Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Stumps of Three Representative Trees in Pingtung County, Taiwan: Leucaena leucocephala, Syzygium samarangense, and Ziziphus jujuba
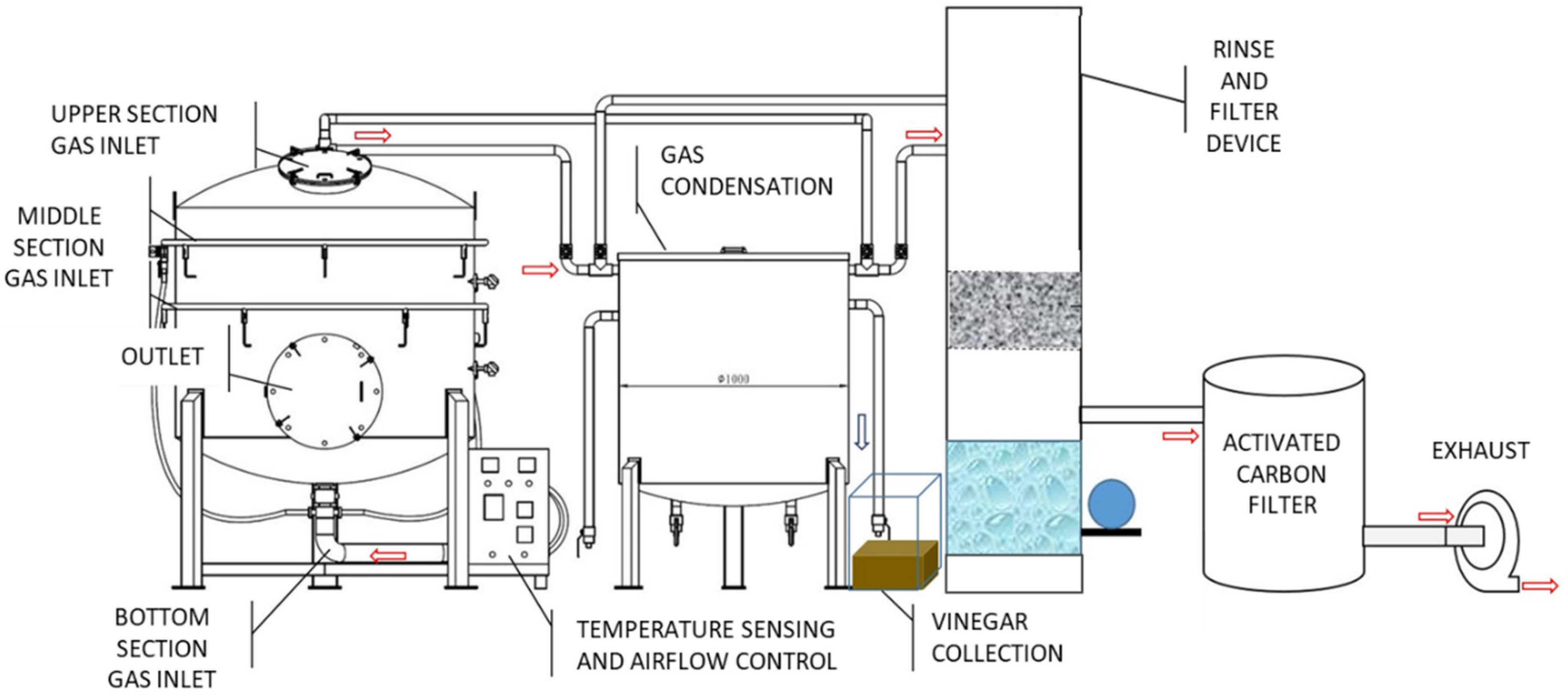
2.2. Carbonization Equipment: Energy-Saving Digital Carbonization System
2.3. Biochar Preparation
2.4. Biochar Activation
2.5. Adjustment of the Acidity of Biochar
2.6. Cation Exchange Capacity (CEC)
2.7. Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.8. Mercury Intrusion Porosimetry
3. Results and Discussion
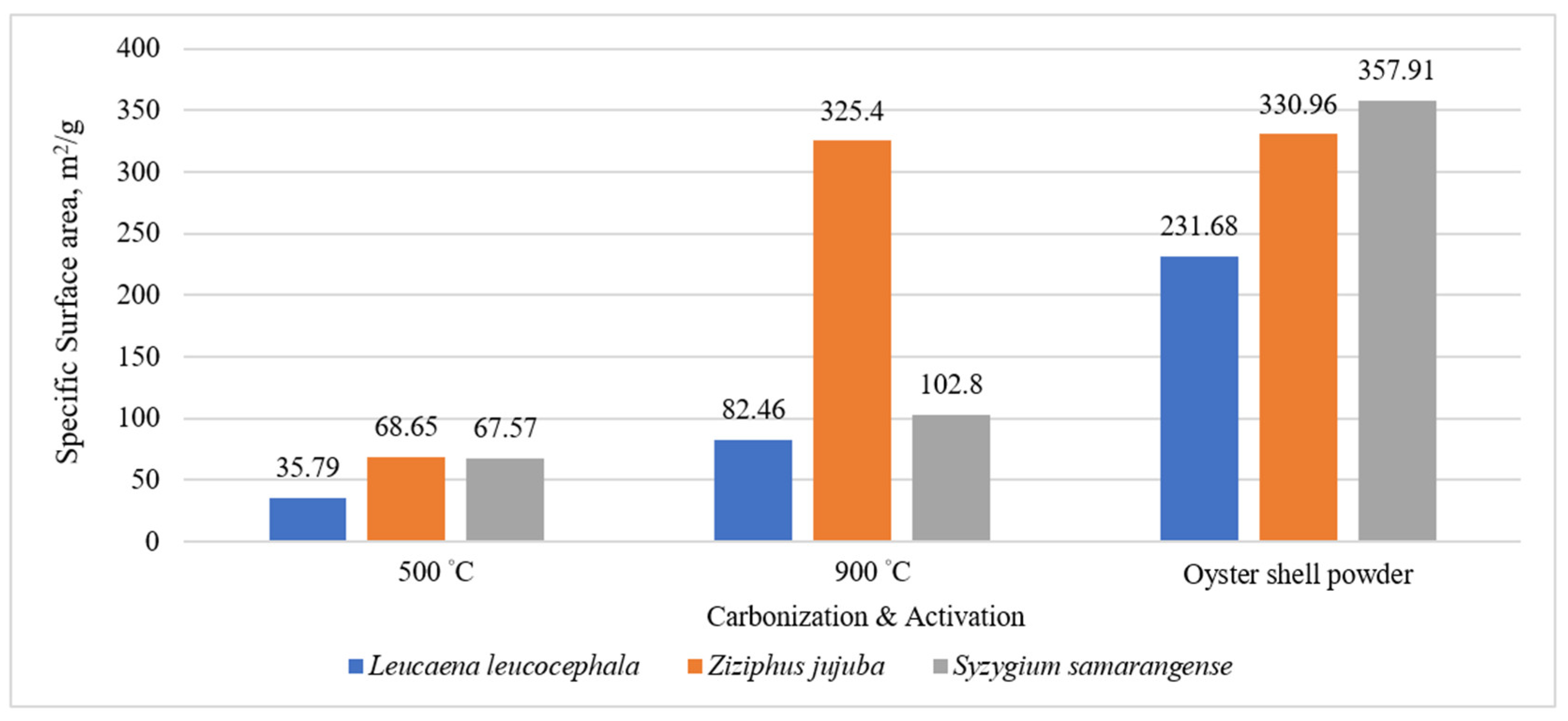
3.1. Analysis and Discussion of Pore Characteristics and Specific Surface Area at Different Carbonization Temperatures
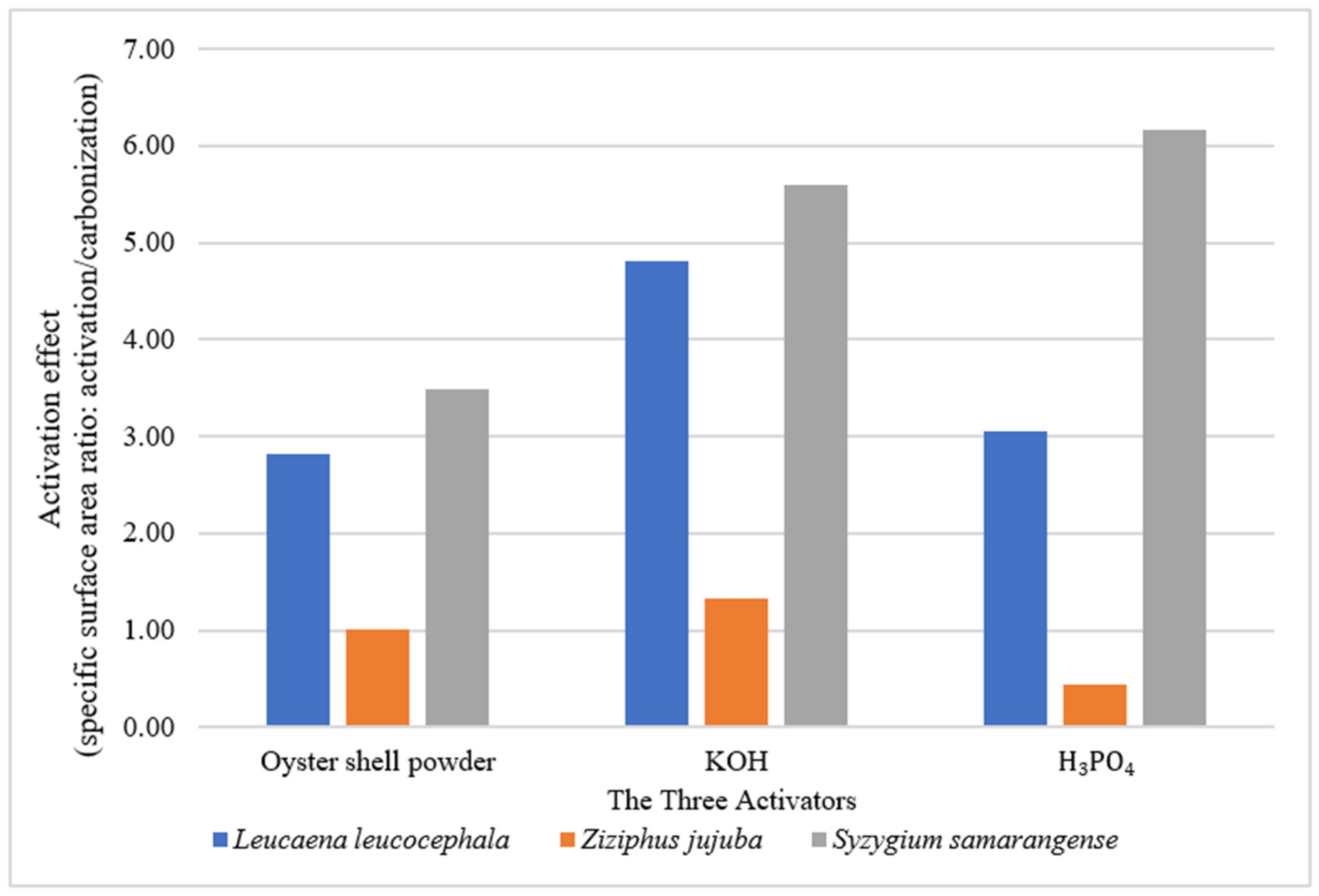
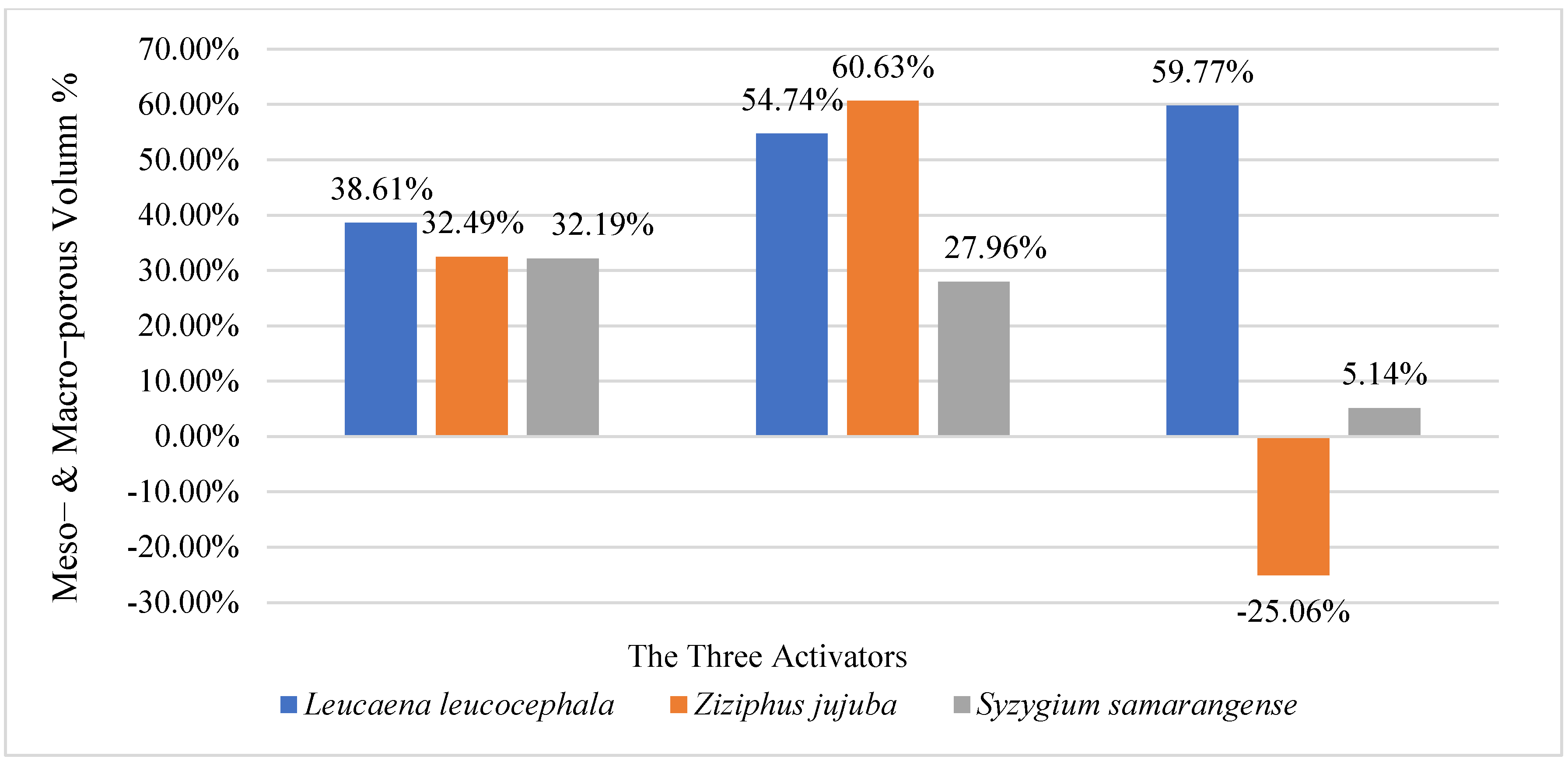
3.2. The Effect of Different Activation Methods on the Pore Characteristics and Specific Surface Area of Biochar Samples
3.3. Analysis of the Properties of Biochar Fertilizers after Modification
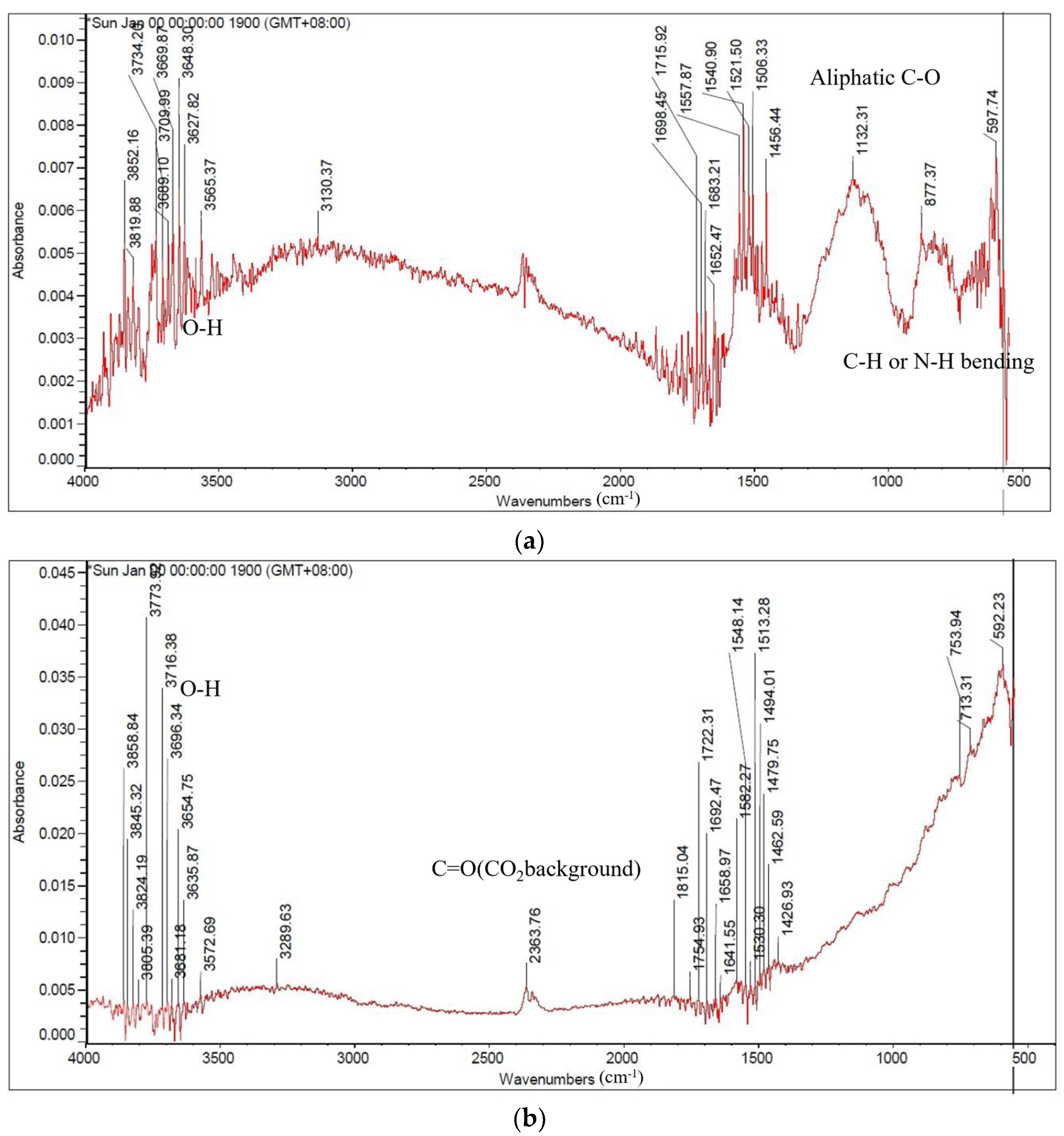
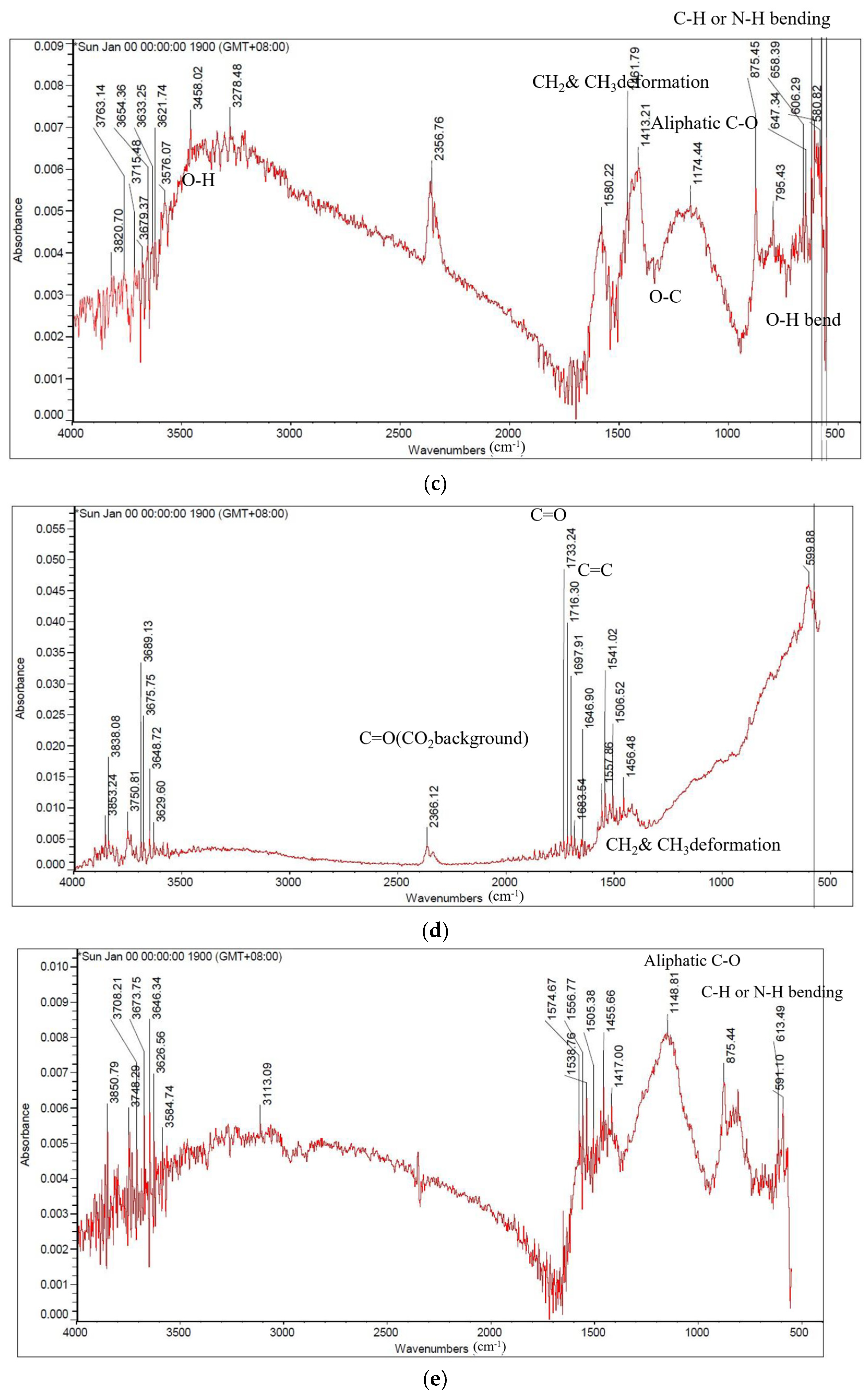
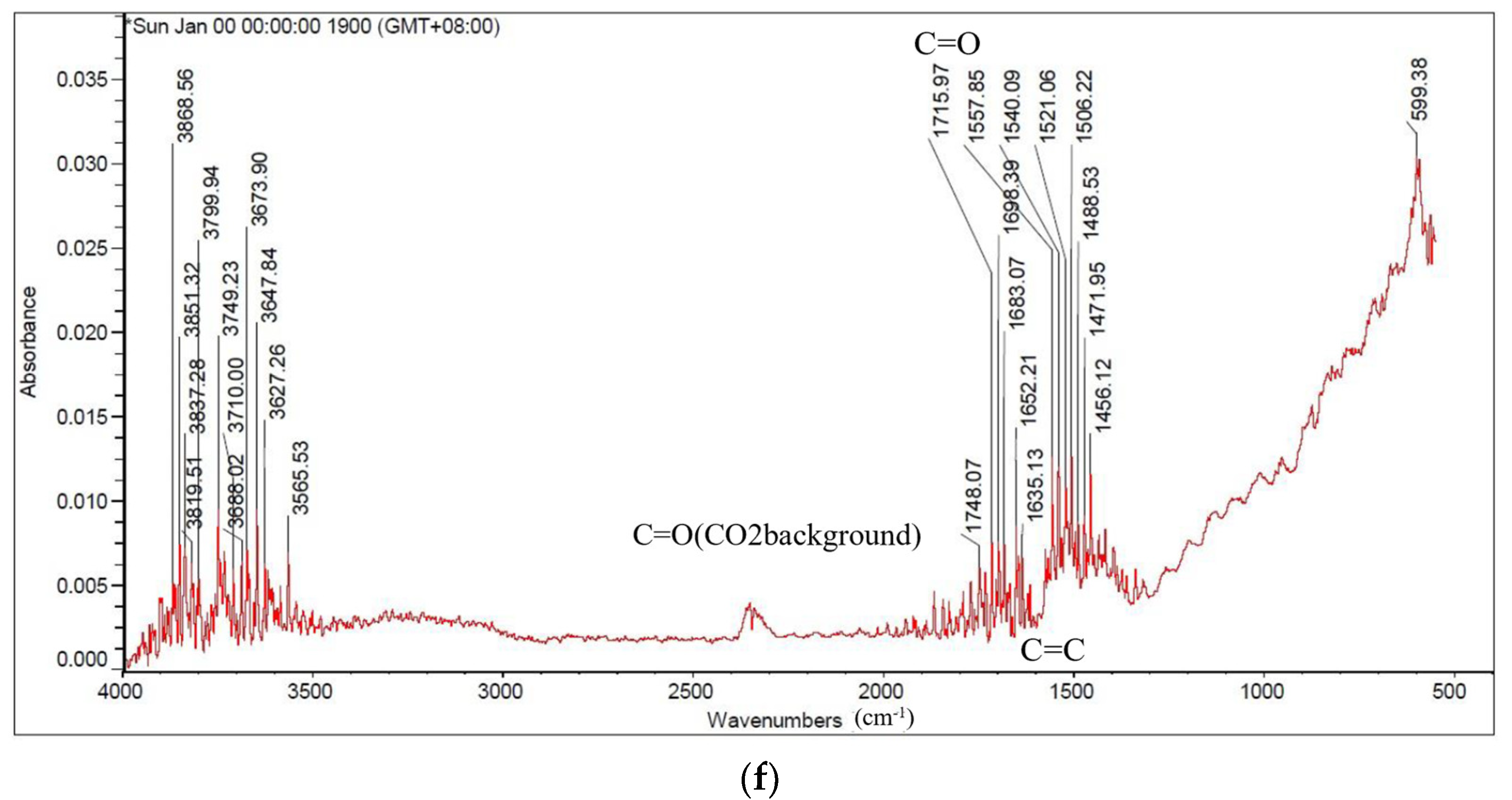
3.4. Investigate the Degree of Carbonization by Functional Group Analysis by FTIR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willer, H.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends 2017; Research Institute of Organic Agriculture FiBL: Frick, Switzerland; IFOAM-Organics International: Bonn, Germany, 2017. [Google Scholar]
- Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S.; Gad, H.M.H. A study of the factors affecting the removal of humic acid by activated carbon prepared from biomass material. Colloids Surf. A Physicochem. Eng. Asp. 2004, 235, 1–10. [Google Scholar] [CrossRef]
- Magdy, Y.H.; Daifullah, A.A.M. Adsorption of a basic dye from aqueous solutions onto sugar-industry-mud in two modes of operations. Waste Manag. 1998, 18, 219–226. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Yu, W.; Cheng, S.; Wang, Y.; Shi, J. Biosorption of methylene blue from aqueous solution by fallen phoenix tree’s leaves. J. Hazard. Mater. 2007, 141, 156–162. [Google Scholar] [CrossRef]
- Gong, R.; Zhu, S.; Zhang, D.; Chen, J.; Ni, S.; Guan, R. Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: Kinetic and thermodynamic profile. Desalination 2008, 230, 220–228. [Google Scholar] [CrossRef]
- Mui, E.L.; Cheung, W.H.; Valix, M.; McKay, G. Dye adsorption onto char from bamboo. J. Hazard. Mater. 2010, 177, 1001–1005. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Gau, D.; Jogi, Q.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Beheiry, H.R.; Sétamou, M.; Simpson, C.R.; Abd El-Mageed, T.A.; Rady, M.M.; Nelson, S.D. Biochar implications for sustainable agriculture and environment: A review. S. Afr. J. Bot. 2019, 127, 333–347. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical Propierties of Biochar. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan Ltd.: London, UK, 2009. [Google Scholar]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Melo, C.A.; Soares, F.H., Jr.; Bisinoti, M.C.; Moreira, A.B.; Ferreira, O.P. Transforming sugarcane bagasse and vinasse wastes into hydrochar in the presence of phosphoric acid: An evaluation of nutrient contents and structural properties. Waste Biomass Valorization 2017, 8, 1139–1151. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Yang, Y.; Wang, J.Y. Utilization of sewage-sludge-derived hydrochars toward efficient cocombustion with different-rank coals: Effects of subcritical water conversion and blending scenarios. Energy Fuels 2014, 28, 6140–6150. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Müller, C.; Grünhage, L.; Koch, C.; Kammann, C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric. Ecosyst. Environ. 2014, 191, 39–52. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, C.S.; Ji, X.; Rainey, T.J. Returning biochar to fields: A review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Huff, M.D.; Marshall, S.; Saeed, H.A.; Lee, J.W. Surface oxygenation of biochar through ozonization for dramatically enhancing cation exchange capacity. Bioresour. Bioprocess. 2018, 5, 18. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Shaaban, M.; Hu, H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 2018, 211, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Mackay, D.M.; Roberts, P.V. The influence of pyrolysis conditions on the subsequent gasification of lignocellulosic chars. Carbon 1982, 20, 105–111. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Ali, W.S.W.; Sulaiman, M.Z. The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon 2000, 38, 1925–1932. [Google Scholar] [CrossRef]
- Kwon, E.E.; Oh, J.I.; Kim, K.H. Polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) mitigation in the pyrolysis process of waste tires using CO2 as a reaction medium. J. Environ. Manag. 2015, 160, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.; Tian, J.; Cai, K. Modified Biochars and Their Effects on Soil Quality: A Review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.; Guo, J.; Wu, J.; Ye, Z.; Liu, D.; Jin, H. Effects of biochar on growth, and heavy metals accumulation of moso bamboo (Phyllostachy pubescens), soil physical properties, and heavy metals solubility in soil. Chemosphere 2018, 219, 510–516. [Google Scholar] [CrossRef]
- Elomaa, H.; Seisko, S.; Lehtola, J.; Lundström, M. A study on selective leaching of heavy metals vs. iron from fly ash. J. Mater. Cycles Waste Manag. 2019, 21, 1004–1013. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Chapter 3. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–38. [Google Scholar]
- Liu, Z.; Niu, W.; Chu, H.; Zhou, T.; Niu, Z. Effect of the carbonization temperature on the properties of biochar produced from the pyrolysis of crop residues. BioResources 2018, 13, 3429–3446. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]

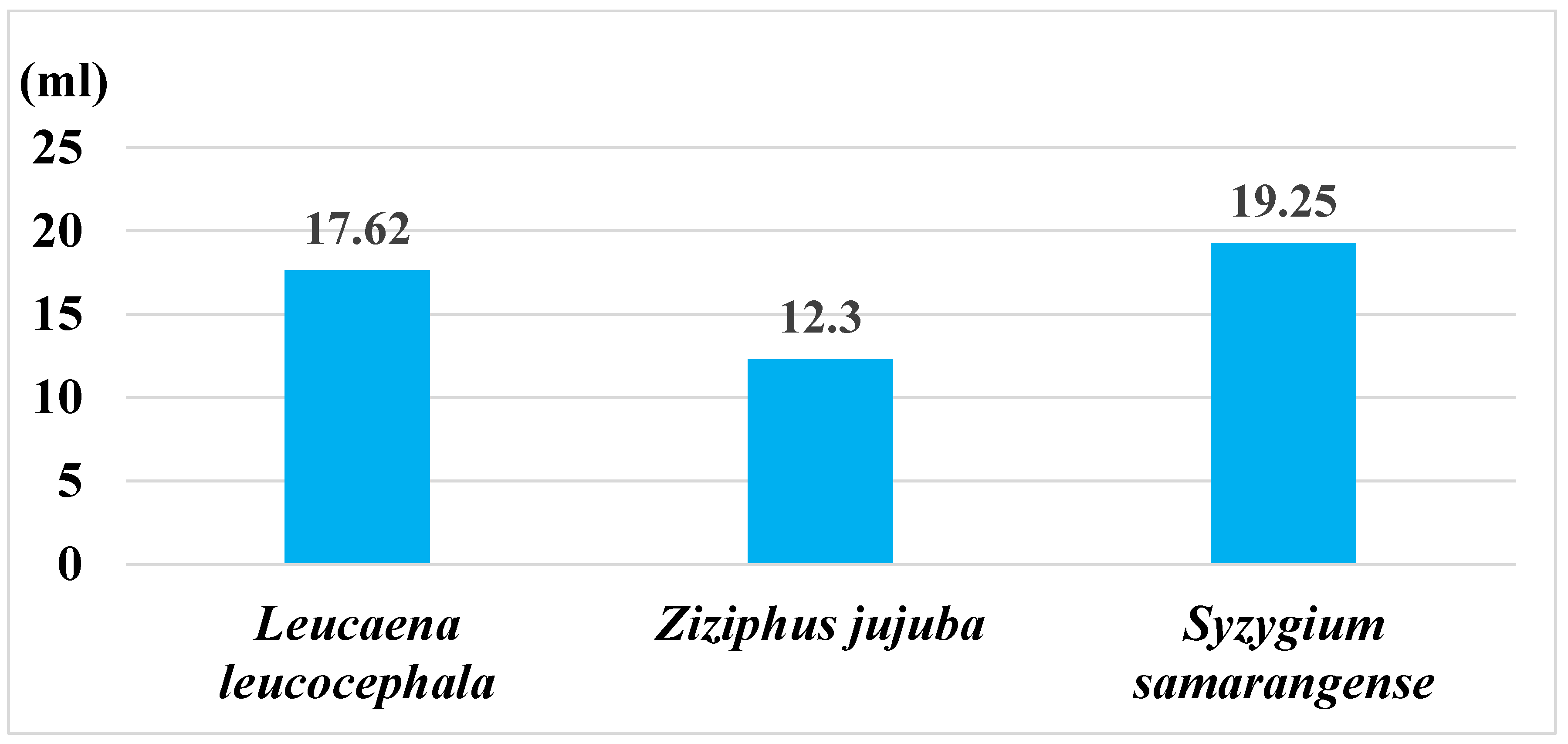
| Sample Source | Carbonization Temp. (°C) | Carbonization Period (min.) | pH | Specific Surface Area (SSA) (m2/g) | Microporous SSA (m2/g) | Meso & Macroporous SSA (m2/g) | Total Pore Volume (mL/g) | Microporous Volume (mL/g) | Meso & Macroporous Volumn (mL/g) | Meso & Macroporous SSA % | Meso & Macroporous Volumn % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucaena leucocephala | 500 | 60 | 9.94 | 35.79 | 0.82 | 34.97 | 0.0775 | 0.0131 | 0.0644 | 97.71 | 83.10 |
| 900 | 30 | 9.98 | 82.46 | 37.34 | 45.12 | 0.0926 | 0.017 | 0.0756 | 54.72 | 81.64 | |
| Ziziphus jujuba | 500 | 60 | 9.64 | 68.65 | 15.43 | 53.22 | 0.0947 | 0.0322 | 0.0625 | 77.52 | 66.00 |
| 900 | 30 | 9.69 | 325.4 | 270.58 | 54.82 | 0.2232 | 0.1636 | 0.0596 | 16.85 | 26.70 | |
| Syzygium samarangense | 500 | 60 | 10 | 67.57 | 1.00 | 66.57 | 0.1089 | 0.0303 | 0.0786 | 98.52 | 72.18 |
| 900 | 30 | 10.03 | 102.79 | 42.70 | 60.09 | 0.1243 | 0.053 | 0.0713 | 58.46 | 57.36 |
| Sample Source | Volatile Components (%) | Fixed Carbon (%) | Ash (%) | pH |
|---|---|---|---|---|
| Leucaena leucocephala | 7.4 | 86.8 | 5.8 | 9.98 |
| Ziziphus jujuba | 5.5 | 90.1 | 4.4 | 9.69 |
| Syzygium samarangense | 4.6 | 86.0 | 9.4 | 10.03 |
| Sample Source | Carbonization Temp. (oC) | Carbonization Period (min.) | Specific Surface Area (SSA) (m2/g) | Microporous SSA (m2/g) | Meso and Macroporous SSA (m2/g) | Total Pore Volume (mL/g) | Microporous Volume (mL/g) | Meso and Macroporous Volumn (mL/g) | Meso and Macroporous SSA % | Meso and Macroporous Volumn % | Activator |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W9-L. leucocephala | 900 | 30 | 82.46 | 37.34 | 45.12 | 0.0926 | 0.017 | 0.0756 | 54.72 | 81.64 | N/A |
| R9-Z. jujuba | 900 | 30 | 325.4 | 270.58 | 54.82 | 0.2232 | 0.1636 | 0.0596 | 16.85 | 26.70 | N/A |
| S9-S. samarangense | 900 | 30 | 102.79 | 42.70 | 60.09 | 0.1243 | 0.053 | 0.0713 | 58.46 | 57.36 | N/A |
| W9P-L. leucocephala | 900 | 60 | 231.683 | 169.150 | 62.540 | 0.192 | 0.108 | 0.084 | 26.99 | 43.71 | oyster shell powder |
| R9P-Z. jujuba | 900 | 60 | 330.960 | 258.330 | 72.630 | 0.248 | 0.219 | 0.029 | 21.95 | 11.52 | oyster shell powder |
| S9P-S. samarangense | 900 | 60 | 357.907 | 278.480 | 79.430 | 0.278 | 0.171 | 0.107 | 22.19 | 38.46 | oyster shell powder |
| W9K-L. leucocephala | 900 | 60 | 396.17 | 326.89 | 69.82 | 0.2782 | 0.1981 | 0.0801 | 17.62 | 28.79 | KOH |
| R9K-Z. jujuba | 900 | 60 | 433.94 | 345.88 | 88.06 | 0.3197 | 0.2172 | 0.1025 | 20.29 | 32.06 | KOH |
| S9K-S. samarangense | 900 | 60 | 575 | 498.11 | 76.89 | 0.3884 | 0.2968 | 0.0916 | 13.37 | 23.58 | KOH |
| W9H-L. leucocephala | 900 | 60 | 251.72 | 179.63 | 72.09 | 0.209 | 0.0898 | 0.1192 | 28.64 | 57.03 | H3PO4 |
| R9H-Z. jujuba | 900 | 60 | 144.89 | 103.81 | 41.08 | 0.1288 | 0.0544 | 0.0744 | 28.35 | 57.76 | H3PO4 |
| S9H-S. samarangense | 900 | 60 | 633.98 | 570.8 | 63.18 | 0.3796 | 0.2746 | 0.105 | 9.97 | 27.66 | H3PO4 |
| Sample Source | CEC (cmol(+)/kg BC) | (CEC/BET) |
|---|---|---|
| Leucaena leucocephala | 6.8 | 0.0293 |
| Ziziphus jujuba | 13.3 | 0.04 |
| Syzygium samarangense | 10.4 | 0.0291 |
| Non-Heavy Metal | N % | P % | K% | Ca (ppm) | Mg (ppm) | Mn (ppm) | Fe (ppm) | B (ppm) | Na (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| Leucaena leucocephala | 1.07 | 0.05 | 0.74 | 13,633.23 | 1838.62 | 22.27 | 187.26 | 53.02 | 677.77 |
| Ziziphus jujuba | 0.87 | 0.29 | 2.05 | 36,618.98 | 4692.50 | 72.57 | 188.10 | 31.81 | 1213.07 |
| Syzygium samarangense | 1.01 | 0.24 | 1.16 | 25,232.43 | 3471.38 | 25.43 | 145.21 | 46.83 | 1162.53 |
| Heavy metal | Cu (ppm) | Zn (ppm) | Cd (ppm) | Cr (ppm) | Ni (ppm) | Pb (ppm) | As (ppm) | Hg (ppm) | |
| Leucaena leucocephala | 6.40 | 12.01 | <0.009 | 32.83 | 20.72 | <0.027 | <0.005 | 0.30 | |
| Ziziphus jujuba | 23.57 | 32.05 | <0.009 | 37.40 | 26.85 | <0.027 | <0.005 | 0.52 | |
| Syzygium samarangense | 14.70 | 12.85 | <0.009 | 6.52 | 5.70 | <0.027 | <0.005 | 0.52 | |
| Basic (Max.) | 100.00 | 400.00 | 1.50 | 90.00 | 50.00 | 150.00 | 13.00 | 1.00 | |
| Premium (Max.) | 100.00 | 400.00 | 1.00 | 80.00 | 30.00 | 120.00 | 13.00 | 1.00 |
| The Stumps of Representative Trees in Taiwan | Carbonization Temperature (°C) | Absorption Peak (cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3400 | 29.00 | 2830–2670 | 1700 | 1600 | 1480–1410 | 1120–1050 | 895–880 | ||
| Alcohols or Phenolic | Aliphatic Hydrocarbons | Carboxylic Group | Hemi- Cellulose | Cellulose & Hemicellulose | Olefins | ||||
| O-H Stretching | C-H Stretching | C-H Bending | C=O Stretching | C=C- Stretching | C-H Deformation | C-O Stretching | C=C Stretching | ||
| Leucaena leucocephala | 500 | + | + | + | + | + | + | + | + |
| 900 | - | - | - | - | - | - | - | - | |
| Syzygium samarangense | 500 | + | + | + | + | + | + | + | + |
| 900 | - | - | - | - | - | - | - | - | |
| Ziziphus jujuba | 500 | + | + | + | + | + | + | + | + |
| 900 | - | - | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-C.; Kitamura, Y.; Chien, C.-C.; Cheng, C.-S.; Cheng, J.-H.; Tsai, S.-H.; Hsieh, C.-C. Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan. Sustainability 2022, 14, 14792. https://doi.org/10.3390/su142214792
Lee S-C, Kitamura Y, Chien C-C, Cheng C-S, Cheng J-H, Tsai S-H, Hsieh C-C. Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan. Sustainability. 2022; 14(22):14792. https://doi.org/10.3390/su142214792
Chicago/Turabian StyleLee, Shih-Chi, Yutaka Kitamura, Chuan-Chi Chien, Chun-Shen Cheng, Jen-Hao Cheng, Shu-Hsien Tsai, and Chin-Cheng Hsieh. 2022. "Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan" Sustainability 14, no. 22: 14792. https://doi.org/10.3390/su142214792
APA StyleLee, S.-C., Kitamura, Y., Chien, C.-C., Cheng, C.-S., Cheng, J.-H., Tsai, S.-H., & Hsieh, C.-C. (2022). Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan. Sustainability, 14(22), 14792. https://doi.org/10.3390/su142214792






