The Potential and Green Chemistry Attributes of Biopesticides for Sustainable Agriculture
Abstract
1. Introduction
2. Biopesticides’ Definition and Suitability as Green Chemistry Agents
- Synthetic processes are safe, green, and use minimal energy;
- The final products are environmentally friendly;
- Wastes are prevented or minimized.
3. Effectiveness of Synthetic Pesticides and Their Disadvantages
4. Biopesticides as a Substitute for Conventional Pesticides
| Product | Manufacturers | Active Agent | Mode of Action | Controlled Pest | Protected Crop | Reference |
|---|---|---|---|---|---|---|
| Biochemical | ||||||
| Aza-Direct | Gowan (USA) | Azadiractin | Growth and moulting disruption | Egg, larvae and pupae of beetles and sucking insects | Cotton, Papua, vegetables | [98] |
| Timorex Gold | STK Stockton (Israel) | Tea tree oil (1,8-cineole and terpinen-4-ol) | Antifeedant, disruption of the fungal cell wall and curative activities | Black Sigatoka, a leaf-spot fungal disease | Bananas, strawberries, tomatoes, grapes, lettuce. | [99] |
| Regalia MAXX | Marrone Bio Innovations (USA) | Extract of Reynoutria sachalinensis | Broad-spectrum antimicrobial activity, resistance against disease | Fungi and bacteria | Hemp, cannabis, tomatoes, apples, blueberries | [19] |
| Nema-Q | Brandt Consolidated (USA) | Saponins | Nematicidal effects, | Root-knot nematodes | Berries, Citrus, Pome Fruit Grapes, Nut Crops | [100] |
| Microbials | ||||||
| Lipel | Agri-Life (India) | Bacillus thuringiensis | Lethal action against eggs and larvae of diamondback moth | Diamondback Moth | Cruciferous vegetables: Collard greens, cauliflower | [101] |
| Cordalene | Agrichem (Australia) | Bacillus thuringiensis | Killing of midgut cells by Cry toxins through signal transduction | Lepidoptera insect pests | Maize, sugarcane, soybeans, peanuts, flax | [102] |
| Daman | International Panaacea Ltd. (India) | Beauveria bassiana | Growth inhibition and larvicidal activities | Larval, pupal and nymphal stages of Spodoptera | Rice, maize, sorghum | [103] |
| MeloCon WG | Certis (USA) | Paecilomyces lilacinus | Colonization of plant roots, egg mass of nematodes and incapacitating second-stage instars | Nematodes | Vegetables, citrus strawberries, grapevines, tomato | [104] |
| Grasshopper Spore | ARBRICO Organics | Nosema locustae | Infects insects at the moulting stage | Grasshopper | Vegetables, fruits, | [105] |
| Littovir | Andermatt Biocontrol AG (Switzerland) | SpliNPV * | Induction of adult, pupa and larval malformation | African cotton leaf worm (Spodoptera littoralis) | Okro, onion, groundnut, beetroot, cabbage | |
| PIPs | ||||||
| Bt-cotton | Chinese Academy of Agricultural Sciences. | Cry1Ac, Cry2Ab toxins | Act as gut poison leading to pore formation | Diptera, beetles, H. armigera | Cotton | [106] |
| 5345 | Monsanto (USA) | Cr y1Ac gene | Kills insets by pore insertion into gut membranes | Lepidopteran pests: Fruitworm, pinworm, hornworm | Tomatoes | [107] |
| At+-Potato | Research-limited | RNAi | Causes post-transcriptional silencing target genes responsible for infection and maintenance | Phytophthora infestans | Potatoes | [108] |
| Natural Enemies | ||||||
| Mealybug Destroyer; Convergent Lady Beetles; Whitefly Predator; Spider Mite Destroyer; Scale Predator; Fungus Gnat Predator | Great Lakes IPM, Inc. (Vestaburg, MI, USA) | Cryptolaemus montrouzieri; Lady beetle; Delphastus; Stethorus; Cybocephalus nipponicus; Rove beetle (Atheta coriaria) | Predation | A broad variety of insects fall victim including slugs, aphids, mealybugs, thrips, whiteflies, scales, spider mites, leafhoppers, fungal growths | Citrus, corn, ornamentals, vegetables, sweet potato | [109,110] |
| Chinese Mantid; Green Lacewings; Aphid Predator Midge; Predatory Mites | Crop King, (Lodi, OH, USA) | Tenodera aridofolia; Chrysoperia spp. larva; Aphidoletes aphidimyza larva; Phytoseiidae | Predation | A broad range of insects including aphids, | Pea, cabbage, cowpea, cucurbits, crucifers, eggplants, okra, lettuce | [111,112] |
5. Sustainability Attributes of Biopesticides
6. Emerging Frontiers of Biopesticide
7. Constraints and Outlook
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Society Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Saranya, G.; Ramachandra, T.V. Life cycle assessment of biodiesel from estuarine microalgae. Energy Convers. Manag. X 2020, 8, 100065. [Google Scholar] [CrossRef]
- Kabbage, M.; Yarden, O.; Dickman, M.B. Pathogenic attributes of Sclerotinia sclerotiorum: Switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 2015, 233, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S. After the famine: Plant pathology, Phytophthora infestans, and the late blight of potatoes, 1845–1960. Hist. Stud. Phys. Biol. Sci. 2005, 35, 341–370. [Google Scholar] [CrossRef]
- Temba, B.A. Occurrence of Mycotoxins in Harvested Maize in Kenya and Tanzania and Postharvest Control by Photosensitisation. Available online: https://www.aflatoxinpartnership.org/ (accessed on 13 August 2022).
- Odeyemi, O.; Ugwu, J. Fall armyworm, Spodoptera frugiperda outbreak in Nigeria: Impacts and management on maize fields-a review. Ethiop. J. Environ. Stud. Manag. 2021, 14, 353–369. [Google Scholar]
- Dutta, T.K.; Khan, M.R.; Phani, V. Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: Current status and future prospects. Curr. Plant Biol. 2019, 17, 17–32. [Google Scholar] [CrossRef]
- Heinrichs, E.A. Management of Rice Insect Pests. Radcliffe’s IPM World Textbook. Available online: https://ipmworld.umn.edu/heinrichs (accessed on 4 January 2021).
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Keifer, M.C.; Firestone, J. Neurotoxicity of pesticides. J. Agromedicine 2007, 12, 17–25. [Google Scholar] [CrossRef]
- Jhala, J.; Baloda, A.S.; Rajput, V.S. Role of bio-pesticides in recent trends of insect pest management: A review. J. Pharmacogn. Phytochem. 2020, 9, 2237–2240. [Google Scholar]
- Constantine, K.L.; Kansiime, M.K.; Mugambi, I.; Nunda, W.; Chacha, D.; Rware, H.; Makale, F.; Mulema, J.; Lamontagne-Godwin, J.; Williams, F.; et al. Why don’t smallholder farmers in Kenya use more biopesticides? Pest Manag. Sci. 2020, 76, 3615–3625. [Google Scholar] [CrossRef]
- Singh, J.K.; Yadav, K.K.; Kumar, V. Integrated pest management: Conservation practices for agriculture and environment. Int. J. Environ. Rehabil. Conserv. 2017, 8, 17–28. [Google Scholar]
- Rao, N.C.; Bathla, S.; Kumar, A.; Jha, G.K. Agriculture and sustainable development goals: An overview and issues. Agric. Econ. Res. Rev. 2018, 31, 1–7. [Google Scholar] [CrossRef]
- Hák, T.; Janoušková, S.; Moldan, B. Sustainable Development Goals: A need for relevant indicators. Ecol. Indic. 2016, 60, 565–573. [Google Scholar] [CrossRef]
- Copping, L.G. Report on the 5th EEN Biopesticide event. Outlooks Pest Manag. 2017, 28, 108–112. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef]
- Oguh, C.E.; Okpaka, C.O.; Ubani, C.S.; Okekeaji, U.; Joseph, P.S.; Amadi, E.U. Natural pesticides (biopesticides) and uses in pest management-a critical review. Asian J. Biotechnol. Genetic. Eng. 2019, 2, 1–18. [Google Scholar]
- Marrone, P.G. An effective biofungicide with novel modes of action. Pestic. Outlook 2002, 13, 193–194. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, 100–129. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Intl. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Bhandari, S.; Kasana, V. Fe3+-Montmorillonite K10 as an Efficient, green and reusable heterogeneous catalyst for synthesis of mannich type reaction under solvent-free condition. Int. Res. J. Pure Appl. Chem. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Mubeen, K.; Shehzad, M.; Sarwar, N.; Rehman, H.U.; Yasir, T.A.; Wasaya, A.; Ahmad, M.; Hussain, M.; Abbas, M.B.; Yonas, M.W.; et al. The impact of horse purslane (Trianthema portulacastrum L.) infestation on soybean [Glycine max (L.) Merrill] productivity in northern irrigated plains of Pakistan. PLoS ONE 2021, 16, e0257083. [Google Scholar] [CrossRef] [PubMed]
- Badhai, S.; Gupta, A.K.; Maurya, S.P.; Koiri, B. Ecological/cultural measures of weed management for sustainable agriculture. J. Wastes Biomass. Manag. 2021, 3, 36–38. [Google Scholar] [CrossRef]
- Schroeder, J.; Thomas, S.H.; Murray, L.W. Impacts of crop pests on weeds and weed–crop interactions. Weed Sci. 2005, 6, 918–922. [Google Scholar] [CrossRef]
- Liebman, M.; Mohler, C.L.; Staver, C.P. Ecological management of agricultural weeds. Cambridge University Press: Cambride, MA, USA, 2001. [Google Scholar]
- Marro, N.; Caccia, M.; López-Ráez, J.A. Are strigolactones a key in plant–parasitic nematodes interactions? An intriguing question. Plant Soil 2021, 462, 591–601. [Google Scholar] [CrossRef]
- Benzonan, N.C.; Dalisay, L.C.; Reponte, K.C.; Mapanao, C.P.; Alvarez, L.V.; Rendon, A.O.; Zurbano, L.Y. Plant-parasitic nematodes associated with pineapple (Ananas comosus) in selected Provinces in Luzon, Philippines. Eur. J. Mol. Clinical. Med. 2021, 8, 945–957. [Google Scholar]
- Charlier, J.; Thamsborg, S.M.; Bartley, D.J.; Skuce, P.J.; Kenyon, F.; Geurden, T.; Hoste, H.; Williams, A.R.; Sotiraki, S.; Höglund, J.; et al. Mind the gaps in research on the control of gastrointestinal nematodes of farmed ruminants and pigs. Transbound. Emerg. Dis. 2018, 65, 217–234. [Google Scholar] [CrossRef]
- Ahmad, M.; Ali, Q.; Hafeez, M.M.; Malik, A. Improvement for biotic and abiotic stress tolerance in crop plants. Biol. Clin. Sci. Res. J. 2021, 2021, 1. [Google Scholar] [CrossRef]
- Budiyanto, G.; Trisnawati, D.W.; Aisyah, S.N. Vegetation analysis of the middle slope geomorphic units on the southern flank of Mount Merapi. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Burrows, M.; Franc, G.; Rush, C.; Blunt, T.; Ito, D.; Kinzer, K.; Olson, J.; O’Mara, J.; Price, J.; Tande, C.; et al. Occurrence of viruses in wheat in the Great Plains region. Plant Health Prog. 2008, 10, 14. [Google Scholar] [CrossRef]
- Deniau, M.; Pihain, M.; Béchade, B.; Jung, V.; Brunellière, M.; Gouesbet, V.; Prinzing, A. Seeds and seedlings of oaks suffer from mammals and molluscs close to phylogenetically isolated, old adults. Ann. Bot. 2021, 127, 787–798. [Google Scholar] [CrossRef]
- Matevski, D.; Glatthorn, J.; Kriegel, P.; Schuldt, A. Non-native Douglas fir (Pseudotsuga menziesii) promotes sentinel prey attack rates in Central European forests. Forest Ecol. Manag. 2021, 489, 119099. [Google Scholar] [CrossRef]
- Witmer, G.W. The ecology of vertebrate pests and integrated pest management (IPM). USDA Natl. Wildl. Res. Cent.-Staff. Publ. 2007, 17, 730. [Google Scholar]
- Sandle, T.; Vijayakumar, R.; Saleh, A.l.; Aboody, M.; Saravanakumar, S. In vitro fungicidal activity of biocides against pharmaceutical environmental fungal isolates. J. Appl. Microbiol. 2014, 117, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Paarlberg, R. African non-adopters. In Handbook on Agriculture, Biotechnology and Development; Edward Elgar Publishing: Chertenham, UK, 2014. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Zviahintseva, A.M. Major cucumber diseases and the crop immunity. Ukr. J. Ecol. 2021, 11, 46–54. [Google Scholar]
- Usta, C. Microorganisms in biological pest control—A review (bacterial toxin application and effect of environmental factors). Curr. Prog. Biol. Res. 2013, 13, 287–317. [Google Scholar]
- Holvoet, K.; Sampers, I.; Seynnaeve, M.; Jacxsens, L.; Uyttendaele, M. Agricultural and management practices and bacterial contamination in greenhouse versus open field lettuce production. Int. J. Environ. Res. Public Health 2015, 12, 32–63. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Growth promotion of the rice genotypes by PGPRs isolated from rice rhizosphere. J. Soil. Sci. Plant Nutr. 2014, 14, 505–517. [Google Scholar] [CrossRef]
- Varanda, C.M.; Félix, M.D.R.; Campos, M.D.; Patanita, M.; Materatski, P. Plant viruses: From targets to tools for CRISPR. Viruses 2021, 13, 141. [Google Scholar] [CrossRef]
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant Sci. 2014, 5, 660. [Google Scholar] [CrossRef]
- Murcia, N.; Serra, P.; Olmos, A.; Durán-Vila, N. A novel hybridization approach for detection of citrus viroids. Mol. Cellular. Probes. 2009, 23, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jarman, W.M.; Ballschmiter, K. From coal to DDT: The history of the development of the pesticide DDT from synthetic dyes till Silent Spring. Endeavour 2012, 36, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Abreu-Villaca, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Wawrzak, Z.; Liu, J.; Waterman, M.R.; Nes, W.D.; Lepesheva, G.I. Structural complex of sterol 14a-demethylase (CYP51) with 14a-methylenecyclopropyl-?7-24, 25-dihydrolanosterol. J. Lipid. Res. 2011, 53, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Stehmann, C.; De Waard, M.A. Relationship between chemical structure and biological activity of triazole fungicides against Botrytis cinerea. Pestic. Sci. 1995, 44, 183–195. [Google Scholar] [CrossRef]
- Kim, D.H.; Rossi, J.J. RNAi mechanisms and applications. Biotechniques 2008, 44, 613–616. [Google Scholar] [CrossRef]
- Linde, C.D. Physico-Chemical Properties and Environmental Fate of Pesticides. 1994. Available online: https://agris.fao.org/agris-search/index.do (accessed on 10 August 2022).
- Jablonowski, N.D.; Linden, A.; Koppchen, S.; Thiele, B.; Hofmann, D.; Mittelstaedt, W.; Putz, T.; Burauel, P. Long-term persistence of various 14C-labeled pesticides in soils. Environ. Pollut. 2012, 168, 29–36. [Google Scholar] [CrossRef]
- Toundou, O.; Palanga, K.K.; Simalou, O.; Abalo, M.; Woglo, I.; Tozo, K. Biopesticide Plants species of the mining area of Tokpli (South-Togo) effects on Okra (Abelmoschus esculentus) protection against Aphtona spp. Int. J. Biol. Chem. Sci. 2020, 14, 225–238. [Google Scholar] [CrossRef]
- Fox, J.E.; Gulledge, J.; Engelhaupt, E.; Burow, M.E.; McLachlan, J. A Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. USA 2007, 104, 10282–10287. [Google Scholar] [CrossRef]
- Han, S.H.; An, J.Y.; Hwang, J.; Kim, S.; Bin, P.B.B. The effects of organic manure and chemical fertilizer on the growth and nutrient concentrations of yellow poplar (Liriodendron tulipifera Lin.) in a nursery system. Forest Sci. Technol. 2016, 12, 137–143. [Google Scholar] [CrossRef]
- Knipe, D.W. Pesticide exposure in Sri Lanka. Int. J. Epidemiol. 2016, 45, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Jose, A.; Alex, R.G.; Mohan, V.R. Chronic pesticide exposure: Health effects among pesticide sprayers in Southern India. Indian J. Occup. Environ. Med. 2015, 19, 95–101. [Google Scholar] [PubMed]
- Crow, W.D.; Catchot, A.L.; Gore, J.; Dodds, D.M.; Cook, D.R.; Allen, T.W. Evaluation of tillage, at-planting treatment, and nematicide on tobacco thrips (Thysanoptera: Thripidae) and reniform nematode (Tylenchida: Hoplolamidae) management in cotton. Agronomy 2020, 10, 300. [Google Scholar] [CrossRef]
- Nguyen, T.T. Pesticide use in rice farming and its impacts on climbing perch (Anabas testudineus) in the Mekong Delta of Vietnam. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2016. [Google Scholar]
- Ramesh, M.; Narmadha, S.; Poopal, R.K. Toxicity of furadan (carbofuran 3% g) in Cyprinus carpio: Haematological, biochemical and enzymological alterations and recovery response. Beni-Seuf Univ. J. Appl. Sci. 2015, 4, 314–326. [Google Scholar] [CrossRef]
- Buser, H.R.; Muuller, M.D.; Buerge, I.J.; Poiger, T. Composition of aldrin, dieldrin, and photodieldrin enantiomers in technical and environmental samples. J. Agri. Food Chem. 2009, 57, 7445–7452. [Google Scholar] [CrossRef]
- Manyilizu, B.W. Pesticides, anthropogenic activities, history and the health of our environment: Lessons from africa. In Pesticides-Use and Misuse and Their Impact in the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Bacmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Swarupa, P.; Kumar, A. Impact of chlorpyrifos on plant growth promoting rhizobacteria isolated from Abelmoschus esculentus. J. Pure Appl. Microbiol. 2018, 12, 2149–2158. [Google Scholar] [CrossRef]
- Okoroiwu, H.U.; Iwara, I.A. Dichlorvos toxicity: A public health perspective. Interdiscip. Toxicol. 2018, 11, 129–137. [Google Scholar] [CrossRef]
- Aggarwal, V.; Deng, X.; Tuli, A.; Goh, K.S. Diazinon—Chemistry and environmental fate: A California perspective. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2013; pp. 107–140. [Google Scholar]
- Johnson, B.; Whitford, F.; Flakne, D.; Bauman, T.; Nice, G.; Frankenberger, J.; Hahn, L.; Bailey, T.; Donald, B.; Mann, C.; et al. Atrazine Use and Weed Management Strategies to Protect Surface Water Quality. 2004. Available online: https://ppp.purdue.edu/wp-content/uploads/2016/08/PPP-67.pdf (accessed on 26 December 2021).
- Hernandez, M.; Jia, Z.; Conrad, R.; Eeger, M. Simazine application inhibits nitrification and changes the ammonia-oxidizing bacterial communities in a fertilized agricultural soil. FEMS Microbiol. Ecol. 2011, 78, 511–519. [Google Scholar] [CrossRef]
- Narayanan, M.; Ranganathan, M.; Subramanian, S.M.; Kumarasamy, S.; Kandasamy, S. Toxicity of cypermethrin and enzyme inhibitor synergists in red hairy caterpillar Amsacta albistriga (Lepidoptera: Arctiidae). J. Basic Appl. Zool. 2020, 81, 1–8. [Google Scholar] [CrossRef]
- Paul, M.; Hossain, M.S.; Rahman, M.M.; Khaliq, Q.A.; Rahman, S. Chemodynamics of cypermethrin insecticide in summer country bean ecosystem in Bangladesh. Res. J. Environ. Toxicol. 2016, 10, 50–62. [Google Scholar]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising natural products in crop protection and food preservation: Basis, advances, and future prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Ujvary, I. Delmethrin: Properties, mode of action and safety issues related to tsetse control. In Proceedings of the WHO/TDR Progress and Planning Meeting, Nairobi, Kenya, 6–9 July 2015. [Google Scholar]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.; Xu, L.; Zhu, J.; Zhao, M.; Munos, S.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2, 4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Intl. 2018, 111, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34–56. [Google Scholar] [CrossRef]
- Fayinminnu, O.O.; Adesiyan, S.O.; Sosanya, S.O. Effect of paraquat as post emergence herbicide on yield of cowpea (Vigna unguiculata (l) walp). J. Agric. Social. Res. 2010, 10, 25–39. [Google Scholar] [CrossRef][Green Version]
- Meyer, C.J.; Norsworthy, J.K. Timing and application rate for sequential applications of glufosinate are critical for maximizing control of annual weeds in LibertyLink® Soybean. Int. J. Agron. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Rajput, V.S.; Jhala, J.; Acharya, V.S. Biopesticides and their mode of action against insect pests: A review. Int. JChe Stud. 2020, 8, 2856–2862. [Google Scholar] [CrossRef]
- Harding, D.P.; Raizada, M.N. Controlling weeds with fungi, bacteria and viruses: A review. Front. Plant Sci. 2015, 6, 659. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Meena, B.R.; Pandey, K.K.; Singh, J. Microbial bioagents in agriculture: Current status and prospects. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Khan, M.R.; Mohiddin, F.A.; Khan, S.M.; Khan, B. Effect of seed treatment with certain biopesticides on root-knot of chickpea [Cicer arietinum L.; India]. Nematol. Mediterr. 2005, 33, 107–112. [Google Scholar]
- Hammami, I.; Smaoui, S.; Hsouna, A.B.; Hamdi, N.; Triki, M.A. Ruta montana L. leaf essential oil and extracts: Characterization of bioactive compounds and suppression of crown gall disease. EXCLI J. 2015, 14, 83. [Google Scholar] [PubMed]
- Ichim, E.; Marutescu, L.; Popa, M.; Cristea, S. Antimicrobial efficacy of some plant extracts on bacterial ring rot pathogen, Clavibacter michiganensis spp. sepedonicus. EuroBiotech J. 2017, 1, 85–88. [Google Scholar] [CrossRef]
- Vuko, E.; Dunkic, V.; Rušcic, M.; Nazlic, M.; Mandic, N.; Soldo, B.; Šprung, M.; Fredotovic, Ž. Chemical composition and new biological activities of essential oil and hydrosol of Hypericum perforatum L. ssp. veronense (Schrank) H. Lindb. Plants 2021, 10, 1014. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar]
- Idrees, A.; Afzal, A.; Qadir, Z.A.; Li, J. Bioassays of Beauveria bassiana isolates against the fall armyworm, Spodoptera frugiperda. J. Fungi 2022, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Zamanizadeh, H.R.; Hatami, N.; Aminaee, M.M.; Rakhshandehroo, F. Application of biofungicides in control of damping disease off in greenhouse crops as a possible substitute to synthetic fungicides. Int. J. Environ. Sci. Technol. 2011, 8, 129–136. [Google Scholar] [CrossRef]
- Kohl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Krif, G.; Lahlali, R.; El Aissami, A.; Laasli, S.E.; Mimouni, A.; Serderidis, S.; Picaud, T.; Moens, A.; Dababat, A.A.; Fahad, K.; et al. Efficacy of authentic bio-nematicides against the root-knot nematode, Meloidogyne javanica infecting tomato under greenhouse conditions. Physiol. Mol. Plant Pathol. 2022, 118, 101803. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evidence-based complementary. Alt. Med. 2011, 2011, 253643. [Google Scholar]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Williamson, S. Understanding natural enemies; a review of training and information in the practical use of biological control. Biocontrol. News Infor. 1998, 19, 117N–125N. [Google Scholar]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.Y. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp.(Hemiptera: Cimicidae). Parasites Vectors 2017, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Damos, P.; Colomar, L.A.E.; Ioriatti, C. Integrated fruit production and pest management in Europe: The apple case study and how far we are from the original concept? Insects 2015, 6, 626–657. [Google Scholar] [CrossRef] [PubMed]
- Tangtrakulwanich, K.; Reddy, G.V. Development of insect resistance to plant biopesticides: An overview. In Advances in Plant Biopesticides; Springer: New Delhi, India, 2014; pp. 47–62. [Google Scholar]
- Sharma, R.; Kole, C. Utilization of neem and neem products in agriculture. In The Neem Genome; Springer: Cham, Switzerland, 2019; pp. 31–48. [Google Scholar]
- Reuveni, M.; Sanches, E.; Barbier, M. Curative and suppressive activities of essential tea tree oil against fungal plant pathogens. Agronomy 2020, 10, 609. [Google Scholar] [CrossRef]
- Lopez-Perez, J.A.; Edwards, S.; Ploeg, A. Control of root-knot nematodes on tomato in stone wool substrate with biological nematicides. J. Nematol. 2011, 43, 110–117. [Google Scholar] [PubMed]
- Rajamani, M.; Negi, A. Biopesticides for pest management. Sustain. Bioecon 2020, 13, 239–266. [Google Scholar]
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Baskar, K.; Muthu, C.; Raj, G.A.; Kingsley, S.; Ignacimuthu, S. Ovicidal activity of Atalantia monophylla (L) Correa against Spodoptera litura Fab.(Lepidoptera: Noctuidae). Asian Pacific. J. Trop. Biomed. 2012, 2, 987–991. [Google Scholar] [CrossRef]
- Hano, P.; Khan, M.R. Evaluation of fungal (Paecilomyces lilacinus) formulations against root knot nematode infecting tomato. Bangladesh J. Bot. 2016, 45, 1003–1013. [Google Scholar]
- Mahmoud, B.S. Effect of X-ray treatments on inoculated Escherichia coli O157: H7, Salmonella enterica, Shigella flexneri and Vibrio parahaemolyticus in ready-to-eat shrimp. Food Microbiol. 2009, 26, 860–864. [Google Scholar] [CrossRef]
- Tian, J.C.; Wang, X.P.; Chen, Y.; Romeis, J.; Naranjo, S.E.; Hellmich, R.L.; Wang, P.; Shelton, A.M. Bt cotton producing Cry1Ac and Cry2Ab does not harm two parasitoids, Cotesia marginiventris and Copidosoma floridanum. Sci. Rep. 2018, 8, 307. [Google Scholar] [CrossRef]
- Baranski, R.; Klimek-Chodacka, M.; Lukasiewicz, A. Approved genetically modified (GM) horticultural plants: A 25-year perspective. Folia Hortic. 2019, 31, 3–49. [Google Scholar] [CrossRef]
- Jahan, S.N.; Åsman, A.K.; Corcoran, P.; Fogelqvist, J.; Vetukuri, R.R.; Dixelius., C. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J. Exp. Bot. 2015, 66, 2785–2794. [Google Scholar] [CrossRef]
- Edirisinghe, H.M.; Leschen, R.A.B.; Dale, J.; Wignall, A.E. Insights into the establishment of introduced species using Coccinellines (Coleoptera: Coccinellidae) as a model system. Coleopt. Bull. 2021, 75, 121–149. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Barratt, B.I.P.; Bell, N.; Goldson, S.L.; Hardwick, S.; Jackson, M.; Wilson, M. Quantifying the economic cost of invertebrate pests to New Zealand’s pastoral industry. New Zeal. J. Agr. Res. 2018, 62, 255–315. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Hassankiadeh, M.N.; Zhang, L. Facile biosynthesis of SnO2/ZnO nanocomposite using Acroptilon repens flower extract and evaluation of their photocatalytic activity. Ceram. Int. 2021, 47, 29303–29308. [Google Scholar] [CrossRef]
- Volesky, N.; Schrumm, Z.R. High Tunnel Pest Management-Aphids. Utah Pest Fact Sheet. Utta State University. 2021. Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=3197&context=extension_curall (accessed on 5 July 2022).
- Ouedraogo, L.; Fuchs, D.; Schaefer, H.; Kiendrebeogo, M. Morphological and molecular characterization of Zanthoxylum zanthoxyloides (Rutaceae) from Burkina Faso. Plants 2019, 8, 353. [Google Scholar] [CrossRef]
- Filho, F.H.; Heldens, W.B.; Kong, Z.; de Lange, E.S. Drones: Innovative technology for use in precision pest management. J. Econ. Entomol. 2019, 113, 1–25. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Bingru, L.; Zhihong, M. Trifluralin residues in soils from main cotton fields of China and associated ecological risk. Chemosphere 2021, 284, 131300. [Google Scholar] [CrossRef]
- Karar, M.E.; Alsunaydi, F.; Albusaymi, S.; Alotaibi, S. A new mobile application of agricultural pests recognition using deep learning in cloud computing system. Alex. Eng. J. 2021, 60, 4423–4432. [Google Scholar] [CrossRef]
- James, B.; Atcha-Ahowé, C.; Godonou, I.; Baimey, H.; Goergen, H.; Sikirou, R.; Toko, M. Integrated Pest Management in Vegetable Production: A Guide for Extension Workers in West Africa. IITA: Ibadan, Nigeria, 2010.
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agro. Sustain. Dev. 2021, 41, 38. [Google Scholar]
- Gaetani, R.; Lacotte, V.; Dufour, V.; Clavel, A.; Duport, G.; Gaget, K.; Calevro, F.; Da Silva, P.; Heddi, A.; Vincent, D.; et al. Sustainable laser-based technology for insect pest control. Sci. Rep. 2021, 11, 11068. [Google Scholar]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil. Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Current status and prospects of botanical biopesticides in Europe and mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, S.; Malik, M.S.; Ahirwar, R.; Shukla, J.P. Identification of suitable sites and structures for artificial groundwater recharge for sustainable groundwater resource development and management. Groundw. Sustain. Dev. 2020, 11, 100388. [Google Scholar] [CrossRef]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287, 132107. [Google Scholar] [CrossRef]
- Lata, R.K.; Divjot, K.; Nath, Y.A. Endophytic microbiomes: Biodiversity, ecological significance and biotechnological applications. Res. J. Biotechnol. 2019, 14, 10. [Google Scholar]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 096–102. [Google Scholar]
- Bartomeus, C. Contribution of Insect Pollinators to Crop Yield and Quality Varies with Agricultural Intensification Vanbergen. A Report to the Department for Environment, Food and Rural Affairs; Defra: London, UK, 2014. [Google Scholar]
- IPBES. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; IPBES: Panama, Germany, 2016. [Google Scholar]
- Adamidis, G.C.; Cartar, R.V.; Melathopoulos, A.P.; Pernal, S.F.; Hoover, S.E. Pollinators enhance crop yield and shorten the growing season by modulating plant functional characteristics: A comparison of 23 canola varieties. Sci. Rep. 2019, 9, 14208. [Google Scholar] [CrossRef]
- Porto, R.G.; de Almeida, R.F.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. Pollination ecosystem services: A comprehensive review of economic values, research funding and policy actions. Food Secur. 2020, 12, 1425–1442. [Google Scholar] [CrossRef]
- Sawe, T.; Nielsen, A.; Eldegard, K. Crop pollination in small-scale agriculture in Tanzania: Household dependence, awareness and conservation. Sustain 2020, 12, 2228. [Google Scholar] [CrossRef]
- Fijen, T.P.; Scheper, J.A.; Boom, T.M.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.P.; Bishop, J.; Degani, E.; Potts, S.G.; Shaw, R.F.; Shi, A.; Roy, S. Insect pollination as an agronomic input: Strategies for oilseed rape production. J. Appl. Ecol. 2018, 55, 2834–2842. [Google Scholar] [CrossRef]
- Centner, T.J. Pesticide usage is compromising people’s health in the United States: Ideas for reducing damages. Agriculture 2021, 11, 486. [Google Scholar] [CrossRef]
- Attina, T.M.; Hauser, R.; Sathyanarayana, S.; Hunt, P.A.; Bourguignon, J.-P.; Myers, J.P.; Trasande, L. Exposure to endocrine-disrupting chemicals in the USA: A population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016, 4, 996–1003. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J. US urban forest statistics, values, and projections. J. For. 2018, 116, 164–177. [Google Scholar] [CrossRef]
- Çelık, D.; Meral, M.E.; Waseem, M. A New Area Towards to Digitalization of Energy Systems: Enables, Challenges and Solutions. In Proceedings of the 2022 14th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Ploiesti, Romania, 30 June–1 July 2022; IEEE; pp. 1–6. [Google Scholar]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kus, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P..; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Klumper, W.; Qaim, M. A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef]
- Doni, F.; Suhaimi, N.S.M.; Mispan, M.S.; Fathurrahman, F.; Marzuki, B.M.; Kusmoro, J.; Uphoff, N. Microbial contributions for rice production: From conventional crop management to the use of ‘omics’ technologies. Int. J. Mol. Sci. 2022, 23, 737. [Google Scholar] [CrossRef]
- Berini, F.; Casciello, C.; Marcone, G.L.; Marinelli, F. Metagenomics: Novel enzymes from non-culturable microbes. FEMS Microbiol. Lett. 2017, 364, fnx211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Chen, H.Y.; Xin, Z.; Cai, X.Z. Induction of hypersensitive response and nonhost resistance by a Cladosporium fulvum elicitor CfHNNI1 is dose-dependent and negatively regulated by salicylic acid. J. Integr. Agric. 2012, 11, 1665–1674. [Google Scholar] [CrossRef]
- Hjort, K.; Presti, I.; Elväng, A.; Marinelli, F.; Sjooling, S. Bacterial chitinase with phytopathogen control capacity from suppressive soil revealed by functional metagenomics. Appl. Microbiol. Biotechnol. 2014, 98, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Prigigallo, M.I.; Ahmed, A.O.; Cacciola, R.; Simona, M.; Sanzani, L.; Schena, L. Metabarcoding analysis of Phytophthora diversity using genus-specific primers and 454 pyrosequencing. Phytopathology 2016, 106, 305–313. [Google Scholar] [CrossRef]
- Cobo-Díaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. A novel metabarcoding approach to investigate Fusarium species composition in soil and plant samples. FEMS Microbiol. Ecol. 2019, 95, fiz084. [Google Scholar] [CrossRef]
- Kemp, N.D.; Vaughan, M.M.; McCormick, S.P.; Brown, J.A.; Bakker, M.G. Sarocladium zeae is a systemic endophyte of wheat and an effective biocontrol agent against Fusarium head blight. Biol. Cont. 2020, 149, 104329. [Google Scholar] [CrossRef]
- Jensen, B.D.; Knorr, K.; Nicolaisen, M. In vitro competition between Fusarium graminearum and Epicoccum nigrum on media and wheat grains. Eur. J. Plant Pathol. 2016, 146, 657–670. [Google Scholar] [CrossRef]
- Del-Frari, G.; Cabral, A.; Nascimento, T.; Boavida-Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef]
- Zaidi, S.S.E.A.; Mansoor, S.; Paterson, A. The rise of cotton genomics. Trends Plant Sci. 2018, 23, 953–955. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef]
- Tyagi, S.; Kumar, R.; Kumar, V.; Won, S.Y.; Shukla, P. Engineering disease resistant plants through CRISPR-Cas9 technology. GM Crops Food 2021, 12, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, R.; Vaslin, M.F. The viral threat in cotton: How new and emerging technologies accelerate virus identification and virus resistance breeding. Front. Plant Sci. 2022, 13, 851939. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, J.K.; Yildiz, A.; Doudna, J.A. Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 2017, 550, 407–410. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Corfas, R.A.; Matthews, B.J.; Ritchie, S.A.; Vosshall, L.B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction on humans. Cell 2014, 156, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Smidler, A.L.; Terenzi, O.; Soichot, J.; Levashina, E.A.; Marois, E. Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS ONE 2013, 8, e74511. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Shin, J.H.; Gumilang, A.; Chung, K.; Choi, K.Y.; Kim, K.S. Effectiveness of different classes of fungicides on Botrytis cinerea causing gray mold on fruit and vegetables. Plant Pathol. J. 2016, 32, 570–574. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-Stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Bachman, P.M.; Bolognesi, R.; Moar, W.J.; Mueller, G.M.; Paradise, M.S.; Ramaseshadri, P. Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against western corn rootworm (Diabrotica virgifera LeConte). Transg. Res. 2013, 22, 1207–1222. [Google Scholar] [CrossRef]
- Jasrotia, P.; Nagpal, M.; Mishra, C.N.; Sharma, A.K.; Kumar, S.; Kamble, U.; Bhardwaj, A.K.; Kashyap, P.L.; Kumar, S.; Singh, G.P. Nanomaterials for postharvest management of insect pests: Current state and future perspectives. Front. Nanotechnol. 2022, 3, 811056. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.; Barba, F.J.; Putnik, P.; Kovacevic, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Janczak, K..; Kosmalska, D.; Kaczor, D.; Raszkowska-Kaczor, A.; Wedderburn, L.; Malinowski, R. Bactericidal and fungistatic properties of LDPE modified with a biocide containing metal nanoparticles. Materials 2021, 14, 4228. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Murad, M.; Masood, M.U.; Bilal, S.; Khan, M.N.; Farooq, Q.; Iqbal, M.O.; Shakir, M.; Naeem, M. Nano-biopesticides as an emerging technology for pest management. In Insecticides; IntechOpen: York, UK, 2021. [Google Scholar]
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on emulsion liquid membrane (ELM) for the treatment of various industrial effluent streams. Rev. Environ. Sci. Bio./Technol. 2019, 18, 153–182. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollution. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef] [PubMed]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium Confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Behera, A. Nanomaterials. In Advanced Materials; Springer: Cham, Switzerland, 2022; pp. 77–125. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques. Boca Raton; CRC Press: London, UK, 2004. [Google Scholar]
- Vilasau, J.; Solans, C.; Gómez, M.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Phase behaviour of a mixed ionic/ nonionic surfactant system used to prepare stable oil-in-water paraffin emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 473–481. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Baba, W.N.; Ahmad, M.; Rahmanian, N.; Jafari, S.M. Nanoencapsulation of agrochemicals, fertilizers, and pesticides for improved plant production. In Advances in Phytonanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 279–298. [Google Scholar]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Patir, K. Nanoemulsion and Its Application in Pesticide Formulation. In Handbook of Research on Nanoemulsion Applications in Agriculture, Food, Health, and Biomedical Sciences; IGI Global: Hershey, PA, USA, 2022; pp. 401–424. [Google Scholar]
- Manzocco, L.; Mikkonen, K.S.; García-González, C.A. Aerogels as porous structures for food applications: Smart ingredients and novel packaging materials. Food Struct. 2021, 28, 100188. [Google Scholar]
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as nanofertilizers and nanopesticides: An overview. ChemistrySelect 2021, 6, 8645–8663. [Google Scholar] [CrossRef]
- Nassar, A.M.K. Pesticide alternatives use in Egypt: The concept and potential. In Sustainability of Agricultural Environment in Egypt: Part II; Springer: Cham, Switzerland, 2018; pp. 111–143. [Google Scholar]
- Anandhi, S.; Saminathan, V.R.; Yasotha, P.; Saravanan, P.T.; Rajanbabu, V. Nano-pesticides in pest management. J. Entomol. Zool. Stud. 2020, 8, 685–690. [Google Scholar]
- Assalin, M.R.; de Souza, D.R.C.; Rosa, M.A.; Duarte, R.R.M.; Castanha, R.F.; Vilela, E.S.D.; Tasic, L.; Durán, N. Thiamethoxam used as nanopesticide for the effective management of Diaphorina citri psyllid: An environmental-friendly formulation. Int. J. Pest. Manag. 2022, 24, 1–9. [Google Scholar] [CrossRef]

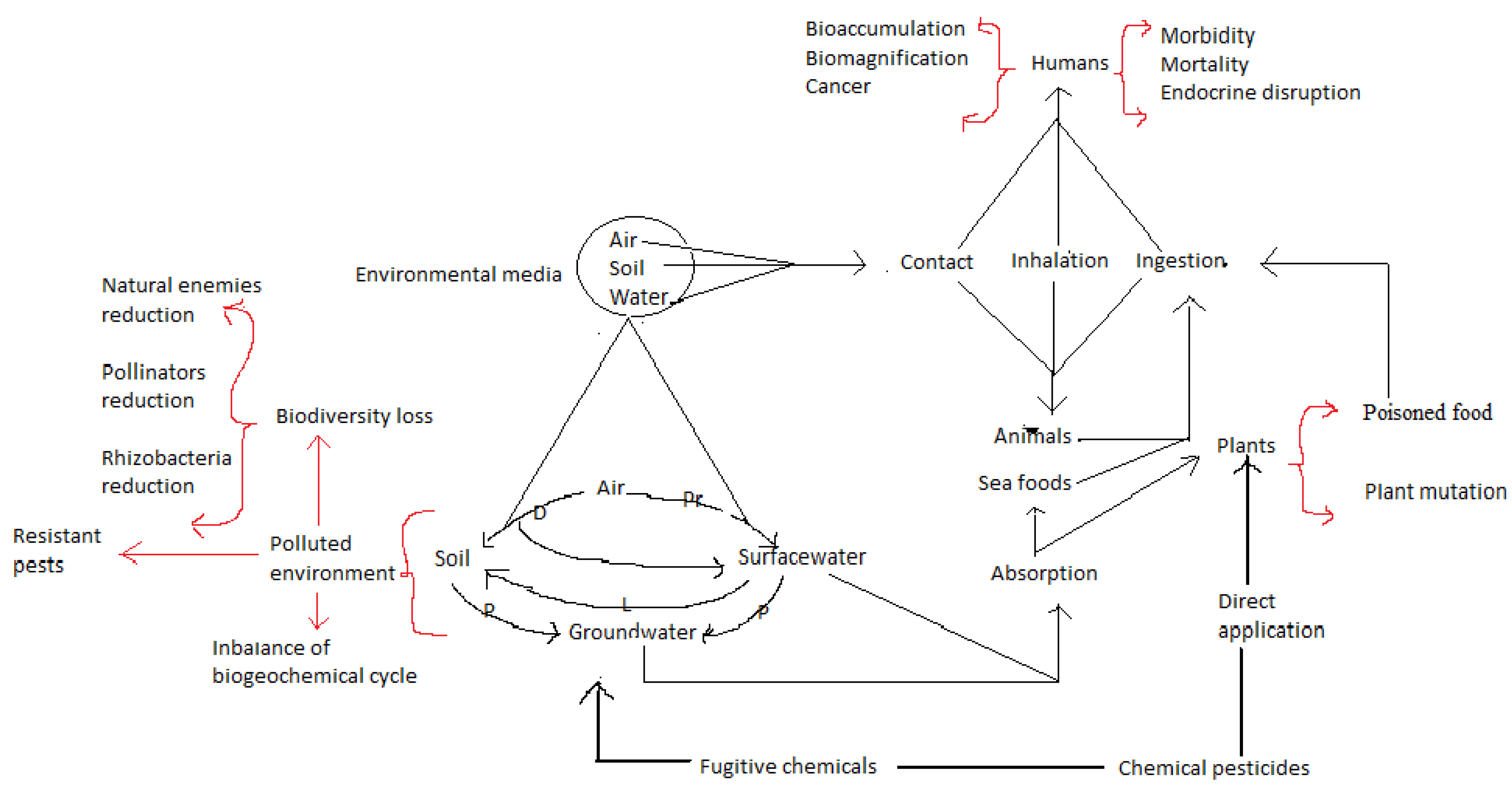

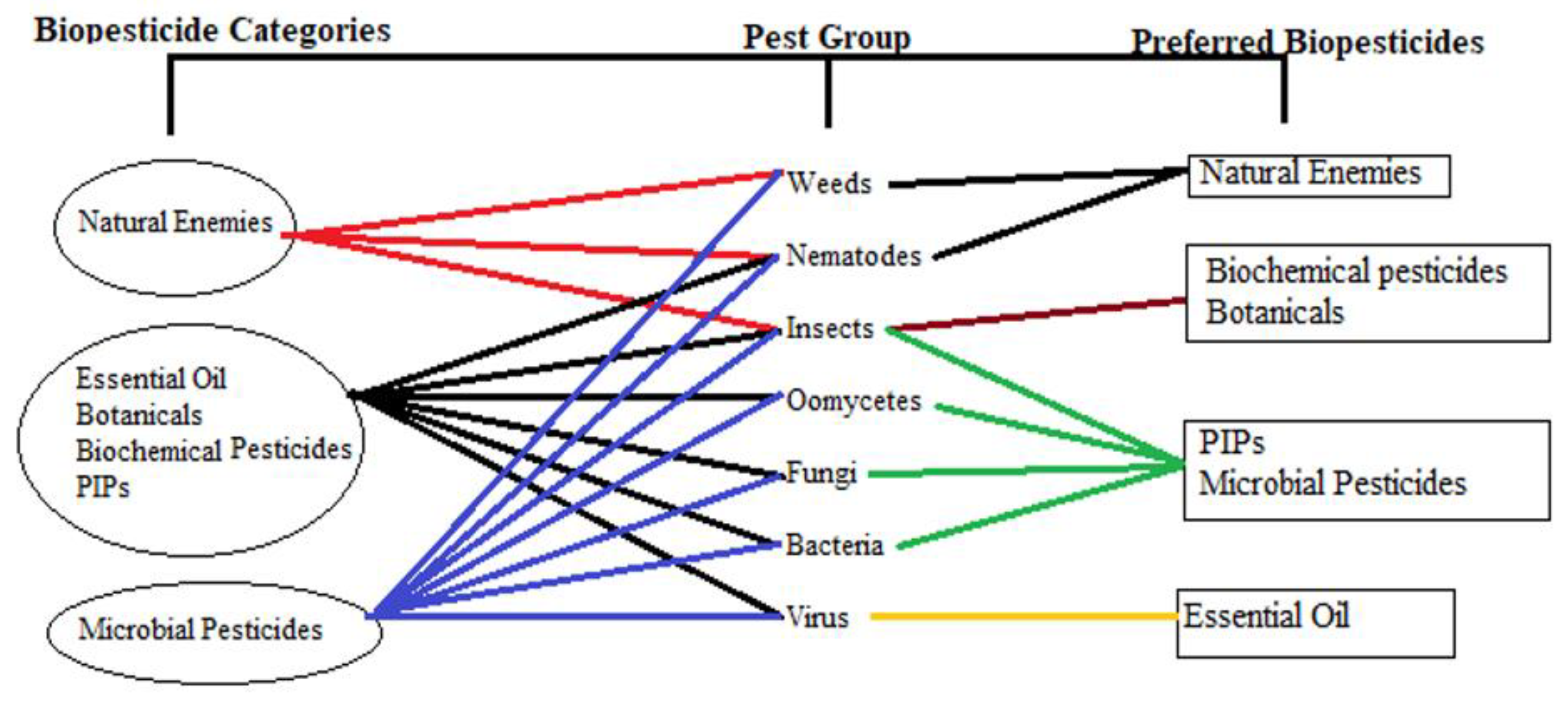
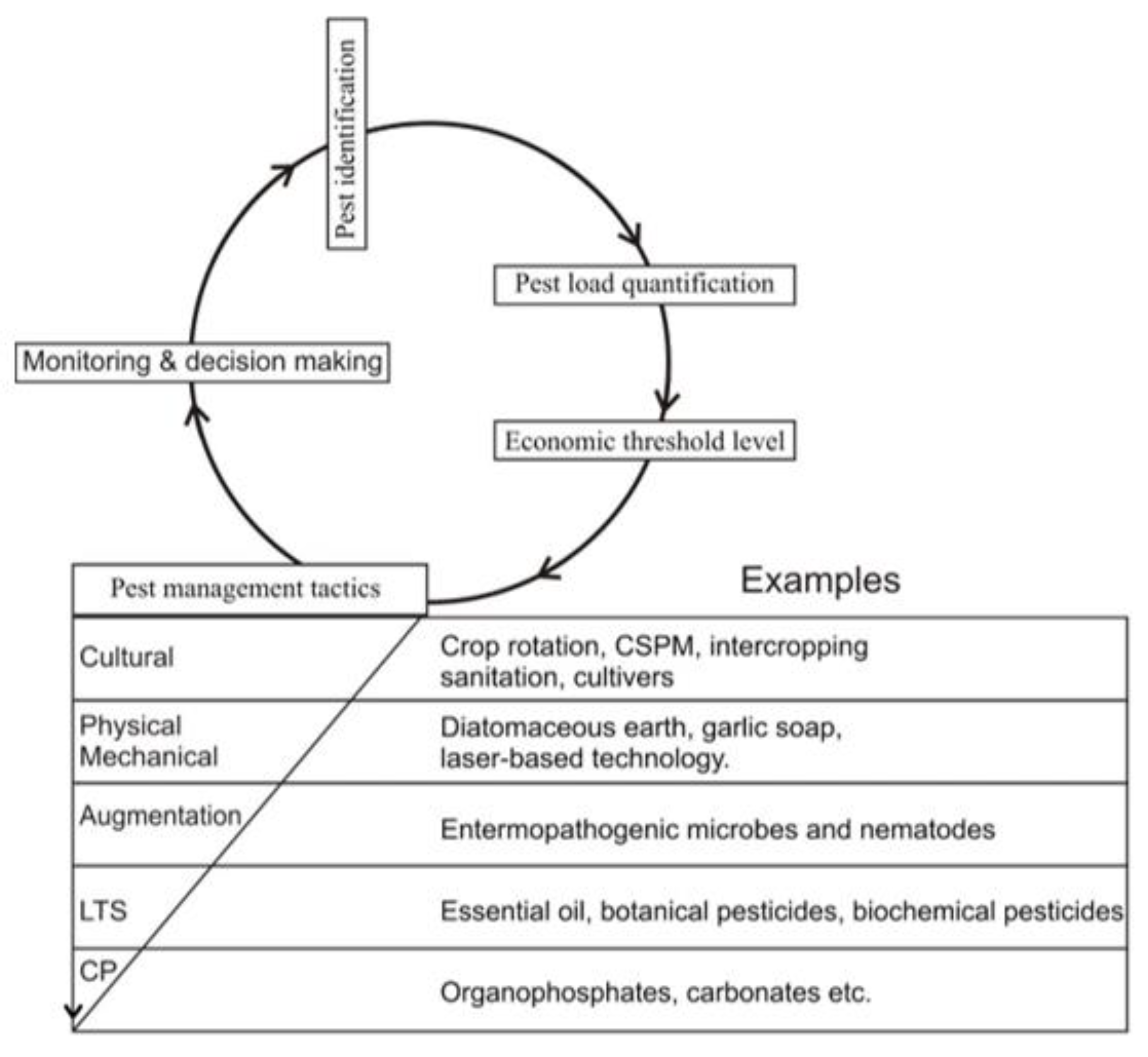
| Pests | Hosts | Impact | Reference |
|---|---|---|---|
| Weeds: Annual: Ambrosia artemisiifolia, Abutilon theophrasti, Chenopodium album (Baconweed), Amaranthus spp. (Pigweeds), Digitaria spp. Biannual: Ailanthus latissimus (Tree-of-heaven), Cirsium vulgare (Bull thistle), Perennial: Convolvulus arvensis, Rubus spp. (Blackberries), Smilax spp. (Greenbrier), Phytolacca Americana (Pokeweed), Toxicodendron radicans (Poison Ivy). | Varieties of crops including grains, wheat, rice, maize, beans, chickpeas, potatoes, vegetables, and cotton. |
| [24,25,26,27] |
| Nematodes: Heterodera spp. and Globodera spp., (Plant-parasitic nematodes (PPN), Meloidogyne spp. (root-knot nematodes), Pratylenchus spp., Heterodera and Globodera spp. (cyst nematodes), Bursaphelenchus xylophilus (pine wilt nematode), Aphelenchoides besseyi, Radopholus similis (burrowing nematodes), Xiphinema index (virus vector nematode), Ditylenchus dipsaci, Nacobbus aberrans, Rotylenchulus reniformis (reniform nematode). | Varieties of crops including peaches, nectarines, tomato, pepper, cucumber, almonds, squash, eggplant, okra, sugarcane, beetroot, and pineapple. |
| [28,29,30] |
| Insects Aphids, Mexican fruit flies (Anastrepha ludens), grasshoppers, whiteflies, spider mites, silkworms, desert locust (Schistocerca gregaria), migratory locust (Locusta migratoria), screwworm fly (Cochliomyia), tsetse flies (Glossina), uzi fly (Exorista bombycis), potato beetle, Banana- spotting bug (Amblypelta lutescens), European corn borer (Pyrausta nubilalis), Japanese beetle (Popillia japonica), alfalfa weevil (Hypera postica), alfalfa aphid (Therioaphis maculata). | Varieties of crops including sugar beets and potatoes, maize, peanuts, chickpeas, and cotton. |
| [31,32,33] |
| Small vertebrates: Field mice, house mice, rats, feral cats, bats, foxes, wild dogs, pigs, rabbits, snakes, dogs, pigeons. | Varieties of crops including potatoes, grains, sugar beets, citrus and succulent fruits, peaches, plums, pears, strawberries, grapes, potatoes, and carrots. |
| [34,35,36] |
| Fungi Pythium and Phytophthora infestans (Fungal-like organisms), Fusarium spp., Fusarium graminearum, Fusarium oxysporum, Rhizoctonia solani, Tilletia spp., Plasmopara viticola, Puccinia graminis var. tritici, Gaeumannomyces graminis var. tritici, Blumeria graminis, Mycosphaerella graminicola, Botrytis cinerea, Ascosphaera spp., Ustilago maydis, Aspergillus spp., Magnaporthe oryzae, Puccinia spp., Colletotrichum spp., Sclerotinia sclerotiorum, Verticillium dahlia, Armillaria spp., Melampsora lini, Phakopsora pachyrhizi Blumeria graminis. | Variety of crops including grains, rice, wheat, sorghum, potatoes, cassava, tomatoes, bananas, cucumber, grapes, strawberries, coffee, cacao, spices, mangos, and several nuts. |
| [37,38,39] |
| Protozoa: Phytomonas leptovasorum, Phytomonas stahelii, Phytomonas françai, Phytomonas serpens | Variety of crops including, coffee beans, Coconut palm, oil palms, cassava, tomatoes. |
| [40,41] |
| Bacteria: Xanthomonas campestris, Psuedomonas syringae, Pseudomonas corrugate, Clavibacter michiganensis, Pseudomonas spp., Erwinia spp., Ralstonia solanacearum, Rhizomonas suberifaciens, Erwinia carotovora, Agrobacterium tumefaciens, Spiroplasma citri | Variety of crops including, lettuce, cucurbits, cucumber, pumpkin, melon, tomatoes, chilli, potatoes, eggplant, rice, beans. |
| [42,43] |
| Viruses: Cassava mosaic begomovirus, Citrus Tristeza closterovirus, Barley yellow dwarf luteovirus, Plum pox potyvirus, Potato leafroll polerovirus, Cacao swollen shoot badnavirus, Potato potexvirus X, Tobacco mosaic tobamovirus, Turnip crinkle carmovirus, Tomato spotted wilt virus, Cucumber green mottle mosaic virus, Pepino mosaic virus | Variety of crops including, cassava, citrus fruits, barley, cucumber, lettuce, tomatoes, peppers |
| [44,45,46] |
| Chemical Group | Trade Name | Pest Controlled | Plant Protected | Toxicity | Reference |
|---|---|---|---|---|---|
| Carbamates | |||||
| Aldicarb | Temix, Standak, Namex | Effective against: thrips, aphids, nematodes | Cotton, potatoes, soya beans | Acute, environmental | [60] |
| Fenobucarb | Wardam, Knock, BioStadt | Effective against: hoppers | Rice field, cotton | Irritant, environmental | [61] |
| Carbofuran | Furadan, Curaterr, Carbosip | Effective against: Mites, nematodes | Corn, soybeans, potatoes | Acute, environmental | [62] |
| Organochlorine | |||||
| Aldrin | Aldrec, Altox, Octalene | Effective against: Termite, weevil, hoppers | Corn, potatoes | Acute, health, environmental | [63] |
| Lindane | Agrocide, benexane, Isotox | Effective against: Beetles, ants, locust | Corn, rice, seeds | Irritant, acute, health, environmental | |
| DDT | Anofex, Cezarex, dicophane | Effective against: Armyworms, mites, soil larvae | Cowpea, cotton | Irritant, acute, health, environmental | [64] |
| Chlorothalonil | Bravo, Daconil, Nopcocide | Effective against: Mold, mildew, algae | Vegetables, trees, ornamental crops | Corrosive, irritant, acute, health, environmental | [65] |
| Organophosphates | |||||
| Chlorpyrifos | Brodan, Scout, Nufos | Effective against: Borers, hoppers, termites | Apples, soybeans, broccoli | Acute, environmental | [66] |
| Dichlorvos | Vapona, Diclogreen | Effective against: Beetles, aphids, larvae | Grains, mushrooms, citrus | Irritant, acute, environmental | [67] |
| Diazinon | Basudin, Gardentox Dazzel | Effective against: Mites, aphis, worms | Vegetables, nuts, fruit trees | Irritant, environmental | [68] |
| Triazines | |||||
| Atrazine | Aatrex, Fenamin, Prozine | Effective against: Weeds, | corn, sugarcane | Irritant, health, environmental | [69] |
| Simazine | Aquazine, Primatol, Simadex | Effective against: Weeds | Seedlings | Health, environmental | [70] |
| Pyrethroids | |||||
| Cypermethrin | Ammo, Basathrin, Arrivo | Spiders, scorpions, bugs | Lettuce, cotton, cowpea | Irritant, environmental | [71] |
| Fenvalerate | Sumicidin, Devifen, Pydrin | Mites, tobacco budworms | Cotton lettuce | Irritant, acute, environmental | [72] |
| Deltamethrin | Decis, Kordon, Sadethrin | Millipedes, fleas, silverfish | Strawberries, ornamental gardens | Acute, environmental | [73] |
| Phenoxy-derivatives | |||||
| 2,4-D | Hi-Dep, Weedar 64, Weed RHAP | Dandelions, clover, and chickweed | Variety of plants | Corrosive, irritant, environmental | [74] |
| 2,4,5-T | Dacamine, Inverton 245, Forron | Broadleaf weeds | Monocotyledonous plants | Irritant, environmental | [75] |
| Carbofuran | Furadan, Curaterr, Carbosip | Mites, nematodes | Corn, soybeans, potatoes | Acute, environmental | [76] |
| Dipyridyl-derivatives | |||||
| Paraquat | Gramoxone, Helmquat, Firestorm | Weeds | Seedlings | Corrosive, irritant, acute, health, environmental | [77] |
| Glycine derivatives | |||||
| Glufosinate | Basta, Rely, Ignite | Weeds | Seedlings | Irritant, health | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenibo, E.O.; Ijoma, G.N.; Nurmahomed, W.; Matambo, T. The Potential and Green Chemistry Attributes of Biopesticides for Sustainable Agriculture. Sustainability 2022, 14, 14417. https://doi.org/10.3390/su142114417
Fenibo EO, Ijoma GN, Nurmahomed W, Matambo T. The Potential and Green Chemistry Attributes of Biopesticides for Sustainable Agriculture. Sustainability. 2022; 14(21):14417. https://doi.org/10.3390/su142114417
Chicago/Turabian StyleFenibo, Emmanuel O., Grace N. Ijoma, Weiz Nurmahomed, and Tonderayi Matambo. 2022. "The Potential and Green Chemistry Attributes of Biopesticides for Sustainable Agriculture" Sustainability 14, no. 21: 14417. https://doi.org/10.3390/su142114417
APA StyleFenibo, E. O., Ijoma, G. N., Nurmahomed, W., & Matambo, T. (2022). The Potential and Green Chemistry Attributes of Biopesticides for Sustainable Agriculture. Sustainability, 14(21), 14417. https://doi.org/10.3390/su142114417








