The Use of Fermented Plant Biomass in Pigs Feeding
Abstract
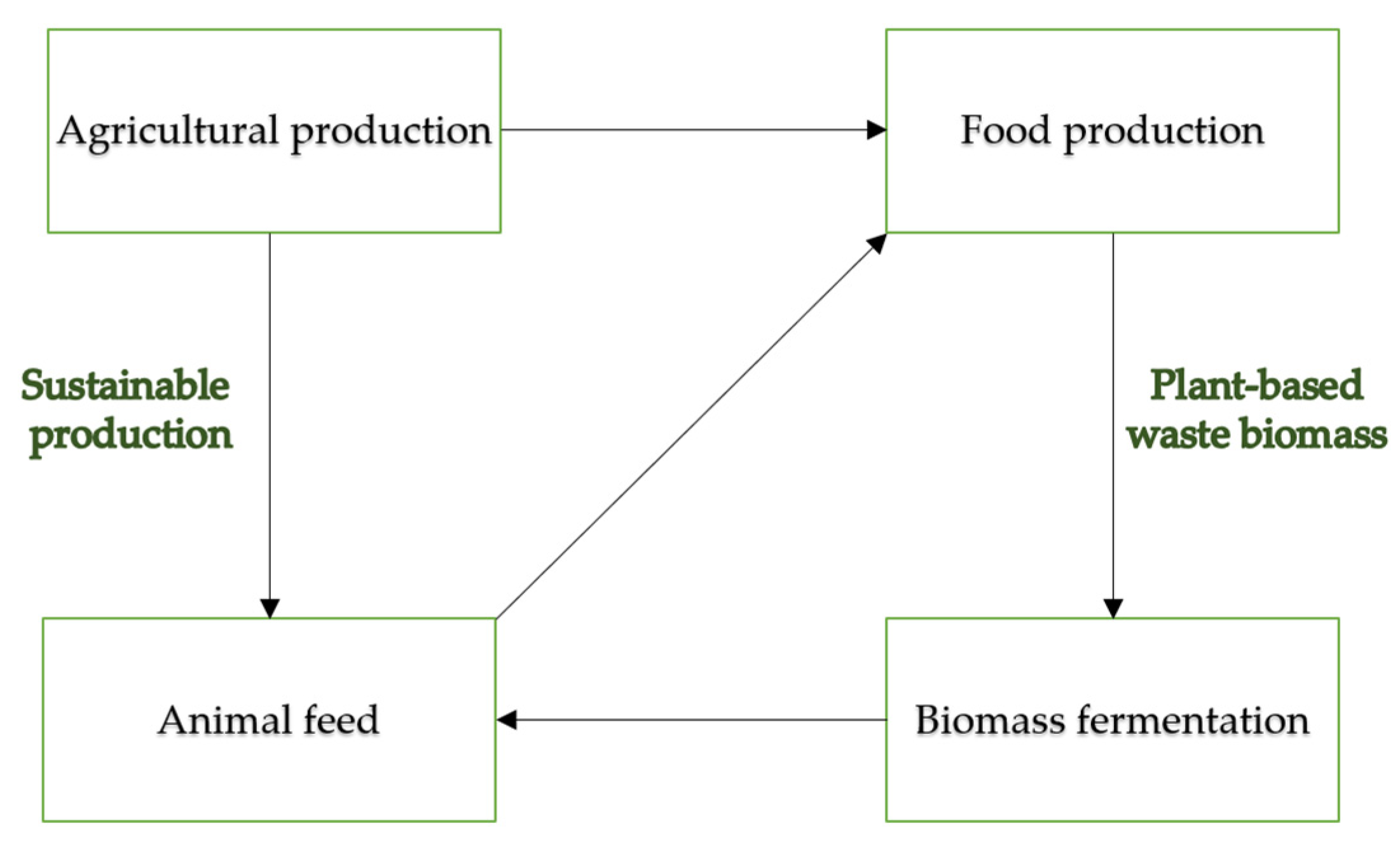
1. Introduction
1.1. Fermented Biomass as an Element of the Circular Economy
1.2. Contemporary Models Related to Pig Feeding
2. Fermented Biomass as a Feed Component
| Type of Biomass | Microorganisms Used for Fermentation | Process Conditions | Results of Fermentation | References |
|---|---|---|---|---|
| Corn grains | Lactobacillus fermentum, Saccharomyces cerevisiae, Bacillus subtilis | Fermentation using a probiotic composition and NSP (nonstarch polysaccharides) with the activity of xylanase, β-glucanase, mannanase, cellulase, and pectanase. | Increased population of each microbial strain, increased fiber degradation, and increased protein contents; the residual contents of dry matter, crude ash, and reducing sugar decreased. | [19] |
| Corn bran | Bacillus subtilis MA139, Saccharomyces cerevisae | Fermentation for 14 days at 30 °C. | Decreased amount of cellulose, hemicellulose, and lignin; increased amount of soluble dietary fiber and nonstarch polysaccharides as well as arabinose, xylose, and glucose. | [20] |
| Narrow-leaved lupine | Candida utilis | Fermentations were carried out under aerobic conditions (natural pH = 5.5) for 24 h in a continuous mixing system. Then, yeast enzymes were deactivated for 10 min at 70 °C. | Increased contents of alkloids, protein, lysine, cystine, and threonine contents but not the methionine. Fermentation also reduced the Acid Detergent Fibre (ADF), NDF, and phytate-P. Fermentation significantly improved the digestibility of protein, asparagine, threonine, tyrosine, histidine, and arginine. Fermented products were characterized also by acidic pH and higher content of yeast and bacteria. | [21] |
| Saccharomyces cerevisiae, Saccharomyces carlsbergensis | Aerobic conditions (natural pH = 5.5) for 24 h in a continuous mixing system. Then, yeast enzymes were deactivated for 10 min at 70 °C. | The content of crude ash and ADF significantly increased in all fermented products, whereas the ether extract and Nitrogen-Free Extract (NFE) contents significantly decreased. | [22] | |
| Yellow lupine | Saccharomyces cerevisiae, Saccharomyces carlsbergensis | Aerobic conditions (natural pH = 5.5) for 24 h in a continuous mixing system. Then, yeast enzymes were deactivated for 10 min at 70 °C. | The content of crude ash and ADF significantly increased in all fermented products, whereas the ether extract and Nitrogen-Free Extract (NFE) contents significantly decreased. The metabolizable energy was similar in all the samples. | [22] |
| Soy | Rhizopus microspores zm. microsporus LU 573 | Soybeans were soaked overnight in tap water, then washed with tap water and boiled for 20 min in fresh tap water at a 1:3 ratio. The cooked seeds were cooled and dried at room temperature, inoculated with a 7-day suspension containing Rhizopus microsporus zm. microsporus LU 573, 0.85% HCl, and 0.1% peptone, then fermented for 72 h at 30 °C. | Increased contests of crude lipid and crude protein in cooked and fermented soya beans. No differences in crude lipid and crude protein between cooked and fermented soya beans, but values for crude fibre were higher for fermented soya beans. Fermentation led to a major increase in nonprotein nitrogen. | [23,24] |
| Rhizopus microspores zm. microsporus LU 573 Bacillus subtilis LU B83 | Fermentation was carried out for 48 h at 37 °C in large vessels containing ± 35 kg of inoculated cooked soybeans. | Increased contests of crude lipid and crude protein in cooked and fermented soya beans. No differences in crude lipid and crude protein between cooked and fermented soya beans, but values for crude fibre were higher for fermented soya beans. Fermentation led to a major increase in nonprotein nitrogen. | [23,24] | |
| L. acidophilus (BCRC 10695), L. delbrueckii (BCRC 10696), L. salivarius (BCRC12574) Clostridium butyricum MIYAIRI 588 | The substrate was inoculated with a 3% inoculum and incubated in a chamber at 37 °C for 2–6 days, depending on the assay. Humidity was 40, 45, and 50%. | Soybean oligosaccharides, including raffinose and stachyose, were more efficiently degraded at the initial moisture of 50% compared with other initial moisture contents. Increased levels of the lactic acid, decreased level of pH, and reduced sugar content. | [25] | |
| Rapeseed cake | Aspergillus niger (CICC 41258) | Solid fermentation at 25, 28, 31, 34, or 37 °C (depending on the test) for 72 h, humidity 43, 50, 56, 62, and 67.5%. | Increased content of crude protein content and the total amino acids (TAA), essential amino acids (EAA), methionine and threonine, no significant difference in lysine. Decrease content of histamine. In vitro TAA and EAA digestibility was improved, the in vitro digestibility of nine amino acids including four essential amino acid (methionine, lysine, arginine, and histamine) also improved. The NDF contents and phityc acid content were reduced but ether extract content increased. | [26] |
| L. plantarum LUHS122, L. casei LUHS210, L. farraginis LUHS206, P. acidilactici LUHS29, L. plantarum LUHS135, L. uvarum LUHS245 | Two-stage fermentation: the first stage was inoculation of the rapeseed cake with a mixture of microorganisms and fermentation for 12 h at 30 °C; in the second stage, 30% of the fermented cake was added to a new batch of rapeseed and fermented for 6 weeks at 30 °C. | Lactic acid bacteria increased, pH decreased, additional essential nutrients were not lost. | [27] | |
| Rapeseed middlings (Brassica napus), wheat bran (Triticum eastivum), two types of brown seaweed (Saccharina latissima and Ascophylum nodosum) | Pediococcus acidilactici (DSM 16243), Pediococcus pentosaceus (DSM 12834), Lactobacillus plantarum (DSM 12837) | Fermentation for 12 days at 38 °C. | Higher lactic acid content and lower pH. | [28] |
| 40% corn, 40% soybean meal (SBM), and 20% wheat bran | Bacillus subtilis ZJU12 | Fermentation at room temperature for 96 h. | Fermented products contained greater concentrations of crude protein, ash, Ca, and total P and more than four times as much Trichloroacetic Acid Soluble Protein (TCA-SP). However, the crude fat decreased. Higher lactic acid content and lower pH. | [29] |
| 12% corn, 20% soybean meal, 48% wheat bran, and 20% soybeans | Bacillus subtilis ZJU12, Pediococcus pentosaceus ZJUAF-4 | 24 h fermentation of biomass at 37 °C with 40% humidity. | Higher lactic acid content and lower pH. | [30] |
| Wheat and barley | L. plantarum DSMZ 8862 and DSMZ 8866, L. buchneri NCIMB 40788 | Wheat and barley were milled, inoculated with a 1:1 mixture of L. plantarum and L. buchneri, and fermented anaerobically for 90 days. Humidity 27%. | Higher lactic acid content and lower pH. | [31] |
| Wheat, barley, and triticale | Natural grain bacteria | The grains of the cereals were mixed with grains of wet wheat stock, whey, and tap water. The mixtures were incubated at 10, 15, or 20 °C. After the 5 days of fermentation, 80% of the contents were replaced with fresh liquids and cereal grains daily for the following 14 days, with 20% left each time as the inoculant for the fresh feed mix. | The cereal grain mix had a more diverse yeast flora, which consisted of Pichia anomala, Rhodotorula glutinis, Sporobolomyces ruberrimus, Aureobasidium pullulans, and Cryptococcus adeliensis. The LAB Pediococcus pentosaceus, L. plantarum, Lactococcus lactis, and Lactococcus garvieae were identified in the cereal grain mix. The LAB population was dominated by L.plantarum both before and after storage. The species composition of yeast and LAB populations did not change during grain mix storage. | [32] |
| Feedtech® F3000 (Delaval International AB, Tumba, Sweden) consisting of a mixture Enterococcus faecium, L.plantarum, Lactococcus lactis, and Pediococcus pentosaceus | Feed mixtures after inoculation with lyophilized microorganisms and hydration with tap water were incubated at 20 °C for 5 days. Then, 4/5 of the contents were replaced with fresh compound feed, which was replaced daily for 5 days. The remaining contents served as the inoculum for the next fresh compound feed. | Increased concentrations of all tested organic acids (acetic acid, lactic acid, succinic acid, propionic acid) and ethanol. | [33] | |
| Wheat, barley, and soybeans | Lactobacillus plantarum DSMZ16627, Pediococcus acidilactici NCIMB3005 | The grains were mixed with water. The starter cultures were added, incubated for 48 h at the optimal temperature, and mixed for 30 min with an interval of 30 min between each mixing. | Higher LAB counts. | [34] |
2.1. Use of Maize for the Production of Fermented Feed
- dry weight: 30–35%;
- starch: minimum 30% in dry matter;
- crude fiber: maximum 20% in dry matter;
- ADF: maximum 25% in dry matter;
- NDF: maximum 45% in dry matter;
- energy content: minimum 6.5 MJ NEL or 0.9 JPM in 1 kg of dry matter.
2.2. Use of Yellow and Narrow-Leaved Lupine in the Production of Fermented Feed
2.3. Other Plant-Based Biomass Used in the Production of Fermented Feed
2.3.1. Soy
2.3.2. Rapeseed
2.3.3. Cereals
3. Biologically Active Substances in Fermented Feed Components
- Primary: carbohydrates, proteins, vegetable fats;
- Secondary: protein compounds (phenolic compounds, glucosinolates, glycosides, phytins, or alkaloids).
| Biomass | Biologically Active Substance | Effects on Pigs | Biologically Active Substance in Fermented Biomass | Additional Benefit/Effects on Pigs |
|---|---|---|---|---|
| Corn | Polyphenols | Improving antioxidant potential; beneficial effects on lipid metabolism; improving intestinal health [47] | Probiotics | Increasing the natural immunity of pigs; positive effects on offspring; increasing the number of beneficial intestinal bacteria of the genus Lactobacillus [19,48,49] |
| β-glucan, food fiber | Reducing the risk of cardiovascular disease [50] | |||
| Resistant starch—an insoluble fraction of dietary fiber | Prebiotic; improving intestinal function; reducing symptoms of diarrhea [49] | Lactic acid | Preventing the proliferation of pathogens along the gastrointestinal tract (e.g., Enterobacteriaceae such as coliforms and Salmonella) [51] | |
| Cathorenoids and flavonoids (anthocyanins) | Reducing the risk of cardiovascular disease in animals [52] | |||
| Lupine | Phenolic antioxidants (caffeic acid and myricetin) | Slowing the oxidation reaction in the body (slowing aging, protects against cancer) [51] | Dietary fiber | Improving the physiology of the gastrointestinal tract and the gut microbiota; may also help maintain intestinal health and prevent postweaning diarrhea [53] |
| Soy | Polyphenols | Antioxidant and fungicidal properties; growth stimulating effects [54] | Free phenolic acids | Antioxidant, antityrosinase, and antiproliferative activities [51] |
| Soy isoflavones | Anti-inflammatory, antioxidative properties at cellular levels, engaging several receptors and pathways, including inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB, which plays a key role in regulating the immune response to infection. Disturbances in the regulation of NF-κB are associated with cancer, inflammation and autoimmune diseases, septic shock, viral infections, and inappropriate development of the immune system) activation and inducible-nitric oxide synthase enzymes, thereby ascribing antiviral properties [51] | Flavonoids | Strong antiproliferative activity against cancer cell lines [51] | |
| Soy saponins | Engaging anti-inflammatory pathways [51] | |||
| Prebiotics (raffinose, stachiosis, inulin, oligofructose) | Stimulating the development of probiotic intestinal flora; reducing the symptoms of hepatic encephalopathy; increasing intestinal peristalsis; lowering the pH and ammonia content in the stool; increasing the amount of short-chain fatty acids [48] | Probiotics | Improving the digestibility of nutrients; improving the composition of the gut microflora of piglets [55] | |
| Soybeans, lupins, beans | Lysine (essential amino acid) | Weight gain in pigs [48] | ||
| Rye/rapeseed | Dietary fiber | Improving the physiology of the gastrointestinal tract and the gut microbiota; may also help maintain intestinal health and prevent postweaning diarrhea [56] |
| Biomass | Antinutritive Substance | Effects on Pigs | References |
|---|---|---|---|
| Legumes | Trypsin inhibitors | Inhibiting the action of trypsin; reducing the digestibility of the protein | [57] |
| Common peas, field beans, and sorghum | Tannins | Protein precipitation; lowering the digestibility of feed | [57] |
| Legumes, cereals | Oligopeptides | Not hydrolyzed in the digestive tract; causing gas and diarrhea | [57] |
| Rapeseed | Glucosinolates | Toxic compounds are formed during decomposition; thyroid hypertrophy; damage to the pancreas and liver | [57,58] |
| Lupins | Alkaloids, lectins, tannins, phytates | Affecting the nervous tissue; damaging the liver | [38,57] |
| Protease inhibitors, alkaloids, lectins, tannins, or phytates | Reducing digestive capacity and the use of protein by animals (piglets) | [58] | |
| Soy | Antigenic protein (glycinin and β-conglycinin) | Abnormal morphology of the small intestine and diarrhea in rearing piglets | [11] |
| Trypsin inhibitor, lectin, α-amylase inhibitory factor, and soybean antigens | Reducing the nutritional value, utilization, and digestibility of soybean bios, which can lead to digestive and metabolic diseases | [25] | |
| Phytoestrogens | Negative effects on the reproductive system in sows; negative effects on animal reproduction | [55] | |
| Wheat and barley | Phytates | Reducing the digestibility of the feed | [31] |
| Rye | Nonstarch polysaccharides | Not hydrolyzed in the digestive tract; causing gas and diarrhea | [44] |
Improving the Nutritional Value of Plant Biomass by Lactic Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Agriculture and Climate Mitigation. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/key_policies/documents/cap-specific-objectives-brief-4-agriculture-and-climate-mitigation_en.pdf (accessed on 5 January 2022).
- International Institute for Sustainable Development. World Population to Reach 9.9 Billion by 2050. Available online: https://sdg.iisd.org/news/world-population-to-reach-9-9-billion-by-2050/ (accessed on 5 January 2022).
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Rydhmer, L.; Kyriazakis, I.; Øverland, M.; Gilbert, H.; Dekkers, J.C.; Hermesch, S.; Bouquet, A.; Izquierdo, E.G.; Louveau, I.; et al. Prospects for sustainability of pig production in relation to climate change and novel feed resources. J. Sci. Food Agric. 2020, 100, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council. Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on Genetically Modified Food and Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32003R1829 (accessed on 18 May 2022).
- Sobotka, W.; Fiedorowicz-Szatkowska, E. The Effect of Replacing Genetically Modified Soybean Meal with 00-Rapeseed Meal, Faba Bean and Yellow Lupine in Grower-Finisher Diets on Nutrient Digestibility, Nitrogen Retention, Selected Blood Biochemical Parameters and Fattening Performance of Pigs. Animals 2021, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Pomar, C.; Kipper, M.; Hauschild, L.; Rivest, J. Feeding behavior of growing–finishing pigs reared under precision feeding strategies1. J. Anim. Sci. 2016, 94, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ba, H.; van Milgen, J.; Taghipoor, M. A procedure to quantify the feed intake response of growing pigs to perturbations. Animal 2020, 14, 253–260. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, L.; Fu, J.; Li, Z.; Wang, Y.; Jin, M. Overall assessment of fermented feed for pigs: A series of meta-analyses. J. Anim. Sci. 2019, 97, 4810–4821. [Google Scholar] [CrossRef]
- Nowak, A.; Śliżewska, K.; Libudzisz, Z. Probiotics—History and Mechanisms of Their Effect. Żywność Nauka Technologia Jakość. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-article-9858acac-d432-46bf-90be-3b553194dbef (accessed on 5 January 2022).
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Von Wright, A. Fermented pig liquid feed: Nutritional, safety and regulatory aspects. J. Appl. Microbiol. 2009, 106, 351–368. [Google Scholar] [CrossRef]
- Santos, A.; Ávila, C.; Pinto, J.; Carvalho, B.; Dias, D.; Schwan, R. Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J. Appl. Microbiol. 2016, 120, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Dou, M.; Wang, X.; Han, Q.; Zhao, B.; Hu, J.; Yang, G.; Shi, X.; Li, X. Fermented corn-soybean meal elevated IGF1 levels in grower-finisher pigs1. J. Anim. Sci. 2018, 96, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Kolver, E.S.; Roche, J.R.; Miller, D.; Densley, R. Maize silage for dairy cows. J. N. Z. Grassl. 2001, 63, 195–201. [Google Scholar] [CrossRef]
- Serva, L.; Andrighetto, I.; Marchesini, G.; Contiero, B.; Grandis, D.; Magrin, L. Prognostic capacity assessment of a multiparameter risk score for aerobic stability of maize silage undergoing heterofermentative inoculation (Lactobacillus buchneri) in variable ensiling conditions. Anim. Feed Sci. Technol. 2021, 281, 115116. [Google Scholar] [CrossRef]
- Lin, B.; Yan, J.; Zhong, Z.; Zheng, X. A Study on the Preparation of Microbial and Nonstarch Polysaccharide Enzyme Synergistic Fermented Maize Cob Feed and Its Feeding Efficiency in Finishing Pigs. BioMed Res. Int. 2020, 2020, 8839148. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, J.; Guo, P.; Lu, W.; Geng, Z.; Levesque, C.L.; Johnston, L.J.; Wang, C.; Liu, L.; Zhang, J.; et al. Dietary Corn Bran Fermented by Bacillus subtilis MA139 Decreased Gut Cellulolytic Bacteria and Microbiota Diversity in Finishing Pigs. Front. Cell. Infect. Microbiol. 2017, 7, 526. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Taciak, M.; Frankiewicz, A. The Effect of Yeast Fermentation of Two Lupine Species on the Digestibility of Protein and Amino Acids, Microflora Composition and Metabolites Production in the Ileum of Growing Pigs. Animals 2021, 11, 2894. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Borowczyk, P.; Zaworska, A.; Nowak, W.; Frankiewicz, A.; Gulewicz, P. The Effect of Dry Yeast Fermentation on Chemical Composition and Protein Characteristics of Blue Lupin Seeds. Food Technol. Biotechnol. 2016, 54, 360–366. [Google Scholar] [CrossRef]
- Kiers, J.; Meijer, J.; Nout, M.; Rombouts, F.; Nabuurs, M.; Van Der Meulen, J. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J. Appl. Microbiol. 2003, 95, 545–552. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, M.R.; Rombouts, F.M.; Van Andel, E.E.; Nabuurs, M.J.; Van Der Meulen, J. Effect of processed and fermented soyabeans on net absorption in enterotoxigenic Escherichia coli-infected piglet small intestine. Br. J. Nutr. 2006, 95, 1193–1198. [Google Scholar] [CrossRef]
- Su, L.-W.; Cheng, Y.-H.; Hsiao, F.S.-H.; Han, J.-C.; Yu, Y.-H. Optimization of Mixed Solid-state Fermentation of Soybean Meal by Lactobacillus Species and Clostridium butyricum. Pol. J. Microbiol. 2018, 67, 297–305. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, D. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Badaras, S.; Klupsaite, D.; Mozuriene, E.; et al. Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock. Animals 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Tamez-Hidalgo, P.; Cieplak, T.; Satessa, G.D.; Kot, W.; Kjærulff, S.; Nielsen, M.O.; Nielsen, D.S.; Krych, L. Supplementation of a lacto-fermented rapeseed-seaweed blend promotes gut microbial- and gut immune-modulation in weaner piglets. J. Anim. Sci. Biotechnol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, C.; Su, W.; Zhang, Y.; Wang, F.; Wang, Y.; Shi, C.; Lu, Z. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J. Anim. Sci. 2018, 96, 206–214. [Google Scholar] [CrossRef]
- Hao, L.; Su, W.; Zhang, Y.; Wang, C.; Xu, B.; Jiang, Z.; Wang, F.; Wang, Y.; Lu, Z. Effects of supplementing with fermented mixed feed on the performance and meat quality in finishing pigs. Anim. Feed Sci. Technol. 2020, 266, 114501. [Google Scholar] [CrossRef]
- Koo, B.; Bustamante-García, D.; Nyachoti, C.M. Energy content and nutrient digestibility of diets containing Lactobacillus-fermented barley or wheat fed to weaned pigs. J. Anim. Sci. 2018, 96, 4802–4811. [Google Scholar] [CrossRef]
- Olstorpe, M.; Lyberg, K.; Lindberg, J.E.; Schnürer, J.; Passoth, V. Population Diversity of Yeasts and Lactic Acid Bacteria in Pig Feed Fermented with Whey, Wet Wheat Distillers’ Grains, or Water at Different Temperatures. Appl. Environ. Microbiol. 2008, 74, 1696–1703. [Google Scholar] [CrossRef]
- Olstorpe, M.; Axelsson, L.; Schnã¼Rer, J.; Passoth, V. Effect of starter culture inoculation on feed hygiene and microbial population development in fermented pig feed composed of a cereal grain mix with wet wheat distillers’ grain. J. Appl. Microbiol. 2009, 108, 129–138. [Google Scholar] [CrossRef]
- Meara, F.M.O.; Gardiner, G.E.; Doherty, J.V.O.; Clarke, D.; Cummins, W.; Lawlor, P.G. Effect of wet/dry, fresh liquid, fermented whole diet liquid, and fermented cereal liquid feeding on feed microbial quality and growth in grow-finisher pigs. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 1–25. [Google Scholar] [CrossRef]
- Sola-Oriol, D. Data sheets: Maize. Available online: https://www.pig333.com/articles/maize-as-a-feed-ingredient-for-pigs_14971/ (accessed on 17 June 2022).
- Bathla, S.; Jaidka, M.; Kaur, R. Nutritive Value. 2020. Available online: https://www.intechopen.com/chapters/70668 (accessed on 5 January 2022).
- Islam, S.; Ma, W. Lupine. Encyclopedia of Food and Health. 2016. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/lupin (accessed on 5 January 2022).
- Kim, J.C.; Pluske, J.R.; Mullan, B.P. Lupins as a protein source in pig diets. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 3. [Google Scholar] [CrossRef]
- Available online: www.tridge.com/intelligences/lupin-bean/production (accessed on 5 January 2022).
- Grela, E.R.; Samolińska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of Minerals and Fatty Acids and Their Correlation with Phytochemical Compounds and Antioxidant Activity of Leguminous Seeds. Biol. Trace Element Res. 2017, 180, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- McGhee, M.L.; Stein, H.H. Apparent and standardized ileal digestibility of AA and starch in hybrid rye, barley, wheat, and corn fed to growing pigs1. J. Anim. Sci. 2018, 96, 3319–3329. [Google Scholar] [CrossRef]
- Roberfroid, M.; Slavin, J. Nondigestible Oligosaccharides. Crit. Rev. Food Sci. Nutr. 2000, 40, 461–480. [Google Scholar] [CrossRef]
- Zaworska-Zakrzewska, A.; Kasprowicz-Potocka, M.; Mikuła, R.; Taciak, M.; Pruszyńska-Oszmałek, E.; Frankiewicz, A. Growth Performance, Gut Environment and Physiology of the Gastrointestinal Tract in Weaned Piglets Fed a Diet Supplemented with Raw and Fermented Narrow-Leafed Lupine Seeds. Animals 2020, 10, 2084. [Google Scholar] [CrossRef]
- Paulsmeyer, M.; Juvik, J. Functional Characterization of an Anthocyanin Dimalonyltransferase in Maize. Molecules 2021, 26, 2020. [Google Scholar] [CrossRef]
- He, X.; Sun, W.; Ge, T.; Mu, C.; Zhu, W. An increase in corn resistant starch decreases protein fermentation and modulates gut microbiota during in vitro cultivation of pig large intestinal inocula. Anim. Nutr. 2017, 3, 219–224. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Bikker, P.; van Wikselaar, P.G.; van Krimpen, M.M. Inoculated Fermented Corn as Dietary Performance and Health Enhancer in Pigs: Literature and In Vitro Study. Available online: https://edepot.wur.nl/384209 (accessed on 18 May 2022).
- Sheng, S.; Li, T.; Liu, R.H. Corn phytochemicals and their health benefits. Food Sci. Hum. 604 Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Smith, B.N.; Dilger, R.N. Immunomodulatory potential of dietary soybean-derived isoflavones and saponins in pigs1. J. Anim. Sci. 2018, 96, 1288–1304. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, R.; Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Bolaños, E.N.A.; Carrillo-Rodríguez, J.C.; Pérez-Herrera, A. Bioactive compounds and antioxidant activities in pigmented maize landraces. Interciencia 2019, 44, 549–556. [Google Scholar]
- Lu, J.; Zhang, X.; Liu, Y.; Cao, H.; Han, Q.; Xie, B.; Fan, L.; Li, X.; Hu, J.; Yang, G.; et al. Effect of Fermented Corn-Soybean Meal on Serum Immunity, the Expression of Genes Related to Gut Immunity, Gut Microbiota, and Bacterial Metabolites in Grower-Finisher Pigs. Front. Microbiol. 2019, 10, 2620. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Ju, H.K.; Kim, S.C.; Park, J.H.; Lim, J.; Kwon, S.W.; Lee, J. Determination of bioactive compounds in fermented soybean products using GC/MS and further investigation of correlation of their bioactivities. J. Chromatogr. B 2012, 880, 42–49. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M. Dietary fermented rapeseed or/and soybean meal additives on performance and intestinal health of piglets. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Ellner, C.; Wessels, A.G.; Zentek, J. Effects of Dietary Cereal and Protein Source on Fiber Digestibility, Composition, and Metabolic Activity of the Intestinal Microbiota in Weaner Piglets. Animals 2022, 12, 109. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Rho, Y.; Wey, D.; Zhu, C.; Kiarie, E.; Moran, K.; Van Heugten, E.; De Lange, C.F.M. Growth performance, gastrointestinal and digestibility responses in growing pigs when fed corn–soybean meal-based diets with corn DDGS treated with fiber degrading enzymes with or without liquid fermentation1. J. Anim. Sci. 2018, 96, 5188–5197. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.; Kim, I. Effects of probiotics containing (Lactobacillus planetarium) and chlortetracycline on growth performance, nutrient digestibility, fecal microflora, diarrhea score and fecal gas emission in weanling pigs. Livest. Sci. 2020, 241, 104186. [Google Scholar] [CrossRef]
- Cebulska, A.; Jankowiak, H.; Weisbauerová, E.; Nevrkla, P. Influence of an increased content of pea and yellow lupin protein in the diet of pigs on meat quality. Porc. Heal. Manag. 2021, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Nutrient Ingredient | Nutritive Value (%) |
|---|---|
| Protein | 8.80 ± 0.49 |
| Ash | 1.17 ± 0.16 |
| Fat | 3.77 ± 0.48 |
| Total fibre | 12.24 ± 0.93 |
| Insoluble fiber | 11.29 ± 0.85 |
| Soluble fiber | 0.94 ± 0.18 |
| Carbohydrates | 64.77 ± 1.58 |
| Lysine | 2.64 ± 0.18 |
| Methionine | 2.10 ± 0.17 |
| Cysteine | 1.55 ± 0.14 |
| Threonine | 3.23 ± 0.29 |
| Tryptophan | 3.23 ± 0.29 |
| Nutrient Ingredient | Concentration in 1 kg of Dry Matter |
|---|---|
| Total protein (%) | 356 |
| Crude fiber (%) | 164 |
| Crude fat (%) | 56 |
| Nitrogen-Free Extracts (%) | 384 |
| Crude ash (%) | 51 |
| Starch (g) | 96 |
| Simple sugars (g) | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płacheta, B.; Motyl, I.; Berłowska, J.; Mroczyńska-Florczak, M. The Use of Fermented Plant Biomass in Pigs Feeding. Sustainability 2022, 14, 14595. https://doi.org/10.3390/su142114595
Płacheta B, Motyl I, Berłowska J, Mroczyńska-Florczak M. The Use of Fermented Plant Biomass in Pigs Feeding. Sustainability. 2022; 14(21):14595. https://doi.org/10.3390/su142114595
Chicago/Turabian StylePłacheta, Barbara, Ilona Motyl, Joanna Berłowska, and Marta Mroczyńska-Florczak. 2022. "The Use of Fermented Plant Biomass in Pigs Feeding" Sustainability 14, no. 21: 14595. https://doi.org/10.3390/su142114595
APA StylePłacheta, B., Motyl, I., Berłowska, J., & Mroczyńska-Florczak, M. (2022). The Use of Fermented Plant Biomass in Pigs Feeding. Sustainability, 14(21), 14595. https://doi.org/10.3390/su142114595







