Abstract
Strawberry is a delicate fruit with a short shelf life after harvest. High metabolic activities and fungal decay deteriorate its quality. In an attempt to extend its shelf life and maintain its quality while retaining its antioxidant potential, the harvested fruits of strawberry cv. Chandler were dipped in 30% eucalyptus leaf extract (ELE). Weight loss, fungal decay index, percentage of decayed fruits, respiration rate, and electrolyte leakage were all reduced in ELE-treated strawberries. The enzymatic activities of catalase in fruits increased initially until the fifth day of storage but then started to decline during the later period while superoxide dismutase and peroxidase activities continued to rise until the 10th day and then declined. With higher contents of total phenolics, ascorbic acid, total antioxidants, and anthocyanin, the application of ELE maintained the general acceptance, firmness, flavor, and marketable fruit percentage. Titrable acidity (TA) and sugar–acid ratio (SAR) were also significantly affected by ELE application. A declining trend was observed in TA during storage, along with an increase in SAR. The pH and soluble solid contents of strawberry juice were not significantly changed by the application of ELE; however, both attribute scores were greater than those for the control. In conclusion, ELE could be used as an environmentally safe method to postpone senescence and maintain postharvest quality for up to 15 days.
1. Introduction
Strawberry is grown in a wide range of regions from mild maritime to severe temperate continental climates [1]. It is considered a minor fruit in Pakistan although its production in Pakistan has almost doubled during the last five years [2]. It is rich in vitamins, fibers, antioxidants, flavonoids, potassium, and phenolic acids such as hydroxybenzoic and hydroxycinnamic acids [3]. As living tissues, fruits and vegetables go through physicochemical changes during postharvest handling and storage, resulting in quality degradation [4]. According to the National Commission on Agriculture of Pakistan, postharvest losses in fruits and vegetables range from 20 to 40% [5]. Due to the strawberry’s extreme perishability, postharvest handling is a significant global issue [6]. It undergoes rapid water loss and microbial degradation while being stored [7]. Such unfavorable variations in the quality of strawberries are undesirable from the perspective of the consumer. The market life of fresh strawberries is one week when kept at 5–13 °C and after cold storage, these commodities have 3–4 days of shelf life at room temperature (20 ± 2.0 °C) [8].
The best postharvest technique to conserve the physio-chemical quality of fresh produce aims to slow down the senescence processes by lessening or preventing the occurrence of physiological disorders and lowering the risk of microbial contamination. The use of chemicals such as calcium chloride, Hydrogen sulfide, hydrogen peroxide, sulfur dioxide, etc. for shelf-life enhancement, on the other hand, is expensive as well as hazardous to human health [9,10]. In recent decades, with the increasing trend of using natural food preservatives along with cold storage due to the limited risk they present to human health, reduced environmental impact, and lower price, these are promising alternatives to traditionally used toxic chemicals. Previous research has proved that the use of plant extracts [11,12] such as propolis [13], chitosan [14], and essential oils [15] can effectively minimize postharvest fruit decay and improve fruit storability. These plant-based postharvest treatments can reduce respiration, decrease moisture migration and loss of volatile compounds, and ultimately postpone textural changes [11]. Additionally, they form a strong barrier to fats and oils and have a highly selective gas permeability ratio of CO2/O2 in comparison to traditional synthetic films [16].
Eucalyptus leaf extract (ELE) has been approved as a safe food additive and can be employed to extend the shelf lives of fruits and vegetables. The leaf extract of E. camaldulensis var. obtusa contains several compounds such as aromadendrene (1.1%), cuminaldehyde (1.9%), terpinen-4-ol (2.5%), thymol (2.7%), phellandral (3.0%), crypton (5.4%), γ-terpinene (6.5%), p-cymene (10.5%), spathulenol (21.2%), and eucalyptol/1,8-cineole (33.0%) [17,18]. When using ELE, their effectiveness against microbes, their composition and quantities, as well as the characteristics and application method of the product should be determined. Plant-based extracts containing ELE or its active ingredient i.e., eucalyptol have been reported to affect physicochemical quality attributes including improving the general appearance of produce, reducing moisture loss, maintaining firmness, reducing respiration rate, delaying oxidative browning, conserving sensory properties (taste, aroma, flavor) and inhibiting microbial growth in various fruits including passion fruit [19], grapes [10], apples [20], pears [21], nectarines [22], and mangoes [23,24]. The use of various natural products including ELE for postharvest preservation of fruits and vegetables has been extensively reported, but studies have rarely described the use of ELE in the extension of storage life and hindrance of decay while conserving the physicochemical quality of strawberries. Therefore, this experiment aimed to examine the effect of ELE on strawberries to prolong storage life by postponing senescence and preserving levels of different bioactive compounds in cold storage settings (2.0 ± 0.3 °C).
2. Materials and Methods
2.1. Fruit Source
Fresh fruits of strawberry cv. Chandler were harvested from the Horticulture Experimental Area (29°22′17.4″ N 71°45′53.6″ E) of The Islamia University of Bahawalpur (IUB) when they were commercially mature (95–100% red) and transferred to the General Laboratory, Department of Horticultural Sciences, IUB within half an hour. A total of 25 clean and healthy fruits were chosen for each of the four replications of both control and ELE treatment, and were rinsed with distilled water to remove dirt. The fruits were stored for 15 days at 2 ± 0.3 °C then analyzed for studying various physicochemical traits.
2.2. Preparation of ELE Solution
Selected leaves (of uniform size, color, freshness, and maturity) of Eucalyptus camaldulensis var. obtusa were collected from the IUB (29°37′17.4″ N 71°76′53.6″ E), Pakistan, and brought to the General Laboratory, Department of Horticultural Sciences, IUB. The leaves were washed under running water, to get rid of dirt, insects, and plankton. The leaves were then dipped for three minutes in 0.1% sodium hypochlorite and thoroughly air-dried at ambient temperature. After that, the eucalyptus leaves were added to the water at a temperature of 55 °C, and the water was heated again until it reached a temperature of 90 °C. It was kept for 10 min at that temperature and then cooled to 55 °C. The extracted juice was filtered through a muslin cloth to eliminate the spongy material. The pH of the extracted juice was maintained below 3.5 by adding 1% (v/w) citric acid [25]. After that, it was pasteurized for 45 min at 70 °C [25]. The ELE solution was then allowed to cool to room temperature before being diluted 1:1 (v/v) with distilled water for further usage. Strawberries were immersed in 30% ELE solution for five minutes. This concentration was chosen after initial testing with ELE concentrations of 0, 30, 60, and 90%. The ELE-treated strawberries were air-dried and stored for 15 days at 10 °C with a RH of 85–90%. Fruits under control conditions (those not treated with ELE) were kept beside the ELE-treated fruits. For both treatments, 250 g of strawberries were weighed, placed in plastic boxes, and this was repeated four times.
2.3. Fruit Weight Loss, Fungal Decay Index, and Decayed Fruits
The weight of the fruits for both treatments was measured using a digital weighing balance (DM-01, ScaleTech, Beijing, China) on the first and last day of each removal, which was at 5, 10, and 15 days of storage. The difference between the final weight and the initial weight, as previously reported by Ali et al. [26], was used to calculate the weight loss of control and ELE-treated strawberries during cold storage. To determine the fungal decay index (FDI), the fruits were visually examined and rated for FDI as a percent of the surface area that had decayed i.e., 1 = no decay; 2 = 5% surface area under decay; 3 = 6–20% surface area under decay; 4 = 21–50% surface area under decay; and 5 = >50% surface area under decay. The weight of decayed fruits and < 100% of decayed fruits were calculated according to the given formula and results were expressed in percentages:
2.4. Respiration Rate and Electrolyte Leakage
To determine the respiration rate of control and treated samples, 100 g strawberry fruit was weighed and added to sealed glass jars of 1 L volume for an hour. The CO2 produced in the jar was measured using a CO2 analyzer (Vaisala MI 70, Vaisala Inc., Helsinki, Finland) and denoted as mmol CO2 kg−1 h−1 as previously described by Razzaq et al. [27]. Electrolyte leakage (EL) was analyzed following the previous procedure as stated by Hasan et al. [11] with slight adjustments. Strawberry fruits were sliced into 14 equally sized discs from randomly selected control and ELE-treated fruit material, and submerged in 20 mL of deionized water for half an hour at room temperature i.e., 25 ± 1 °C. After that, the EL meter was used to obtain the initial reading (Lt) of leakage of ions from the tissues of the strawberry. Following a five-minute boil, the strawberry samples were subjected to a similar measurement procedure for final leakage (Lo). The following formula was used to determine the relative electrolyte leakage from strawberry tissues, and the results were expressed as a percentage:
2.5. Antioxidative Enzyme Activities
The stored strawberries were extracted in 2 ml of phosphate buffer (pH 7.2) with the help of a cold mortar and pestle, then centrifuged at 10,000× g for five minutes at 4 °C. Antioxidative enzyme activities were measured after the supernatant was collected. Catalase (CAT) (EC 1.11.1.6), peroxidase (POD) (EC 1.11.1.7), and superoxide dismutase (SOD) (EC 1.15.1.1) activities were assessed using the method previously described by Ali et al. [26], and samples were read at different wavelengths, i.e., 240, 470, and 560 nm, respectively.
2.6. Biochemical Quality
For the assessment of the biochemical quality of strawberry fruits, total soluble solids were determined from the extracted juice with a digital refractometer (RX-5000, Atago, Tokyo, Japan) and expressed as a percentage. Titratable acidity was determined using a titration method as previously described by Hassan et al. [16], in which strawberry juice was titrated against a 0.4% sodium hydroxide solution with phenolphthalein as an indicator. The sugar–acid ratio—also known as the ripening index—was calculated by dividing values of SSC over TA. The pH of the strawberry juice was tested with a digital pH meter (HI98130, Hanna, Nușfalău, Romania) [28]. Total phenolic contents (TPC) in strawberry fruits were quantified using the Folin–Ciocalteu assay [29], and their concentration was reported as gallic acid equivalent mg GAE kg−1 of FW after noting the absorbance at 765 nm. For the ascorbic acid quantification, strawberry fruits were homogenized and centrifuged at 10,000× g for 15 min and the supernatant was collected. The ascorbic acid quantification was carried out through a titrimetric approach with 2,6-dichlorophenol indophenol dye, and the results were expressed as mg 100 g−1 fresh fruit weight. The method described by Ali et al. [30] was used to measure 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and the results were represented as % inhibition. The total anthocyanin content was determined using the method reported by Ali et al. [26]. A single gram of supernatant was taken in a falcon tube with 15 ml of an extraction mixture made up of 0.15% HCl and 95% methanol (15:85). The extracted sample was spun at 4000× g in a temperature-controlled centrifuge (Rotofix 46, Hettich, Kirchlengern, Germany) for 4 h at 25 °C. The absorbance of the collected supernatant was measured at 530, 620, and 650 nm with a UV-Vis spectrophotometer (2326K, Hermle Labortechnik GmbH, Wehingen, Germany). The anthocyanin content was calculated using a formula that expressed total anthocyanins per gram of fresh weight:
ΔA g−1 = (A530 nm − A620 nm) − 0.1 (A650 nm − A620 nm)
2.7. General Appearance, Firmness, Flavor, and Marketable Fruits
The general appearance was assessed by analyzing the samples under scrutiny of a 5-member panel of experts in strawberry sensory analysis. The samples’ placement and labelling were at random on individual plates. Every sample was graded using the score system provided by Kader et al. [31]: 1 = unsalable, 3 = poor, 5 = fair, 7 = good, and 9 = excellent. Fruit firmness was assessed by measuring the compression force of the samples using a fruit pressure tester (53205N, Lutron Worldwide, Coopersburg, PA, USA). For flavor assessment, 12 samples of whole strawberry fruits were scored for aroma and taste using pre-defined descriptors [7]. Individual scores for aroma and taste were averaged and reported as flavor [32]. Fruit weighing more than 10 g fresh weight and free of any defect was considered marketable [5], and is reported as a percentage of total fruit inspected.
2.8. Statistical Analysis
All data were processed on Microsoft Excel 2016. The statistical software Statistix 9® (Analytical Software, Tallahassee, CA, USA) was employed to determine the differences between treatments, using two-factor analysis of variance (ANOVA, general linear model) followed by the Tukey HSD test for p ≤ 0.05. Four replicates (n = 4) were used for statistical analysis of all parameters. The Pearson correlation was assessed using Corrplot R package version 0.84.
3. Results and Discussion
3.1. Fruit Weight Loss (%), Fungal Decay Index (Score), and Decayed Fruits (%)
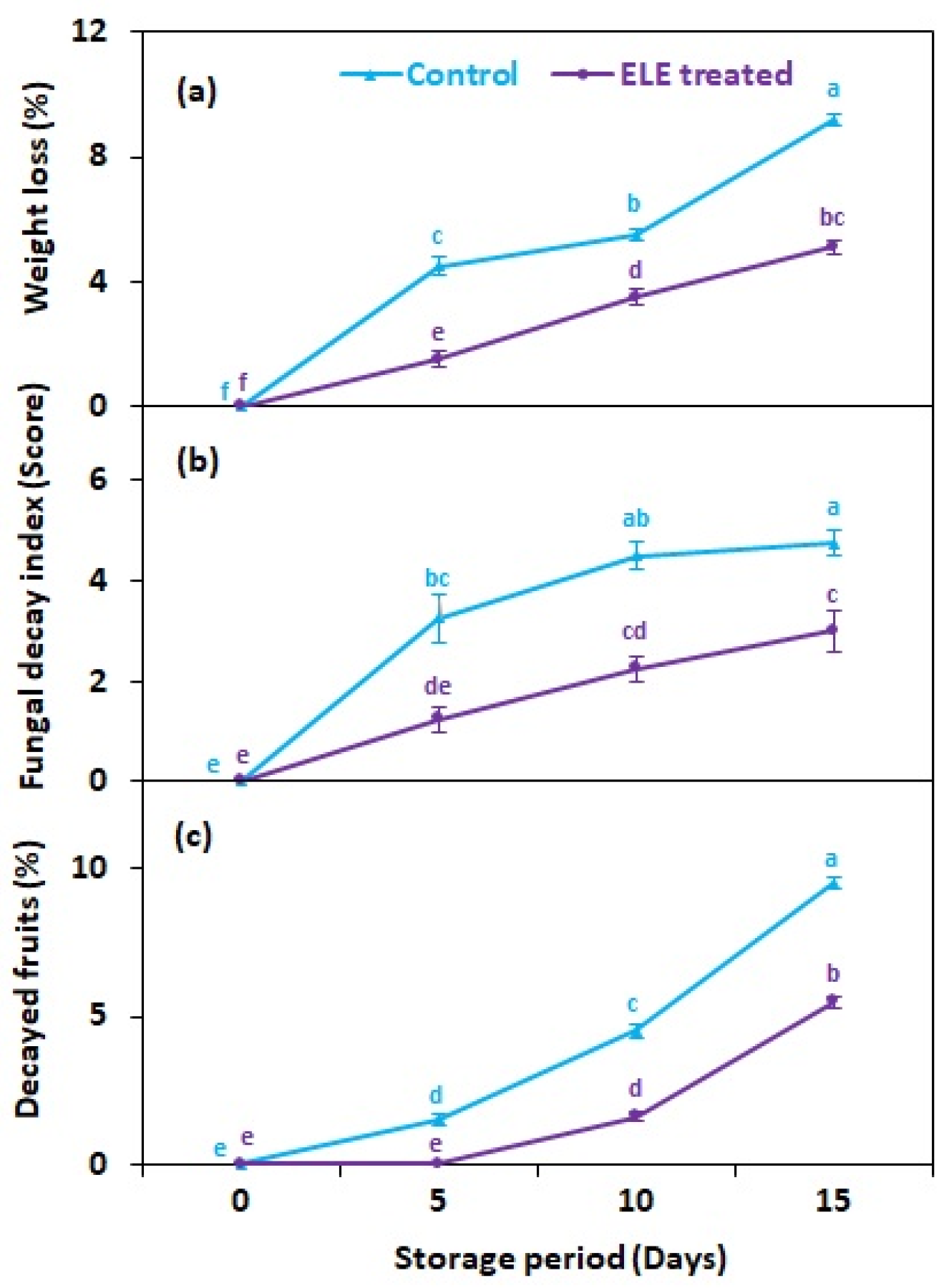
Weight loss in fresh fruit is mostly caused by the respiration rate and moisture evaporation between the fruit tissues and surrounding air. In the present study, the weight loss of strawberry fruits significantly increase with the increase in storage period regardless of treatments (Figure 1). However, ELE-treated fruits lost significantly less weight than the untreated control fruits after cold storage (Figure 2a). On 15th day of storage, the weight loss of the ELE-treated fruit was 5.1% more than that of untreated control strawberries (9.2%) (Figure 2a). Freshly harvested strawberry is a perishable commodity that loses a lot of moisture quickly, resulting in reduced consumer acceptance. ELE treatment has been shown to significantly prolong the shelf-life of freshly harvested fruits or their juices by delaying their senescence or/and preventing contamination. For example, in passion fruit juice, when the concentration of ELE increased from 0–40%, it resulted in extension of the juice’s shelf life by hinderance of microbial growth by 42.2% [19]. Similarly, in grapes, exposure to rosemary essential oil reduced weight loss and incidence of gray mold, and enhanced shelf life compared to the control [10]. Apple fruits treated with 1000 µL L−1 essential oil of rosemary, cinnamon, citronella, and clove had reduced weight loss and reduced growth, number, and viability of Penicillium expansum spores when stored for 30 days [20]. The application of 100 and 300 µL L−1 eucalyptus and rosemary essential oils on pear fruits inhibited blue rot in fruits and improved their shelf-life [21]; moreover, two nectarine cultivars treated with Aloe vera (ALV) alone or in combination with thymol showed reduced weight loss, less Penicillium digitatum contamination, and hence improved shelf life [22]. In mangoes, when the fruits were dipped in aqueous plant extracts, a significant reduction in disease development and an extension of shelf life was observed [23,24].

Figure 1.
The appearance of control and ELE-treated strawberries after at 0, 5, 10, and 15 days of storage at 2.0 ± 0.3 °C.
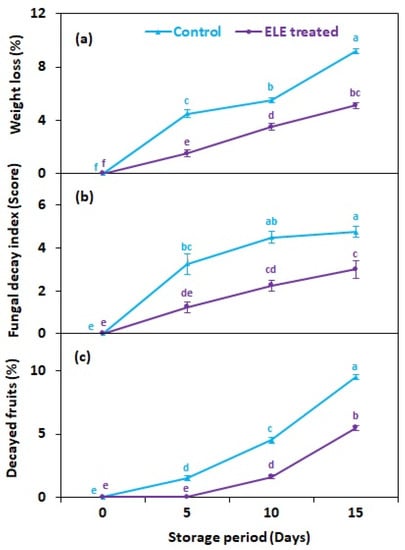
Figure 2.
Effect of eucalyptus leaf extract (ELE) on (a) fruit weight loss, (b) fungal decay index, and (c) decayed fruits of strawberries during cold storage. The treatment means sharing same letter were not significantly different (p < 0 .05) by the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean and the data is the average of four replicates.
In this study, treated and untreated fruits of strawberry were stored at 2.0 ± 0.3 °C to observe the fungal growth visually (Figure 1). The control and treated fruits showed significant (p ≤ 0.05) changes in fungal decay index (FDI) with extended storage time. About 6–20% of the surface area of control fruits started showing mycelial growth five days after storage, while strawberry fruits treated with ELE showed a comparable fungal decay index 15 days after storage (Figure 2b). Similar findings were recently reported by Manzi et al. [19], indicating that the use of ELE led to microbial decrease in passion fruit. Similarly, Tyagi et al. [33] found that the growth of molds and bacterial colonies was 25–40% inhibited after treating apple fruits with 2.25–4.5 mgL−1 ELE. In another study, Atress et al. [4] observed that the application of soy protein containing thymol resulted in microbial reduction in strawberry fruits. Ponce et al. [34] reported ELE application with greatest antimicrobial effect at a minimum bactericidal concentration of 0.093–1.5 mL/100 (v/v) and minimum inhibitory concentration of 0.049–0.13 mL/100 (v/v). These concentrations caused 99% and 90% of bacterial reduction, respectively. The percentage of decayed fruit among both control and ELE-treated strawberries increased with the increase in storage time, but the strawberries treated with ELE had a lower percentage of decaying fruit (Figure 2c). Recent studies indicated that the thymol present in ELE solution significantly reduced the percentages of decayed fruits in nectarines [12] and grapes [35]. This might be related to the accumulation of CO2 during storage, which accelerated fruit senescence and hence hastened the postharvest decay of strawberry fruits.
3.2. Respiration Rate (CO2 mmol kg−1 h−1) and Electrolyte Leakage (%)
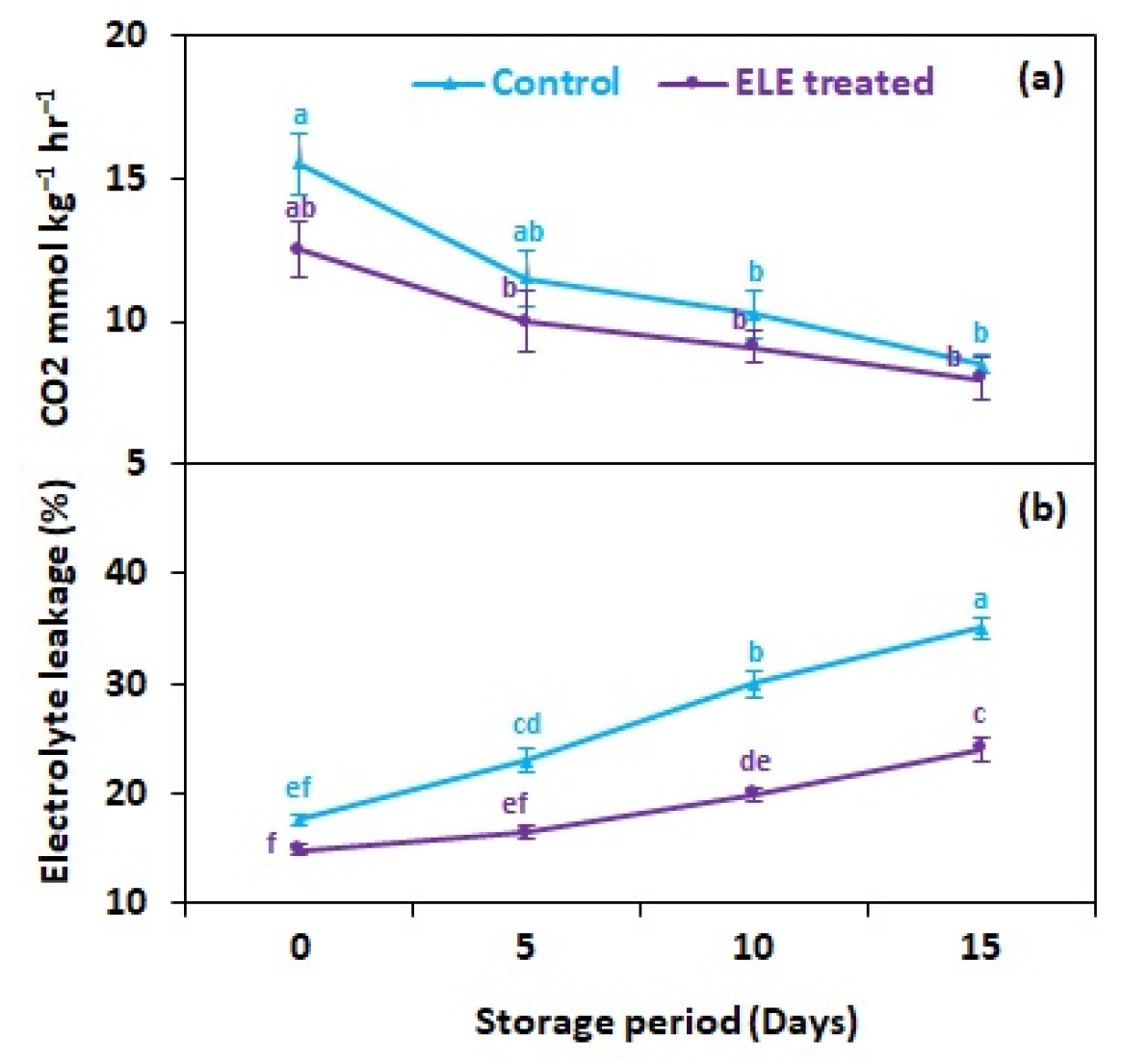
Respiration rate affects the metabolism of strawberry fruits, and is influenced by postharvest treatments and storage temperature. Our observations indicated that the respiration rate of strawberry fruits significantly decreased after the ELE application (Figure 3a). On the last day of storage (15th day), the respiration rate was lower (8.0 mmol kg−1 h−1) in ELE-treated strawberries in comparison with control fruits (8.5 mmol kg−1 h−1) (Figure 3a). Furthermore, the slower respiration rate in the treated fruits also allowed the conservation of their color and shape. Delay in the ripening and senescence of strawberries might be attributed to restricted respiration in treated fruits. There has been clear evidence in the previous research carried out by González et al. [36] that eucalyptol alters tomato fruit metabolism. Furthermore, in the current investigation, CO2 production was high in control and ELE-treated fruits at the start of storage but then decreased over time. The CO2 production remained higher in the control fruits than in the ELE-treated throughout the storage period. Observations of respiration rate comparable to those one made in the current work were indicated by Vargas et al. [37], who assessed the quality of coated and controlled strawberries stored at 4 °C. The authors noted that the usage of chitosan-based edible coatings decreased the respiration rate of strawberries. In accordance, Lee et al. [38] observed that the use of edible coatings of carrageenan and whey-protein concentrate decreased the respiration rate of minimally processed apples kept at 25 °C. Recent findings by Nourozi and Sayyari [39] in apricots, Ozturk et al. [40] in cherries, Martínez-Romero et al. [41] in plums, Chauhan et al. [42] in tomatoes, Paladines et al. [43] in peaches, and Ratra et al. [44] in bananas showed that coating with Aloe vera gel promisingly delays respiration rate, confirming the results of the present study. This could be explained by the fact that respiration was initially elevated during an earlier storage period and then declined afterwards. Under cold conditions, the electrolyte leakage (EL) of ELE-treated strawberries fell. A high EL was associated with increasing storage time, comparatively less so in ELE-treated fruits than in the untreated control. After 15 days, treated strawberries showed lower EL (24.1%) than the control (35.0%) (Figure 3b). Membrane damage in fresh fruits is mostly caused by environmental oxidative stress, which raises malondialdehyde levels and substantially reduces marketable life during storage [45]. ELE treatment could help to lower EL by preserving biochemical quality and the antioxidative potential to lessen lipid peroxidation and cellular damage during storage [46,47].
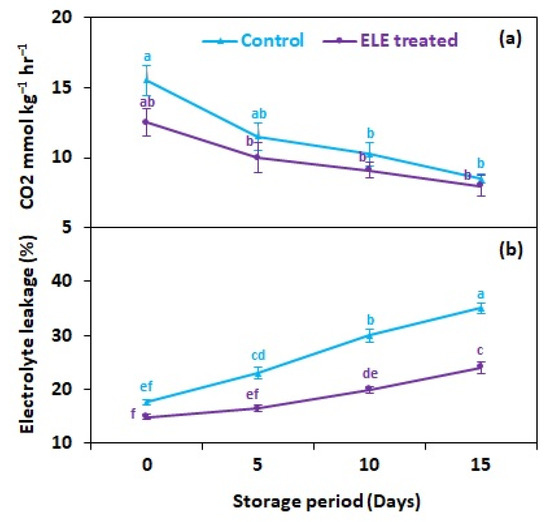
Figure 3.
Effect of eucalyptus leaf extract (ELE) on (a) fruit CO2 mmol kg−1 h−1 and (b) relative electrolyte leakage of strawberries during cold storage. The treatment means sharing same letter were not significantly different (p < 0 .05) according to the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean, and the data is the average of four replicates.
3.3. CAT, SOD, and POD Enzyme Activities (U mg−1 Protein)
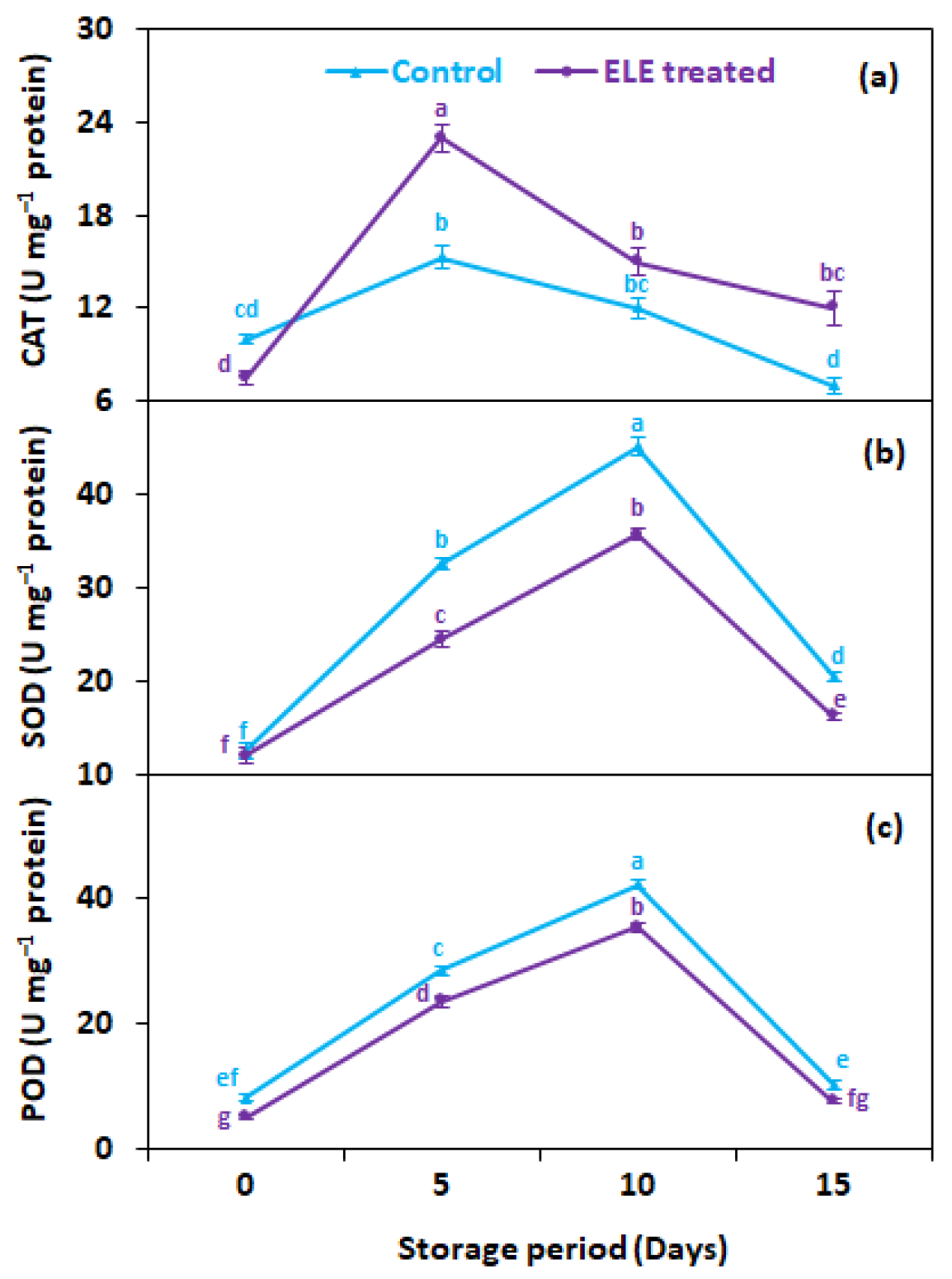
CAT activity progressively increased by day 5 of storage in treated (23.0 U mg−1 proteins) and non-treated (15.3 U mg−1 proteins) strawberries, but subsequently decreased for both treatments of fruits, until the last day of storage (day 15) (Figure 4a). Generally, strawberries treated with ELE showed 1.7 times more CAT activity 15 days after storage than untreated control fruits (Figure 4a). Likewise, the activities of SOD (Figure 4b) and POD (Figure 4c) were also elevated by day 10 of storage but then declined until the end of storage, regardless of treatment. In general, ELE-treated strawberries showed 1.3- and 1.4-fold higher SOD (Figure 4b) and POD (Figure 4c) activities, respectively, than untreated control fruits at 15 days from storage. Few studies have been reported on the effect of ELE application on the changes in enzymatic antioxidant activities. Similar results were noted by Badawy et al. [48], who found that using EOs with thymol (0.02%) or geraniol (0.04%) enhanced CAT and POD activities. Mangena and Muyima [49] discovered in their research on mushrooms that SOD plays a significant role in preserving the quality of mushrooms by destroying free oxygen radicals to support membrane integrity against oxidative stress. According to these findings, the usage of EOs raises levels of oxyradical detoxification enzymes i.e., POD, CAT, and SOD. The postharvest senescence is delayed by activities of CAT, SOD, and POD through detoxification of the H2O2 radical that increases the oxidative damage, and may delay the browning of tissues and discoloration [26]. Thus, ELE treatment has been proven effective in maintaining membrane integrity by maintaining the activities of CAT, SOD, and POD, enzymes thus improving the quality and storage potential of fresh strawberries.
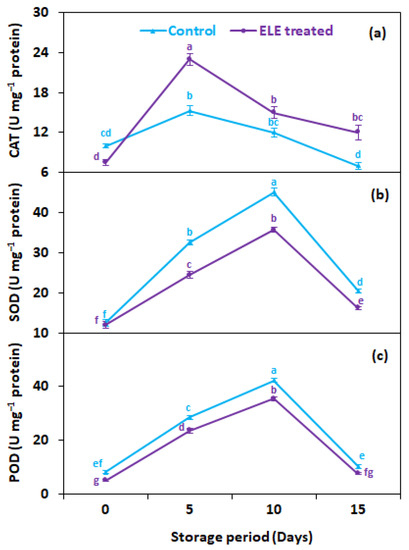
Figure 4.
Effect of eucalyptus leaf extract (ELE) on (a) catalase, (b) superoxide dismutase, and (c) peroxidase of strawberries during cold storage. The treatment means sharing the same letter were not significantly different (p < 0 .05) according to the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean and the data is the average of four replicates.
3.4. SSC (%), TA (%), Sugar Acid Ratio, and Juice pH
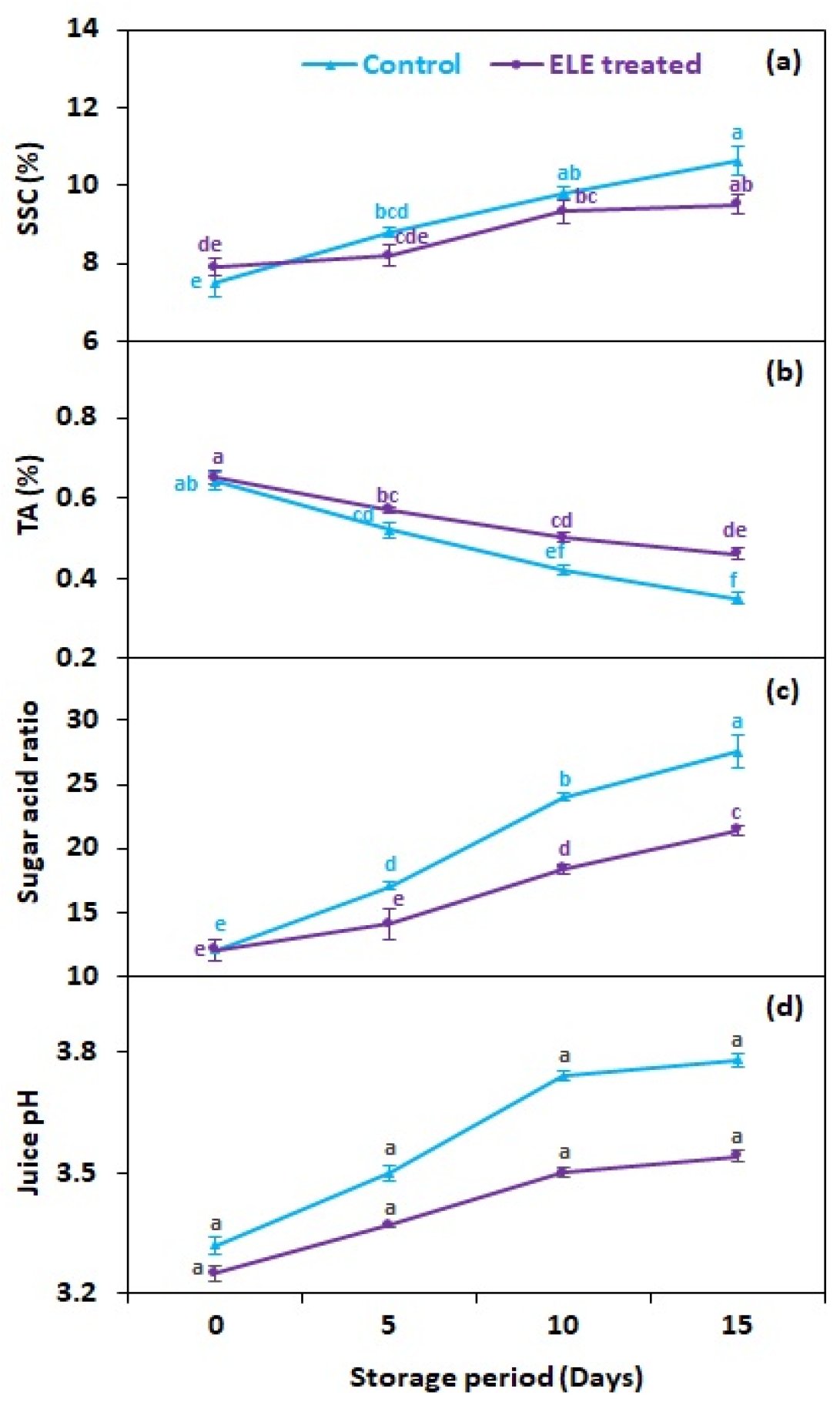
Total soluble solids in strawberry fruits were not significantly affected by the application of ELE (Figure 5a), although SSC was recorded as slightly lower in ELE-treated strawberries than in control ones (Figure 5a). A study by Vieira et al. [20] revealed that the SSC values of apples inoculated with Penicillium expansum ‘Fuji’ were unaffected by the application of citronella, cinnamon, rosemary, and clove essential oils (Eos). In our study, the titrable acidity (TA) reduced with storage time but the reduction was lower in ELE-treated strawberries (0.46%) than in the control (0.35%) on the 15th day (Figure 5b). Vieira et al. [20] found non-significant variations in apples’ TA after the application of citronella, cinnamon, rosemary, and clove. Tzortzakis [50] likewise found no significant variations in the TA of strawberries and tomatoes exposed to cinnamon and eucalyptus fumes for up to 10 days. The sugar–acid ratio increased throughout the storage period but it was comparatively lower in ELE-treated fruits (21.4) than in the control (27.6) on the 15th day of storage (Figure 5c). ELE treatment provided a lower sugar–acid ratio in apples, pears, grapes, and mangoes throughout storage [10,20,21,22,23]. Moreover, fruits treated with ELE also exhibited reduced juice pH compared to the control (Figure 5d), which is considered in contrast with previous results [40,51].
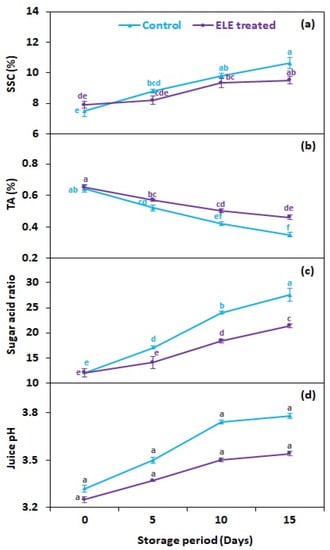
Figure 5.
Effect of eucalyptus leaf extract (ELE) on (a) soluble solid contents, (b) titrable acidity, (c) sugar–acid ratio, and (d) juice pH of strawberries during cold storage. The treatment means sharing the same letter were not significantly different (p < 0 .05) according to the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean and the data is the average of four replicates.
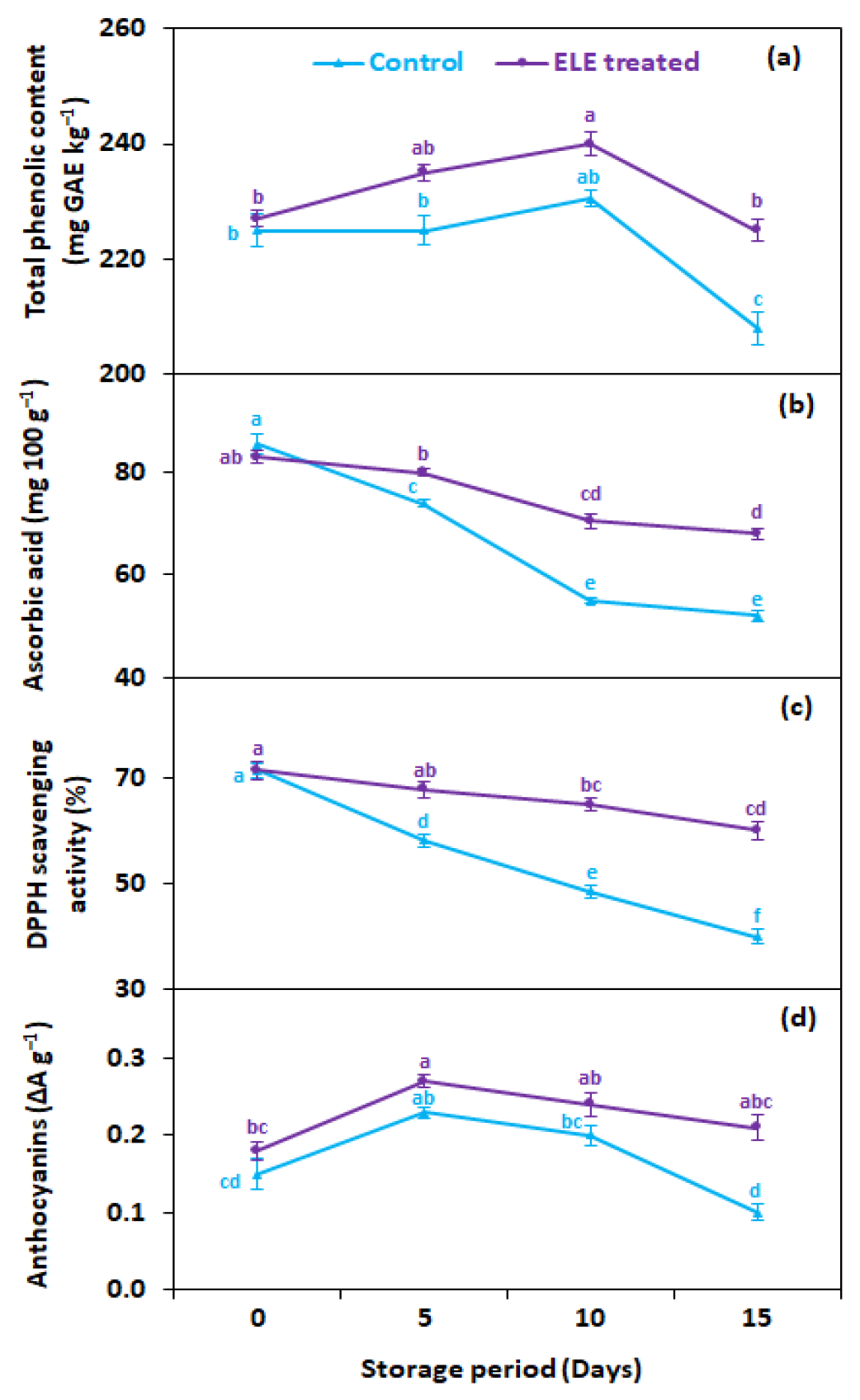
3.5. Total Phenolic Contents (mg GAE kg−1), Ascorbic Acid (mg kg−1), Anthocyanins (ΔA g−1), and Total Antioxidants (DPPH Scavenging Activities)
Total phenolic contents (TPC) gradually declined during the entire storage period regardless of ELE application (Figure 6a). On average, ELE application significantly reduced the decrease in TPC of strawberry fruits compared with untreated ones. After 15 days of cold storage, higher TPC (225 mg GAE kg−1) was recorded in ELE-treated strawberries in contrast to the untreated control (208 mg GAE kg−1) (Figure 6a). Ascorbic acid content gradually reduced during cold storage, both in ELE-treated and control strawberry fruits; however, ELE-treated fruits had higher levels of ascorbic acid than control (Figure 6b). After 15 days of storage, the ascorbic acid content in the control decreased from 85.5 to 52.0 mg 100 g−1, while it decreased from 83.0 to 67.9 mg 100 g−1 in ELE-treated fruits (Figure 6b). The lowering of ascorbic acid content in control fruits can be attributed to the increased respiration rate of fruits, which increased the deteriorative oxidation reaction of ascorbic acid. Anthocyanins increased during the early storage period, but their levels decreased after the fifth day both in control and ELE-treated fruits (Figure 6c). The rate of reduction in total anthocyanins was relatively high in the control (0.1 ΔA g−1) compared with the ELE-reated (0.21 ΔA g−1) (Figure 6b). The changes in phenolic, ascorbic acid, and anthocyanin contents were consistent with earlier studies carried out on the postharvest preservation effects of ALV on strawberries, plums, and peaches, and ELE on passion fruits and nectarines [19,22,41,43,50]. Total antioxidants in terms of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity showed a similar pattern of change as total phenolic contents, ascorbic acid, and anthocyanins in response to ELE during storage at 2 °C for 15 days. However, the ELE-treated strawberries showed higher DPPH compared with untreated fruits. On the 15th day of storage, the DPPH was higher (60.1%) in ELE-treated strawberries than in control fruits (40.0%) (Figure 5a). Likewise, previous investigations also reported DPPH preservation with exogenous application of chitosan for strawberries [49,52], grapes [53], and tomatoes [54]; ALV preserved DPPH in litchi [45], sapodilla [55], and raspberry [47]; and salt solution preserved higher DPPH in grapes [56] and Mesembryanthemum [57]. The decline in total antioxidants in the untreated fruits at the end of storage might be due to the release of free radicals leading to senescence and decay. This finding indicated that ELE treatment not only can extend shelf life but can also retain higher antioxidant activity in strawberries after prolonged storage. So, it can be assumed that increased DPPH after longer storage in strawberries treated with ELE is probably triggered by a decrease in the formation of free radicals and postponed senescence.
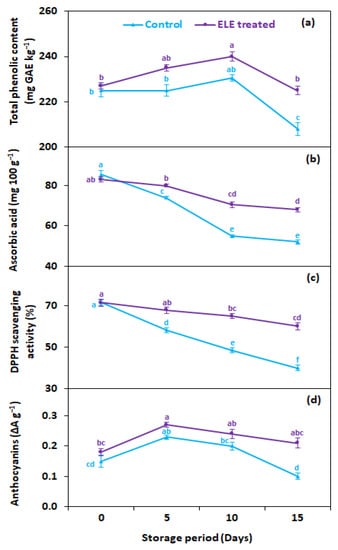
Figure 6.
Effect of eucalyptus leaf extract (ELE) on (a) total phenolic content, (b) ascorbic acid, (c) total antioxidants, and (d) anthocyanins of strawberries during cold storage. The treatment means sharing same letter were not significantly different (p < 0 .05) according to the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean, and the data is the average of four replicates.
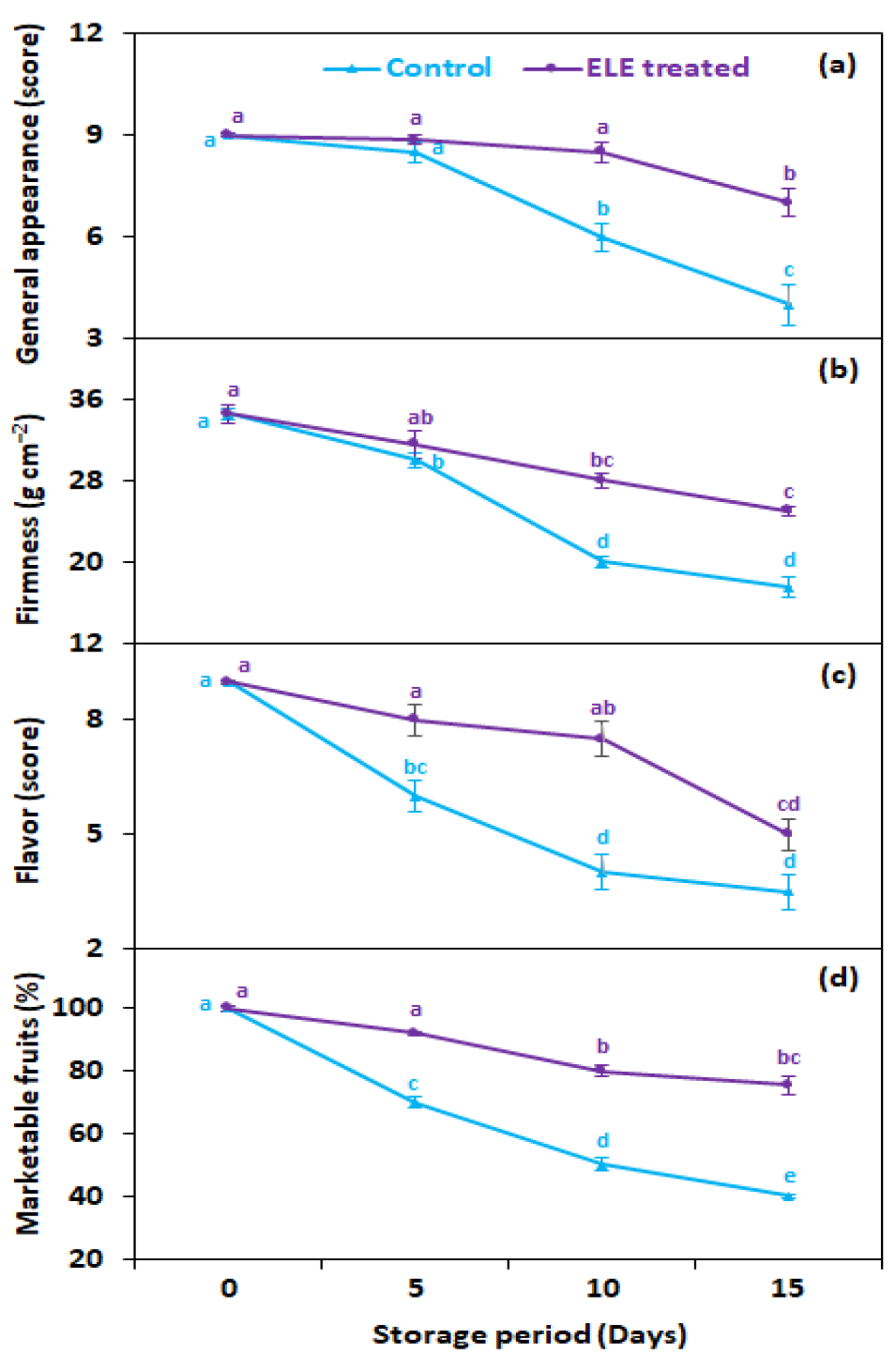
3.6. General Appearance, Flavor, Firmness, and Marketable Fruits
As expected, ELE-treated fruits had better aesthetic quality, flavor, and firmness, and as a consequence a higher proportion of marketable fruits compared with the untreated control (Figure 7a–d). The general appearance at 15 days from storage was 1.8-fold better in ELE-treated strawberries, as compared with the untreated control (Figure 7a). The fruit firmness was 1.4 times higher than the untreated control on the last day of storage (Figure 7b). All the strawberry fruits showed a progressive drop in flavor, but fruits treated with ELE remained above the threshold score for 10 days whereas untreated (control) strawberry fruits lost acceptable flavor quality after 5 days in storage (Figure 7c). The strawberries treated with ELE had more marketable fruits (82.2%) compared with the control fruits (57.8%) at the last removal (15th day) from storage (Figure 7d).
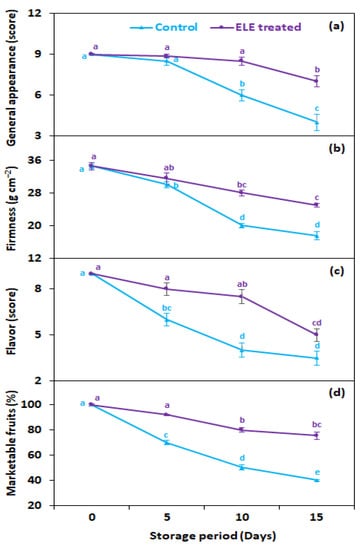
Figure 7.
Effect of eucalyptus leaf extract (ELE) on (a) general appearance, (b) firmness, (c) flavor, and (d) marketable fruits of strawberries during cold storage. The treatment means sharing same letter were not significantly different (p < 0 .05) as indicated by the honestly significant difference test. The vertical bars indicate the (±) standard error of the mean and the data is the average of four replicates.
4. Conclusions
The use of various natural products for postharvest preservation of strawberry fruits has been extensively reported, but the use of eucalyptus leaf extract in storage-life extension has rarely been investigated. The present study revealed that pre-storage application of ELE substantially extended the storage life of strawberries by reducing weight loss, fungal decay index, percentage of decayed fruits, respiration rate, electrolyte leakage, soluble solid contents, sugar–acid ratio, and juice pH. It furthermore maintained antioxidative qualities (phenolic content, ascorbic acid content, DPPH scavenging activity, CAT, SOD, and POD activities of enzymes) of strawberries during storage. ELE application conserved the general appearance, firmness, and flavor of strawberries, attaining a higher percentage of marketable fruits. In order to maintain the postharvest quality of strawberries during storage at 2.0 ± 0.3 °C, ELE [30% (v/v)] could be used as an ecologically sound, non-chemical, and non-toxic treatment.
Author Contributions
Conceptualization, M.W.H., M.N. and M.V.; methodology, M.W.H. and R.I.; software, H.U.A. and H.N.F.; validation, M.N., M.A., J.I. and M.A.S.; formal analysis, S.A.; Data curation, M.V.; resources, M.W.H.; M.N.; Supervision, writing—review and editing, All authors; visualization, M.N.; funding acquisition, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
The authors are highly grateful to the Islamia University of Bahawalpur, Pakistan for providing fruit material under the project “Establishment of Commercial Multistorey Orchard and Fruit Research Unit” which mainly focused on maximizing sustainable fruit production per unit area.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akhtar, I.; Rab, A. Effect of irrigation intervals on the quality and storage performance of strawberry fruit. J. Anim. Plant Sci. 2015, 25, 669–678. [Google Scholar]
- Agriculture Statistics of Pakistan. Ministry of Food and Agriculture (Economic Wing); Government of Pakistan: Islamabad, Pakistan, 2015.
- Khan, M.N.; Sarwar, A.; Bhutto, S.; Wahab, M.F. Physicochemical characterization of the strawberry samples on regional basis using multivariate analysis. Int. J. Food Prop. 2010, 13, 789–799. [Google Scholar] [CrossRef]
- Atress, A.S.H.; El-Mogy, M.M.; Anean, H.E.A.; Alsannius, B.W. Improving strawberry fruit storability by edible coatings as a carrier of Thymol or calcium chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Mahmood, M.A.; Sheikh, A.D. Citrus export system in Pakistan. J. Agric. Res. 2006, 44, 229–238. [Google Scholar]
- Nunes, M.C.N.; Morais, A.M.M.B.; Brecht, J.K.; Sargent, S.A. Fruit maturity and storage temperature influence response of strawberries to controlled atmospheres. J. Am. Soc. Hortic. Sci. 2002, 127, 836–842. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Raheem, M.I.U.; Khan, A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined aqueous ozone and ultrasound application inhibits microbial spoilage, reduces pesticide residues and maintains storage quality of strawberry fruits. J. Food Meas. Charact. 2021, 15, 1437–1451. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Salem, E.A.; Zahran, A.A. Efficacy of coupling gamma irradiation with calcium chloride and lemon grass oil in maintaining guava fruit quality and inhibiting fungal growth during cold storage. Folia Hortic. 2020, 30, 67–78. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-Based coating enriched with hairy fig (Ficus hirta Vahl.) fruit extract for “Newhall” Navel Orange Preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Hasan, M.U.; Malik, A.U.; Anwar, R.; Khan, A.S.; Haider, M.W.; Riaz, R.; Ali, S.; Rehman, R.N.U.; Ziaf, K. Postharvest Aloe vera gel coating application maintains the quality of harvested green chillies during cold storage. J. Food Biochem. 2021, 45, e13682. [Google Scholar] [CrossRef] [PubMed]
- Kahramanoğlu, I.; Aktaş, M.; Gündüz, Ş. Effects of fludioxonil, propolis and black seed oil application on the postharvest quality of “Wonderful” pomegranate. PLoS ONE 2018, 13, e0198411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Long, Y.; Wang, Q.; Li, J.; Wu, X.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Wahab, H.A.; Malek, A.; Ghobara, M. Effects of some plant extracts, bioagents, and organic compounds on Botrytis and Sclerotinia molds. Acta Agrobot. 2020, 73, 19–25. [Google Scholar] [CrossRef]
- Hassan, J.; Anwar, R.; Khan, A.S.; Ahmad, S.; Malik, A.U.; Nafees, M.; Hussain, Z.; Inam-ur-Raheem, M. Chitosan-based edible coating delays fungal decay and maintains quality of strawberries during storage. Int. J. Agric. Biol. 2020, 24, 486–492. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, S.; Gautam, B. Phytochemical analysis of Eucalyptus leaves extract. J. Pharmacogn. Phytochem. 2019, 8, 2442–2446. [Google Scholar]
- Abd-Elkader, D.Y.; Salem, M.Z.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-harvest enhancing and Botrytis cinerea control of strawberry fruits using low cost and eco-friendly natural oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Manzi, H.P.; Niyigena, V.; Matsiko, F.; Niyigaba, T.; Twagirayezu, G.; Irumva, O.; Nyiranshuti, A. Investigation of the effects of eucalyptus extracts on shelf-life of Passion fruit juice. Pol. J. Environ. Stud. 2022, 3, 2729–2736. [Google Scholar] [CrossRef]
- Vieira, A.M.F.D.; Steffens, C.A.; Argenta, L.C.; Amarante, C.V.T.D.; Oster, A.H.; Casa, R.T.; Amarante, A.G.M.; Espíndola, B.P. Essential oils for the postharvest control of blue mold and quality of ‘Fuji’ apples. Pesqui. Agropecu. Bras. 2018, 53, 547–556. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.R.; Tzortzakis, N. Application of rosemary and eucalyptus essential oils and their main component on the preservation of apple and pear fruits. Horticulturae 2021, 7, 479. [Google Scholar] [CrossRef]
- Navarro, D.; Díaz-Mula, H.M.; Guillén, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Alemu, K.; Ayalew, A.; Woldetsadik, K. Antifungal activity of plant extracts and their applicability in extending the shelf-life of mango fruits. Food Sci. Qual. Manag. 2014, 33, 47–53. [Google Scholar]
- Khaliq, G.; Nisa, M.; Ramzan, M.; Koondhar, N. Textural properties and enzyme activity of mango (Mangifera indica L.) fruit coated with chitosan during storage. J. Agric. Stud. 2017, 5, 32–50. [Google Scholar] [CrossRef][Green Version]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays post-cut surface browning and maintains quality of cold stored lotus (Nelumbo nucifera Gaertn.) root slices. Sci. Hortic. 2019, 256, 108612. [Google Scholar] [CrossRef]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M. Ripening period influences fruit softening and antioxidative system of ‘Samar Bahisht Chaunsa’ mango. Sci. Hortic. 2013, 160, 108–114. [Google Scholar] [CrossRef]
- Malik, A.U.; Hasan, M.U.; Hassan, W.U.; Khan, A.S.; Shah, M.S.; Rajwana, I.A.; Latif, M.; Anwar, R. Postharvest quarantine vapour heat treatment attenuates disease incidence, maintains eating quality and improves bioactive compounds of “Gola” and “Surahi” guava fruits. J. Food Meas. Charact. 2020, 15, 1666–1679. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic contents and other oxidation substrates in plant tissue using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Kader, A.A.; Lipton, W.J.; Morris, L.L. Systems for scoring quality of harvested Lettuce. HortScience 1973, 8, 408–409. [Google Scholar] [CrossRef]
- Kelly, K.; Whitaker, V.M.; Nunes, M.C.N. Physicochemical characterization and postharvest performance of the new Sensation® ‘Florida127’ strawberry compared to commercial standards. Sci. Hortic. 2016, 211, 283–294. [Google Scholar] [CrossRef]
- Tyagi, K.A.; Bukvicki, D.; Gottardi, D.; Tabanelli, G.; Montanari, C.; Malik, A.; Guerzoni, M.E. Eucalyptus essential oil as a natural food preservative: In vivo and in vitro antiyeast potential. BioMed. Res. Int. 2014, 2014, 969143. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT-Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Castillo, S.; Navarro, D.; Zapata, P.J.; Guillén, F.; Valero, D.; Serrano, M.; Martínez-Romero, D. Antifungal efficacy of Aloe vera in-vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol. Technol. 2010, 57, 183–188. [Google Scholar] [CrossRef]
- González, L.A.; Torres, F.; Quiñones, W. Changes in Tomato Metabolism by Applying 1,8 Cineole. J. Microb. Biochem. Technol. 2015, 7, 323–326. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martinez, C. Quality of cold stored strawberries as affected by chitosan-oleic acid edible coating. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT-Food Sci. Technol. 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Ozturk, B.; Karakaya, O.; Yıldız, K.; Saracoglu, O. Effects of Aloe vera gel and MAP on bioactive compounds and quality attributes of cherry laurel fruit during cold storage. Sci. Hortic. 2019, 249, 31–37. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Zapata, P.J.; Guillén, F.; Paladines, D.; Castillo, S.; Valero, D.; Serrano, M. The addition of rosehip oil to Aloe gels improves their properties as postharvest coatings for maintaining quality in plum. Food Chem. 2017, 217, 585–592. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P.S. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2015, 52, 1200–1205. [Google Scholar] [CrossRef]
- Paladines, D.; Valero, D.; Valverde, J.M.; Mula, H.M.; Serrano, M.; Romero, D.M. The addition of rosehip oil improves the beneficial effect of Aloe vera gel on delaying ripening and maintaining postharvest quality of several stone fruit. Postharvest Biol. Technol. 2014, 92, 23–28. [Google Scholar] [CrossRef]
- Ratra, S.; Kaur, L.; Thukral, B. Effect of Aloe vera and wheat grass juice as an edible coating to prolong the shelf life of bananas. Int. J. Eng. Res. Technol. 2016, 3, 2648–2655. [Google Scholar]
- Li, T.; Shi, D.; Wu, Q.; Zhang, Z.; Qu, H.; Jiang, Y. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT-Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 2016, 17, 117–136. [Google Scholar] [CrossRef]
- Mangena, T.; Muyima, N.Y.O. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosemarinus officinalis on selected bacteria and yeast strains. Lett. Appl. Microbiol. 1999, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.G. Maintaining postharvest quality of fresh produce with volatile compounds. Innov. Food Sci. Emerg. Technol. 2007, 8, 111–116. [Google Scholar] [CrossRef]
- Peyro, H.; Abbas, M.S.A.; Kavoosi, B. Effect of salicylic acid and Aloe vera gel on postharvest quality of table grapes (Vitis vinifera). Trakia J. Sci. 2017, 2, 154–159. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria × aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Badawy, M.E.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe vera gel coating enriched with Fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Roberto, S.R.; Tiepo, A.N.; Constantino, L.V.; Vilela de Resende, J.T.; Abo-Elyousr, K.A.M. Salt solution treatments trigger antioxidant defense response against gray mold disease in table grapes. J. Fungi 2020, 6, 179. [Google Scholar] [CrossRef]
- Falleh, H.; Jalleli, I.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Magné, C.; Abdelly, C. Effect of salt treatment on phenolic compounds and antioxidant activity of two Mesembryanthemum edule provenances. Plant Physiol. Biochem. 2012, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).