris, J.T.; Sundberg, K.; Hopkinson, C.S. Salt marsh primary production and its responses to relative sea level and nutrients in estuaries at Plum Island, Massachusetts, and North Inlet, South Carolina, USA. Oceanography 2013, 26, 78–84. [Google Scholar] [CrossRef]
- Hanganu, J.; Mihail, G.; Coops, H. Responses of ecotypes of Phragmites australis to increased seawater influence: A field study in the Danube Delta, Romania. Aquat. Bot. 1999, 64, 351–358. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, Y.F.; Cai, Y.H.; Xie, Y.H.; Deng, Z.M.; Li, F.; Hou, Z.Y. Differential Strategies to Tolerate Flooding in Polygonum hydropiper Plants Originating from Low- and High-Elevation Habitats. Front. Plant Sci. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hameed, M.; Hamid, A.; Nawaz, F.; Kiani, B.H.; Ahmad, M.S.A.; Deng, J.; Ahmad, F.; Hussain, I.; Fatima, S. Beating cold by being tough: Impact of elevation on leaf characteristics in Phleum himalaicum Mez. endemic to Himalaya. Acta Physiol. Plant. 2018, 40, 56. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms Underlying the Environmentally Induced Plasticity of Leaf Morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Radersma, R.; Noble, D.W.A.; Uller, T. Plasticity leaves a phenotypic signature during local adaptation. Evol. Lett. 2020, 4, 360–370. [Google Scholar] [CrossRef]
- Miljković, D.; Čortan, D. Morphometric and morphological analysis of Populus nigra L. leaves in flooded regions. Šumarski List 2020, 144, 139–147. [Google Scholar] [CrossRef]
- Yoshinaka, K.; Nagashima, H.; Yanagita, Y.; Hikosaka, K. The role of biomass allocation between lamina and petioles in a game of light competition in a dense stand of an annual plant. Ann. Bot. 2018, 121, 1055–1064. [Google Scholar] [CrossRef]
- Tsukaya, H. Leaf shape diversity with an emphasis on leaf contour variation, developmental background, and adaptation. Semin. Cell Dev. Biol. 2018, 79, 48–57. [Google Scholar] [CrossRef]
- Lou, Y.; Pan, Y.; Gao, C.; Jiang, M.; Lu, X.; Xu, Y.J. Response of Plant Height, Species Richness and Aboveground Biomass to Flooding Gradient along Vegetation Zones in Floodplain Wetlands, Northeast China. PLoS ONE 2016, 11, e0153972. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Gao, Y.; Tong, S. Emergence and growth performance of Bolboschoenus planiculmis varied in response to water level and soil planting depth: Implications for wetland restoration using tuber transplantation. Aquat. Bot. 2018, 148, 10–14. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z.; Bachofen, C.; Lou, Y.; Jiang, M.; Tang, X.; Lu, X.; Buchmann, N. The effect of saline-alkaline and water stresses on water use efficiency and standing biomass of Phragmites australis and Bolboschoenus planiculmis. Sci. Total Environ. 2018, 644, 207–216. [Google Scholar] [CrossRef]
- Ma, X.z.; Wang, X.p. Aboveground and belowground biomass and its’ allometry for Salsola passerina shrub in degraded steppe desert in Northwestern China. Land Degrad. Dev. 2020, 32, 714–722. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Kim, J.G. The optimal balance between sexual and asexual reproduction in variable environments: A systematic review. J. Ecol. Environ. 2016, 40, 12. [Google Scholar] [CrossRef]
- Lastrucci, L.; Gigante, D.; Vaselli, O.; Nisi, B.; Viciani, D.; Reale, L.; Coppi, A.; Fazzi, V.; Bonari, G.; Angiolini, C. Sediment chemistry and flooding exposure: A fatal cocktail for Phragmites australis in the Mediterranean basin? Ann. Limnol. Int. J. Limnol. 2016, 52, 365–377. [Google Scholar] [CrossRef]
- Ye, X.H.; Yu, F.H.; Dong, M. A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Ann. Bot. 2006, 98, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bargali, K.; Tewari, A. Growth and water relation parameters in drought-stressed Coriaria nepalensis seedlings. J. Arid. Environ. 2004, 58, 505–512. [Google Scholar] [CrossRef]
- Rose, L.; Buitenwerf, R.; Cramer, M.; February, E.C.; Higgins, S.I. Effects of nutrient supply on carbon and water economies of C(4) grasses. Funct. Plant Biol. 2018, 45, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sun, T.; Zhu, M.S.; Qi, M.; Yang, W.; Shao, D.D. Salt marsh vegetation distribution patterns along groundwater table and salinity gradients in yellow river estuary under the influence of land reclamation. Ecol. Indic. 2018, 92, 82–90. [Google Scholar] [CrossRef]
- Schenck, F.R.; Hanley, T.C.; Beighley, R.E.; Hughes, A.R. Phenotypic variation among invasive Phragmites australis populations does not influence salinity tolerance. Estuaries Coasts 2018, 41, 896–907. [Google Scholar] [CrossRef]
- Posey, M.H.; Alphin, T.D.; Meyer, D.L.; Johnson, J.M. Benthic communities of common reed Phragmites australis and marsh cordgrass Spartina alterniflora marshes in Chesapeake Bay. Mar. Ecol. Prog. Ser. 2003, 261, 51–61. [Google Scholar] [CrossRef][Green Version]
- Price, A.L.; Fant, J.B.; Larkin, D.J. Ecology of native vs. introduced Phragmites australis (common reed) in Chicago-area wetlands. Wetlands 2014, 34, 369–377. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Hou, A.; Han, G.; Wang, G.; Qu, F.; Xia, J.; Wang, X. The ecological adaptability of Phragmites australis to interactive effects of water level and salt stress in the Yellow River Delta. Aquat. Ecol. 2016, 51, 107–116. [Google Scholar] [CrossRef]
- Łuczak, K.; Czerniawska-Kusza, I.; Rosik-Dulewska, C.; Kusza, G. Effect of NaCl road salt on the ionic composition of soils and Aesculus hippocastanum L. foliage and leaf damage intensity. Sci. Rep. 2021, 11, 5309. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, P.W.; Nowell, C.F.; Leatherman, L.S.T.; Gong, W.; Cruz, B.C.; Burge, D.O.; Delgado-Salinas, A. Leaf adaptations and species boundaries in North American Cercis: Implications for the evolution of dry floras. Am. J. Bot. 2018, 105, 1577–1594. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F.; et al. Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Fazlioglu, F.; Bonser, S.P. Phenotypic plasticity and specialization in clonal versus non-clonal plants: A data synthesis. Acta Oecol. 2016, 77, 193–200. [Google Scholar] [CrossRef]
- Palacio-López, K.; Beckage, B.; Scheiner, S.; Molofsky, J. The ubiquity of phenotypic plasticity in plants: A synthesis. Ecol. Evol. 2015, 5, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, Y.; Lou, Y.; Jiang, M.; Li, H.; Lü, X. How soil ion stress and type influence the flooding adaptive strategies of Phragmites australis and Bolboschoenus planiculmis in temperate saline-alkaline wetlands? Sci. Total Environ. 2021, 771, 144654. [Google Scholar] [CrossRef] [PubMed]
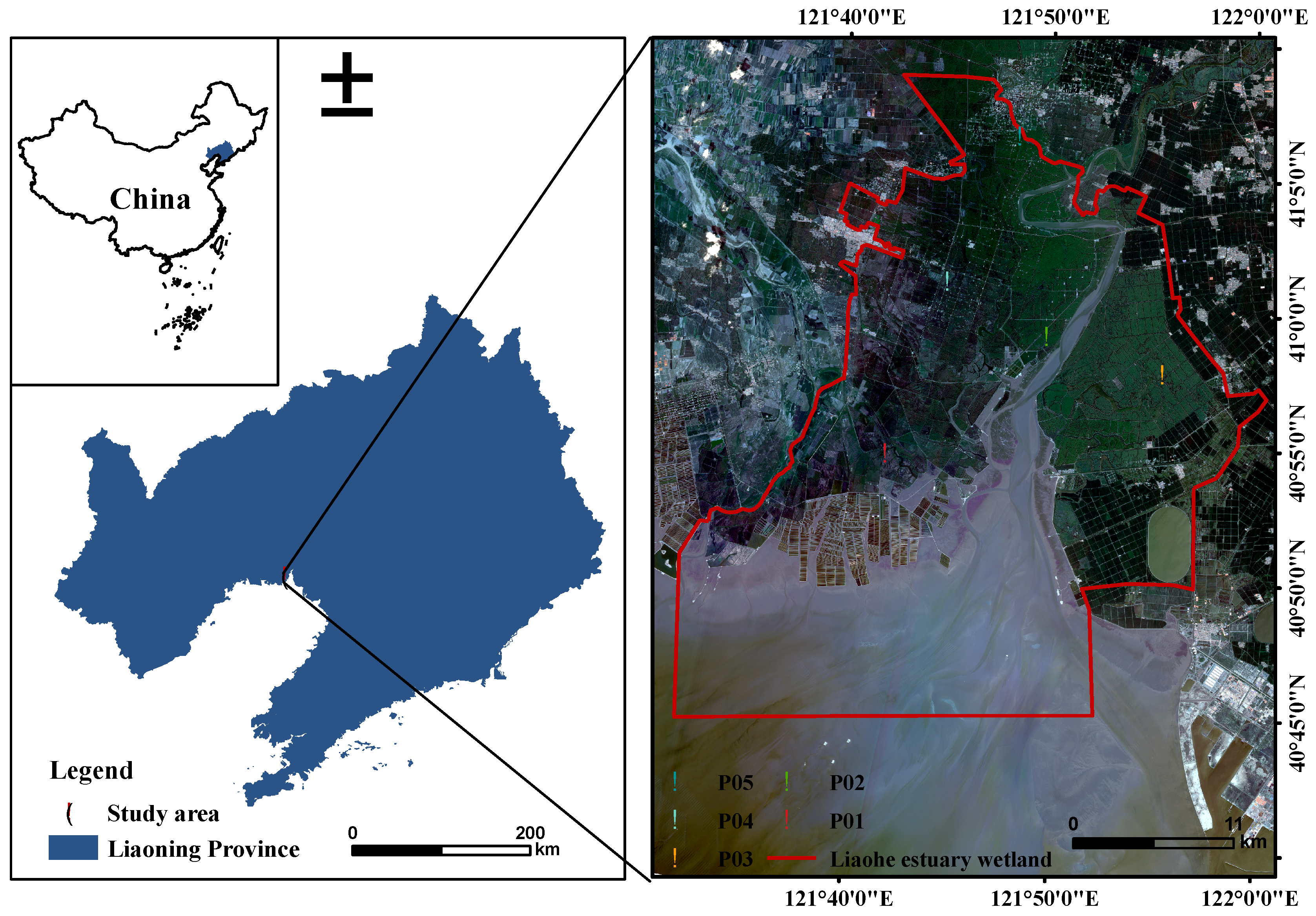

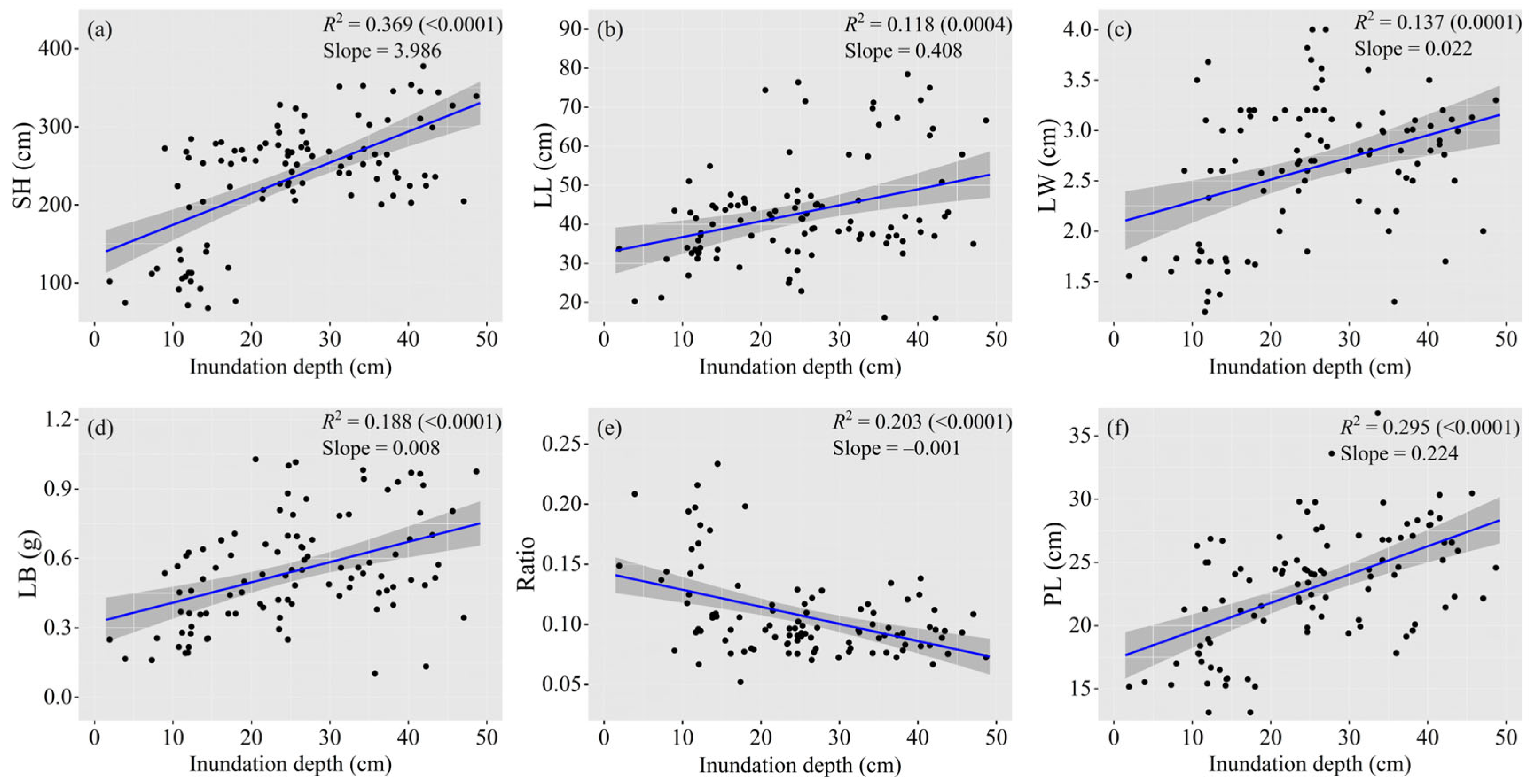
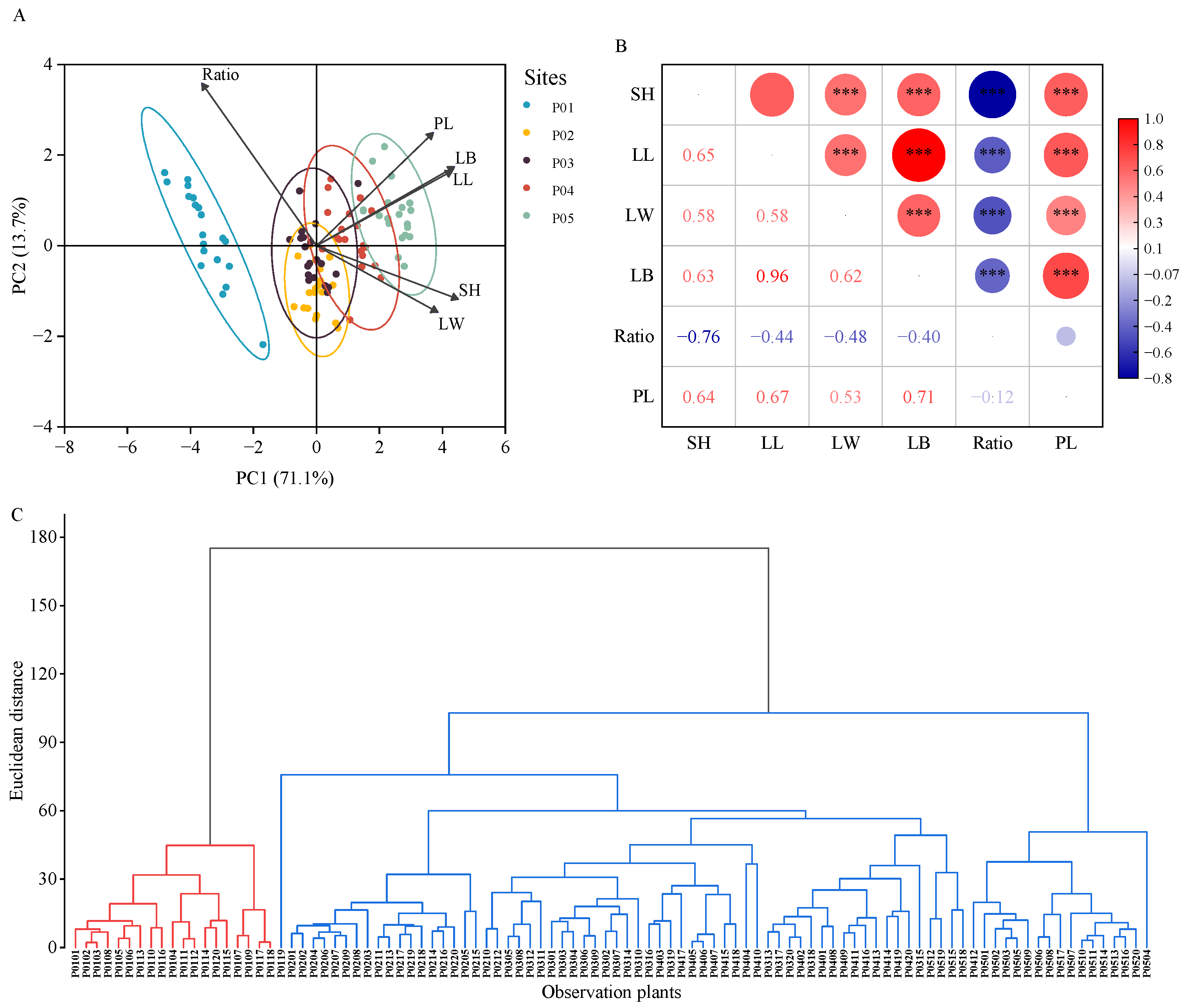
| Sites | Habitat | Depth (cm) | Coexistence of Plant Species |
|---|---|---|---|
| P01 | The intertidal zone with Suaeda salsa invasion | 11.44 ± 3.87 d | Suaeda salsa, Scirpus planiculmis |
| P02 | The intertidal zone near the Liaohe River | 22.90 ± 9.16 c | Suaeda salsa, Scirpus planiculmis, Cirsium setosum, Suaeda glauca |
| P03 | The supralittoral zone closed to paddy field | 30.56 ± 8.55 ab | Suaeda salsa, Scirpus planiculmis, Cirsium setosum, Suaeda glauca, Lagedium sibiricum,Glycine soja |
| P04 | Abandoned oil field after stoppage | 25.40 ± 8.38 bc | Cirsium setosum, Suaeda glauca, Lagedium sibiricum,Glycine soja, Kochia scoparia, |
| P05 | The inland river zone in Dongguo Town | 36.18 ± 7.79 a | Kochia scoparia, Chenopodium Acuminatum, Cynanchum chinense, Imperata cylindrica, Polygonum sibiricum, Artemisia Annua, Calamagrostis epigeios |
| Traits | P01 | P02 | P03 | P04 | P05 | F |
|---|---|---|---|---|---|---|
| Shoot height (cm) | 111.19 ± 29.90 | 267.91 ± 14.81 | 235.65 ± 24.01 | 245.93 ± 29.76 | 315.66 ± 39.69 | 145.66 *** |
| Leaf length (cm) | 35.79 ± 8.78 | 41.66 ± 4.99 | 34.23 ± 7.95 | 39.43 ± 5.61 | 63.81 ± 10.89 | 47.93 *** |
| Leaf width (cm) | 1.65 ± 0.23 | 2.91 ± 0.44 | 2.39 ± 0.43 | 3.22 ± 0.42 | 2.95 ± 0.32 | 52.98 *** |
| Leaf biomass (g) | 0.45 ± 0.05 | 0.66 ± 0.10 | 0.69 ± 0.13 | 0.86 ± 0.05 | 0.96 ± 0.10 | 91.72 *** |
| Ratio | 0.16 ± 0.04 | 0.09 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.01 | 32.29 *** |
| Panicle length (cm) | 16.62 ± 1.81 | 23.30 ± 2.34 | 22.45 ± 3.62 | 24.62 ± 3.33 | 27.92 ± 2.93 | 40.84 *** |
| Site | CVSH | CVLL | CVLW | CVLB | CVR | CVPL | Mean |
|---|---|---|---|---|---|---|---|
| P01 | 0.27 | 0.25 | 0.14 | 0.11 | 0.25 | 0.11 | 0.19 |
| P02 | 0.05 | 0.12 | 0.15 | 0.15 | 0.11 | 0.10 | 0.11 |
| P03 | 0.10 | 0.23 | 0.18 | 0.19 | 0.20 | 0.16 | 0.18 |
| P04 | 0.12 | 0.14 | 0.13 | 0.06 | 0.20 | 0.14 | 0.13 |
| P05 | 0.13 | 0.17 | 0.11 | 0.10 | 0.11 | 0.10 | 0.12 |
| Mean | 0.13 | 0.18 | 0.14 | 0.34 | 0.22 | 0.12 | 0.19 |
| P01 | P02 | P03 | P04 | P05 | |
|---|---|---|---|---|---|
| P01 | 0 | ||||
| P02 | 156.986 | 0 | |||
| P03 | 124.618 | 33.120 | 0 | ||
| P04 | 135.039 | 22.140 | 11.750 | 0 | |
| P05 | 206.700 | 52.838 | 85.478 | 73.946 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, P.; Su, F.; Zhou, F. Inundation Depth Shape Phenotypic Variability of Phragmites australis in Liaohe Estuary Wetland, Northeast China. Sustainability 2022, 14, 14911. https://doi.org/10.3390/su142214911
Cui P, Su F, Zhou F. Inundation Depth Shape Phenotypic Variability of Phragmites australis in Liaohe Estuary Wetland, Northeast China. Sustainability. 2022; 14(22):14911. https://doi.org/10.3390/su142214911
Chicago/Turabian StyleCui, Panpan, Fangli Su, and Fang Zhou. 2022. "Inundation Depth Shape Phenotypic Variability of Phragmites australis in Liaohe Estuary Wetland, Northeast China" Sustainability 14, no. 22: 14911. https://doi.org/10.3390/su142214911
APA StyleCui, P., Su, F., & Zhou, F. (2022). Inundation Depth Shape Phenotypic Variability of Phragmites australis in Liaohe Estuary Wetland, Northeast China. Sustainability, 14(22), 14911. https://doi.org/10.3390/su142214911





