Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Suspension Preparation
2.2. Determination of Rheological Properties
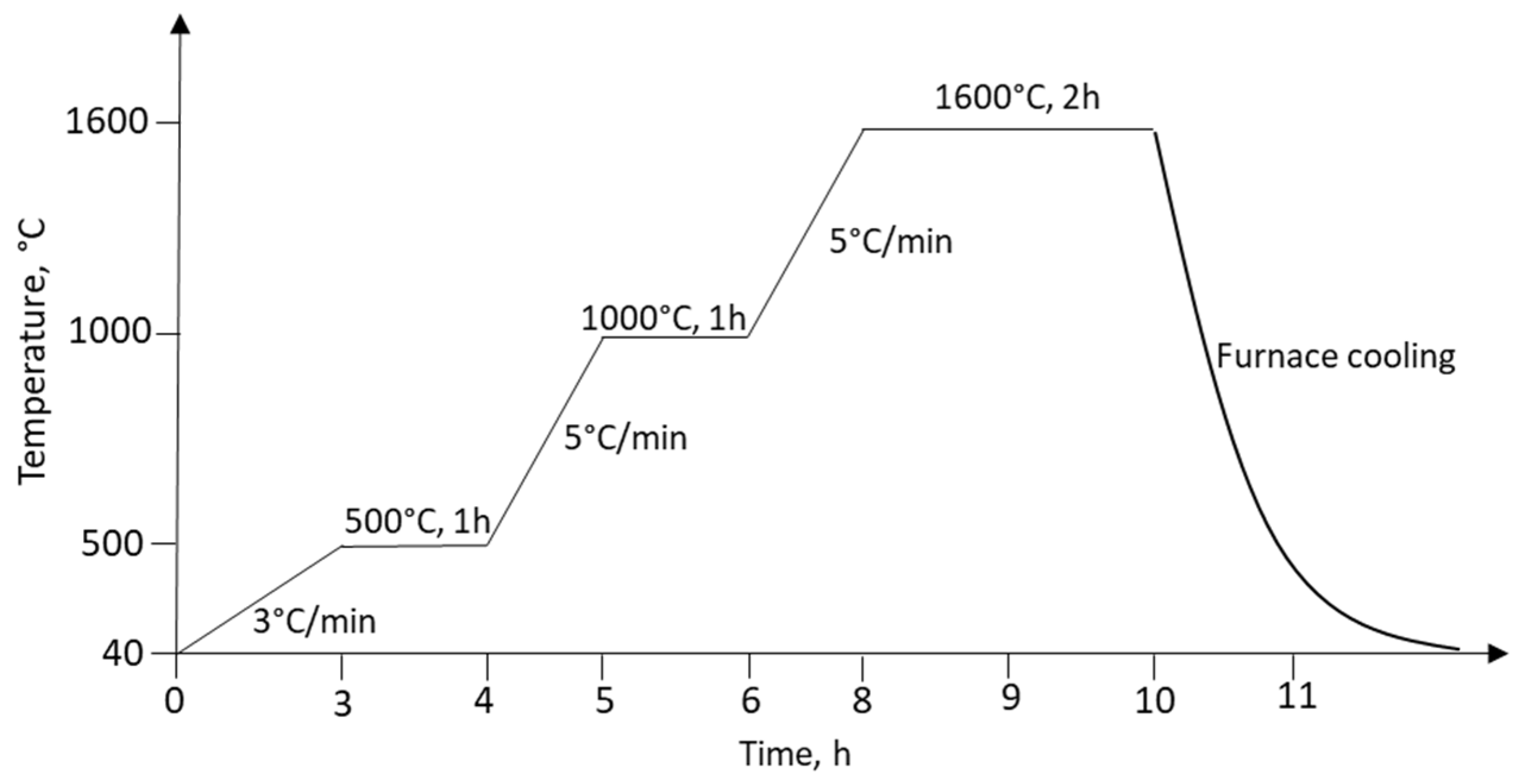
2.3. Sintering of Green Bodies
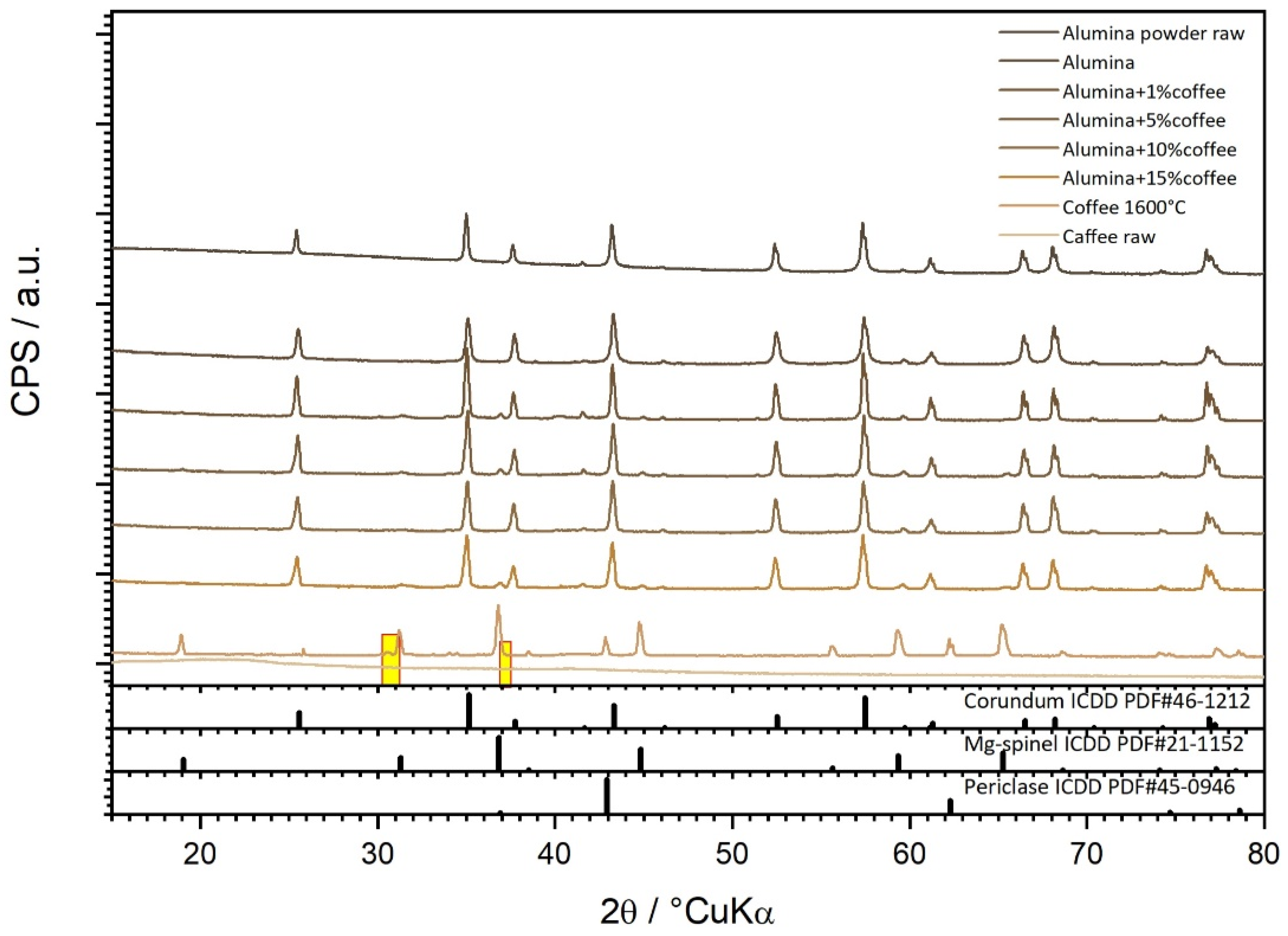
2.4. Characterization of Sintered Alumina Ceramics
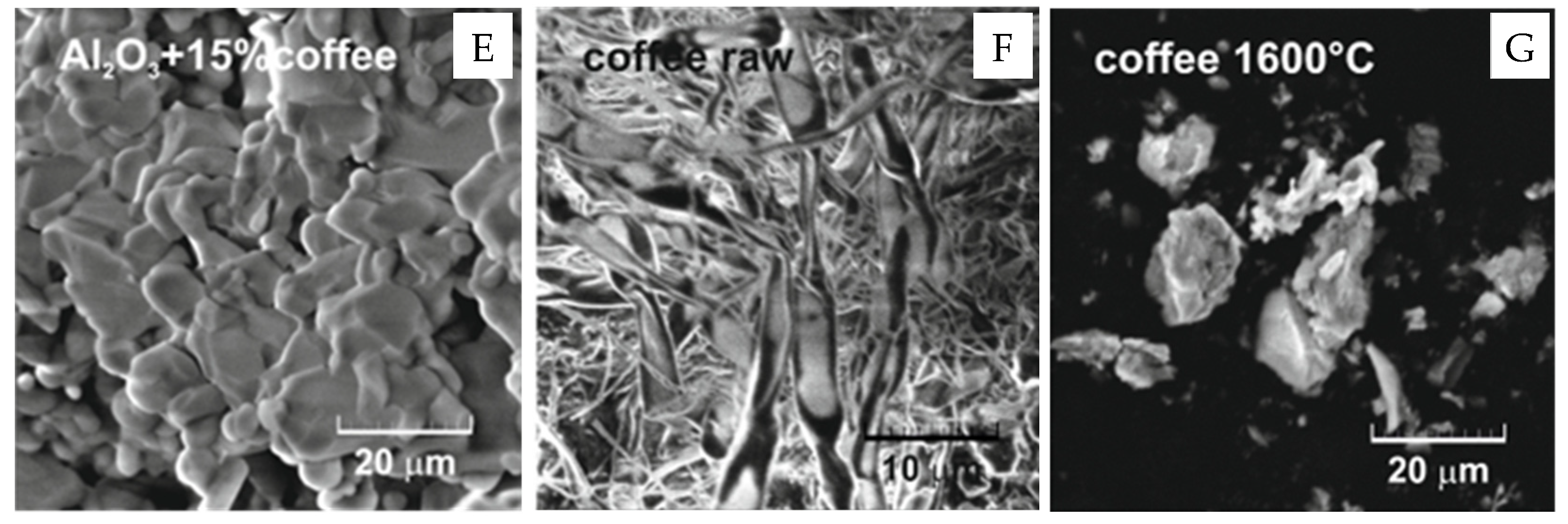
3. Results and Discussion
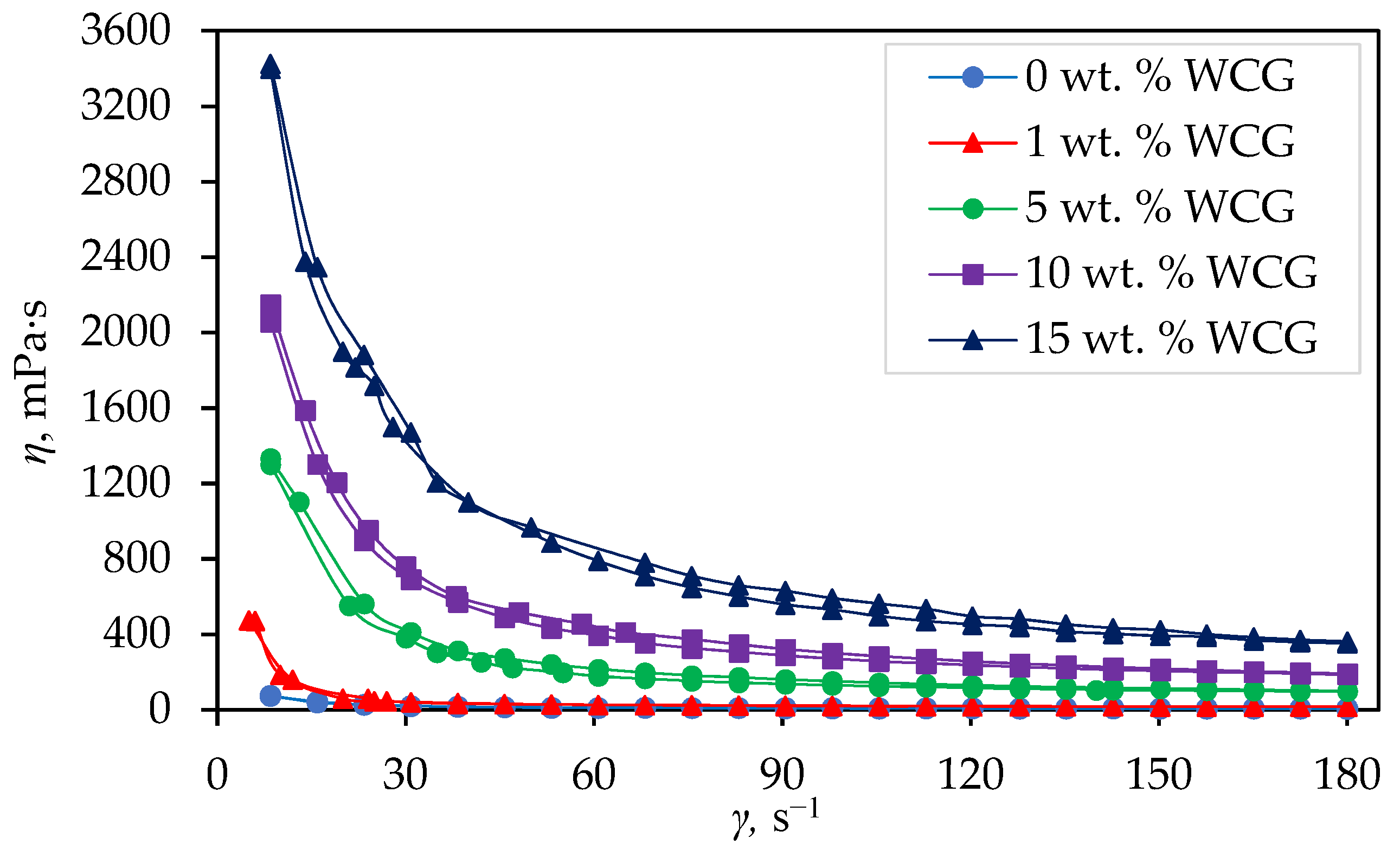
3.1. Rheological Measurements
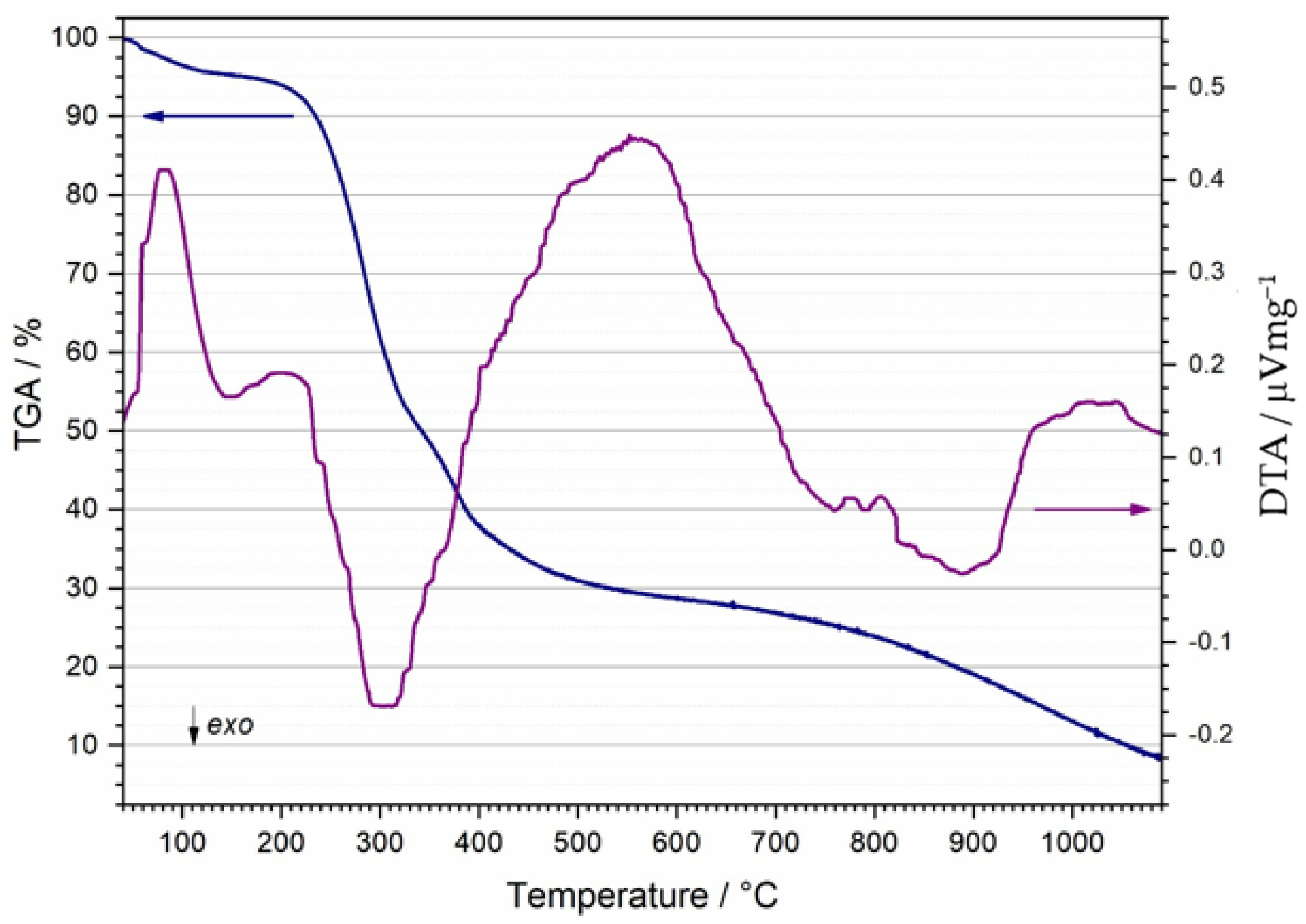
3.2. Differential Thermal Analysis (DTA) and Thermo-Gravimetric Analysis (TGA)
4. Conclusions
- All suspensions show a decrease in apparent viscosity with an increase in shear rate, which means that the suspensions belong to the group of non-Newtonian fluids, that is, the suspensions show pseudoplastic behavior.
- From the obtained diagrams of the dependence of apparent viscosity (η, mPa s) on shear rate (γ, s−1), it is evident that samples with 0 wt. % waste coffee grounds at a shear rate of 50 s−1 have viscosity values of 11.8 mPa s, while samples with 15 wt. % of waste coffee grounds at a shear rate of 50 s−1 have values of around 967.6 mPa s, from which it can be concluded that increasing the waste coffee ground amount also increases the apparent viscosity.
- DTA/TGA analyses of waste coffee grounds revealed multistep endothermic and exothermic events related to the evaporation and release of the moisture, burning of the organic material, the subsequent release of the decomposition residuals, and carbonaceous residuals burnouts accompanied by mass loss. According to DTA/TGA measurements, a suitable course of thermal treatment for sintering was implemented.
- It was found that increasing the waste coffee ground amount increases the porosity of sintered Al2O3 ceramics.
- The crystallite size of alumina grains grows upon the addition of 1 wt. % of WCG.
- By increasing the waste coffee ground amount, the density and relative density of Al2O3 ceramics decrease.
- The porosity of the Al2O3 ceramic samples increased significantly with the waste coffee ground amount, and it is assumed that these pores are formed by the combustion of waste coffee grounds during sintering.
- The shrinkage of the obtained alumina ceramic samples is barely affected by the amount of the waste coffee grounds.
- The mechanical and tribological properties of alumina ceramics with addition different amounts of WCG will be investigated in future research work.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, K.K.O.S.; Paskocimas, C.A.; Oliveira, F.R.; Nascimento, J.H.O.; Zille, A. Development of porous alumina membranes for treatment of textile effluent. Desalin. Water Treat. 2016, 57, 2640–2648. [Google Scholar] [CrossRef]
- Švagelj, Z.; Mandić, V.; Ćurković, L.; Biošić, M.; Žmak, I.; Gaboardi, M. Titania-Coated alumina foam photocatalyst for memantine degradation derived by replica method and sol-gel reaction. Materials 2020, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J. Investigating the effect of SPRM on mechanical strength and thermal conductivity of highly porous alumina ceramics. Ceram. Int. 2017, 43, 16430–16435. [Google Scholar] [CrossRef]
- Da Fonseca, B.S.; Vilão, A.; Galhano, C.; Simão, J.A.R. Reusing coffee waste in manufacture of ceramics for construction. Adv. Appl. Ceram. 2014, 113, 159–166. [Google Scholar] [CrossRef]
- Wei, Z.P.; Li, S.; Li, Y.; Li, X.S.; Xiang, R.; Xu, N.N. Porous alumina ceramics with enhanced mechanical and thermal insulation properties based on sol-treated rice husk. Ceram. Int. 2018, 44, 22616–22621. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Cui, H.; He, Q.; Zhang, Z.; Zhan, X. Biotemplated fabrication of porous alumina ceramics with controllable pore size using bioactive yeast as pore-forming agent. Ceram. Int. 2015, 41, 7042–7047. [Google Scholar] [CrossRef]
- Alzukaimi, J.; Jabrah, R. The preparation and characterization of porous alumina ceramics using an eco-friendly pore-forming agent. Int. J. Appl. Ceram. Technol. 2019, 16, 820–831. [Google Scholar] [CrossRef]
- Liu, J.; Huo, W.; Ren, B.; Gan, K.; Lu, Y.; Zhang, X.; Tang, X.; Yang, J. A novel approach to fabricate porous alumina ceramics with excellent properties via pore-forming agent combined with sol impregnation technique. Ceram. Int. 2018, 44, 16751–16757. [Google Scholar] [CrossRef]
- Ali, M.S.; Hanim, M.A.A.; Tahir, S.M.; Jaafar, C.N.A.; Norkhairunnisa, M.; Matori, K.A. Preparation and characterization of porous alumina ceramics using different pore agents. J. Ceram. Soc. Jpn. 2017, 125, 402–412. [Google Scholar] [CrossRef]
- Salman, M.M.; Radhi, N.S.; Sabr, O.H.; Nhabih, H.T. Utilization of diverse cheap materials as pore generating agent to manufacture low-cost porous ceramic. Ceramica 2020, 66, 179–185. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, X.Q.; Meng, G.Y. Porous α-Al2O3 Ceramics Prepared by Gelcasting. Mater. Res. Bull. 1997, 32, 1705–1712. [Google Scholar] [CrossRef]
- Ali, M.S.; Hanim, M.A.A.; Tahir, S.M.; Jaafar, C.N.A.; Norkhairunnisa, M.; Matori, K.A. The Effect of Commercial Rice Husk Ash Additives on the Porosity, Mechanical Properties, and Microstructure of Alumina Ceramics. Adv. Mater. Sci. Eng. 2017, 2017, 2586026. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Martínez-García, C.; Martínez-Cartas, M.L.M.; Cotes-Palomino, M.T.C.; Pérez-Villarejo, L.; Cruz-Pérez, N.; Corpas-Iglesias, F.A. The use of different forms of waste in the manufacture of ceramic bricks. Appl. Clay Sci. 2011, 52, 270–276. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Andreola, F.; Borghi, A.; Pedrazzi, S.; Allesina, G.; Tartarini, P.; Lancellotti, I.; Barbieri, L. Spent coffee grounds in the production of lightweight clay ceramic aggregates in view of urban and agricultural sustainable development. Materials 2019, 12, 3581. [Google Scholar] [CrossRef]
- Le Ferrand, H. Magnetic slip casting for dense and textured ceramics: A review of current achievements and issues. J. Eur. Ceram. Soc. 2021, 41, 24–37. [Google Scholar] [CrossRef]
- Torman, K.M.; Erdoğan, G.; Yavas, A.; Guler, S.; Sutcu, M. Fabrication of porous anorthite ceramics using eggshell waste as a calcium source and expanded polystyrene granules. J. Polytech. 2022, 25, 1235–1241. [Google Scholar]
- Collivignarelli, M.C.; Cillari, G.; Ricciardi, P.; Miino, M.C.; Torretta, V.; Rada, E.C.; Abbà, A. The Production of Sustainable Concrete with the Use of Alternative Aggregates: A Review. Sustainability 2020, 12, 7903. [Google Scholar] [CrossRef]
- Faridmehr, I.; Bedon, C.; Huseien, G.F.; Nikoo, M.; Baghban, M.H. Assessment of Mechanical Properties and Structural Morphology of Alkali-Activated Mortars with Industrial Waste Materials. Sustainability 2021, 13, 2062. [Google Scholar] [CrossRef]
- Khitab, A.; Riaz, M.S.; Jalil, A.; Khan, R.B.N.; Anwar, W.R.; Khan, A.; Arshad, M.T.; Kirgiz, M.S.; Tariq, Z.; Tayyab, S. Manufacturing of Clayey Bricks by Synergistic Use of Waste Brick and Ceramic Powders as Partial Replacement of Clay. Sustainability 2021, 13, 10214. [Google Scholar] [CrossRef]
- Hossain, S.; Roy, P.K. Sustainable ceramics derived from solid wastes: A review. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Alzukaimi, J.; Jabrah, R. Preparation and characterization of porous alumina ceramics using sunflower seed shells as fugitive material. Cerâmica 2020, 66, 208–220. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Cho, K.S.; Min, J.H.; Lee, H.K.; Kim, H.D. Reducing the density deviation in alumina by pressure-vacuum hybrid slip casting by employing powders with different particle sizes. J. Asian Ceram. Soc. 2020, 8, 407–415. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Gröss und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen. Nachr. Ges. Wiss. Göttingen Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Alonso-Santurde, R.; Coz, A.; Viguri, J.R.; Andre, A. Recycling of foundry by-products in the ceramic industry: Green and core sand in clay bricks. Constr. Build. Mater. 2012, 27, 97–106. [Google Scholar] [CrossRef]




| wt. % | |||||
|---|---|---|---|---|---|
| Al2O3 + WCG | H2O | Al2O3, in the Powder Mixture | WCG, in the Powder Mixture | DOLAPIX CE64 | PVA |
| 60 | 40 | 100 | 0 | 0.2 | 0.5 |
| 60 | 40 | 99 | 1 | 0.2 | 0.5 |
| 60 | 40 | 95 | 5 | 0.2 | 0.5 |
| 60 | 40 | 90 | 10 | 0.2 | 0.5 |
| 60 | 40 | 85 | 15 | 0.2 | 0.5 |
| wt. (WCG in Powder Mixture), % | η (mPa∙s) | |
|---|---|---|
| γ, 50 s−1 | γ, 100 s−1 | |
| 0 | 11.8 | 8.9 |
| 1 | 29.4 | 19.2 |
| 5 | 241.4 | 143.4 |
| 10 | 518.1 | 283.9 |
| 15 | 967.6 | 563.4 |
| wt. (Waste Coffee Grounds in Alumina), % | 0 | 1 | 5 | 10 | 15 |
| Crystallite Size, nm | 135 | 198 | 158 | 148 | 164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerolli Mustafa, M.; Gabelica, I.; Mandić, V.; Veseli, R.; Ćurković, L. Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics. Sustainability 2022, 14, 14244. https://doi.org/10.3390/su142114244
Kerolli Mustafa M, Gabelica I, Mandić V, Veseli R, Ćurković L. Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics. Sustainability. 2022; 14(21):14244. https://doi.org/10.3390/su142114244
Chicago/Turabian StyleKerolli Mustafa, Mihone, Ivana Gabelica, Vilko Mandić, Rea Veseli, and Lidija Ćurković. 2022. "Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics" Sustainability 14, no. 21: 14244. https://doi.org/10.3390/su142114244
APA StyleKerolli Mustafa, M., Gabelica, I., Mandić, V., Veseli, R., & Ćurković, L. (2022). Reusing Waste Coffee Grounds in the Preparation of Porous Alumina Ceramics. Sustainability, 14(21), 14244. https://doi.org/10.3390/su142114244








