Abstract
The unwanted occurrence of antibiotics in the environment is an emerging concern of non-target toxicity and antimicrobial resistance. Floating treatment wetland (FTW) is a low-cost and ecofriendly wastewater remediation strategy; however, the effect of immobilized bacteria on its efficacy during the remediation of ciprofloxacin (CIP)-contaminated water has not been documented. In this study, Phragmites australis was planted to develop FTW, and it was augmented with a bacterial consortium (Acinetobacter lwoffii ACRH76, Bacillus pumulis C2A1, and Acinetobacter sp. HN3), with and without immobilization for the remediation of CIP-contaminated (100 mg L−1) water. The augmentation of bacteria (immobilized or in suspension) in the FTWs significantly increased the elimination of CIP from the water. Maximum removal of CIP (97%), COD (92%), BOD (93%), and TOC (90%) from the water was observed in the FTWs having immobilized bacteria. This research revealed that the FTWs have tremendous potential to remove the CIP from the water and its removal efficiency can be enhanced via immobilized bacterial augmentation strategies.
1. Introduction
Antibiotics are among the most commonly used medications in healthcare systems, whose application is particularly increasing in the developing world [1]. However, the absence of treatment systems results in a vast fraction of antibiotics being directly released into natural streams. The River Ravi in Pakistan has already been declared as a highly polluted river due to pharmaceutical pollution [2]. The situation raises concerns related to antimicrobial resistance (AMR), and disappearance of sensitive but beneficial communities from the ecosystem [3,4]. The global statistics have shown that nearly 700,000 people died in 2016 as a result of AMR and ineffective medications, while the number is expected to rise up to 10 million by 2050 [5,6].
Ciprofloxacin (CIP, C17H18FN3O3), a fluoroquinolone antibiotic with fractions of piperazine, is extensively used to prevent gastrointestinal infections, urinary tract infections, and lung and skin infections for both humans and animals [7,8,9,10]. It has a broad-spectrum antibacterial activity that inhibits the synthesis of essential bacterial enzymes such as topoisomerase IV and DNA gyrase [11]. CIP accounts for 73% of the total consumption of second-generation fluoroquinolones [12,13]. With the rapid increase in the human population and farm animals, use of antibiotics has increased all over the world. This results in a significant increase in antibiotic load in surface waters [14,15]. CIP has been found in drinking water (0.032 µg L−1), lake water (6.5 mg L−1) [16], sewage (11–99 µg L−1) [17], hospital wastewater (150 µg L−1) [18], and pharmaceutical industry effluent (31–50 mg L−1) [19,20].
The unwanted occurrence of antibiotics in the environment can affect the biogeochemical cycling of the nutrients (carbon, nitrogen, sulfur, etc.) driven by indigenous microbial activities in a particular (micro)ecosystem. This is because microbial community structures are changed upon exposure to antibiotics. Precisely, in the presence of antimicrobials, susceptible or sensitive members of the microbial communities are eliminated/inhibited while opportunistic microbes take competitive advantage followed by an increase in abundance, cf. the concept of intrinsic resistance [4]. Some fluoroquinolones could also cause physiologically teratogenic and genotoxic effects in living organisms.
Various physicochemical and biological methods such as advanced oxidation, ozonation, reverse osmosis, membrane filtration, electrochemical, and biodegradation are known to remove antibiotics from contaminated water effectively [21,22,23,24]. However, most of these technologies are expensive and energy/chemical intensive in nature [25]. The use of FTWs, however, is a suitable, economic, and passive treatment alternative that could also remove antibiotics from contaminated water similar to other contaminants [25,26]. In this approach, plants and associated microorganisms work synergistically and eliminate organic pollutants from the contaminated water [25,27].
Plants absorb contaminants from the environment and store them in their tissues [28]. Plant-associated microorganisms are typically involved in the degradation of organic contaminants [29]. Among microorganisms, bacteria play a crucial role in the removal of organic chemicals including antibiotics from soil and water [30,31,32]. Floating treatment wetland (FTW) has recently become one of the most popular and cost-effective methods for removing contaminants from water [33,34,35]. Plants and their associated microbes are used to filter, remove, accumulate, metabolize, adsorb, absorb, and/or degrade organic compounds present in water [36]. In FTWs, plants are planted in a buoyant mat with their roots in water and shoots above the mat [37]. The roots and mat provide a wide surface area for bacterial growth and multiplication, resulting in biofilm formation on the roots and mat [37]. The majority of nutrients are absorbed and organic pollutants are degraded by the biofilm. Certain enzymes, such as cytochrome P450, are found in microorganisms and are involved in the intracellular biodegradation of ciprofloxacin [38].
Immobilized cells have been widely used in recent years for a variety of biotechnological applications, including antibiotic synthesis, biodegradation, and wastewater treatment [39,40,41,42]. The immobilized microbes have several benefits over traditional suspension systems such as high productivity, increased metabolic activity, and potent resistance to harmful substances [40,43]. However, the potential of FTWs augmented with immobilized bacteria for the remediation of CIP-contaminated water has not been documented previously.
The intention of the current investigation was to assess the capability of FTWs having bacteria immobilized on plant roots and shoots for the treatment of CIP-contaminated water. The concentration of CIP in Pakistani streams is found in the range of 42–332 μg mL−1 followed by ofloxacin > ampicillin > levofloxacin > sulfamethoxazole [44]. The River Ravi in Pakistan is already recognized as a highly polluted river due to pharmaceuticals [2]; nevertheless, no current treatment infrastructure exists in the country which signifies the importance of cost-effective and scalable treatment solutions in the country. Previously, it was argued that antimicrobials may negatively affect in planta bacterial communities in wetlands [45]; therefore, the scope of alternative approaches considering microbial colonization in fixed beds or via immobilization strategies should be investigated. We further used an indigenous wetland plant, Phragmites australis, which is a helophytic grass and known to withstand local harsh environmental conditions of Pakistan [46]. The species also allows adsorption of organics on to root surface, without being translocated to aboveground plant parts, allowing organics to be available for degradation by immobilized bacteria. Further, helophytic grasses can diffuse oxygen from the air into the rhizosphere which helps create a redox cone in the rhizosphere for efficient pollutant mineralization [45]. This study hypothesizes that bacterial immobilization on a floating raft could be a useful strategy to treat high-concentration pharmaceutical-contaminated wastewater.
2. Materials and Methods
2.1. Chemicals and Reagents
All regents and solvents were of analytical grade and were used without further purification. Ciprofloxacin (CIP) was purchased from a local pharmacy (Sami Pharmaceuticals, Private Limited, Karachi, Pakistan).
2.2. Macrophyte
In this study, Phragmites australis (common reed) was used to develop FTWs. This plant has shown potential to tolerate and grow in different types of wastewaters [37,46]. Due to its extensive root system, it promotes bacterial growth and pollutant degradation [34].
2.3. Bacterial Strains
A consortium consisting of three bacterial strains, A. lwoffiii [47], B. pumilus [48], and Mesorihizobium sp. [49], was used in the current study. The strain A. lwoffii was previously isolated from the rhizosphere of Acacia ampliceps, whereas B. pumulis and Mesorihizobium sp. were isolated from pesticide-contaminated soil. These strains and their consortium showed in vitro potential to degrade CIP (100 mg L−1) in a minimal salt medium (data not shown).
2.4. Immobilization of Bacterial Consortium on Floating Raft
Each bacterium was grown in Luria–Bertani (LB) broth at 35 °C for 24 h. The bacterial cells were centrifuged at 4 °C, and the pellets were resuspended in 0.9% NaCl solution [37]. According to the turbid metric approach [50], the bacterial consortium (109 colony forming units mL−1) was made by taking equal numbers (1:1:1) of each bacterium. A polystyrene sheet was cut into a round shape with a diameter of 20 cm and a thickness of 2.54 cm. Twenty small holes (1.5 cm diameter) were made in each sheet (Figure 1). The sheet was surface sterilized with a bleach (5%) solution and 70% ethanol. The sheet was put in 100 mL of inoculum overnight so that the consortium was immobilized uniformly on the sheet [40].

Figure 1.
Establishing FTW for the remediation of ciprofloxacin-contaminated water. Buoyant mat (A), biocarrier (B), vegetation of Phragmites australis in plastic container (C), placement of the container in the mat (D), fixing of the container in the mat (E), and placing planted mat in the tank (F).
2.5. Experimental Setup
The study was performed in April 2021 at NIBGE, Faisalabad, Pakistan under natural environmental conditions. A polystyrene sheet (10 cm thickness) was used as a floating mat, and polyethylene containers were utilized to establish eighteen FTW mesocosms. The sheet was cut into a round shape (23 cm diameter) with a hole (5 cm diameter) in the center. Healthy saplings of P. australis (2) were planted in each hole (Figure 1c–f). The seedlings were then placed in tap water to establish root networks. After two months, the containers and saplings were cleaned with a 5% solution of bleach. Thereafter, 20 L of CIP (100 mg L−1)-contaminated water was added in to each tank (Figure 2). The following treatments were used in the experiment.
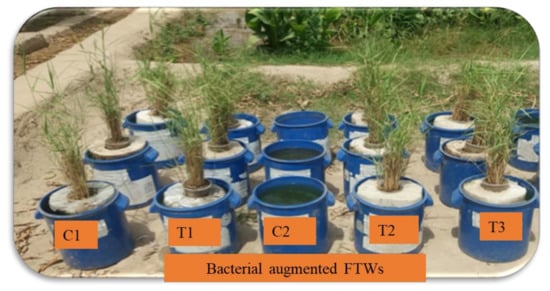
Figure 2.
Experimental setup of bacterially augmented FTWs for the remediation of ciprofloxacin-contaminated water. Only tap water with Phragmites australis (C1), only CIP-contaminated water (C2), CIP-contaminated water treated by FTWs planted with P. australis (T1), CIP-contaminated water treated by FTWs planted with P. australis and augmented with bacterial consortium (T2), and CIP-contaminated water treated by FTWs planted with P. australis and augmented with bacterial consortium, immobilized on polystyrene sheet.
Control: CIP-contaminated water having only mat without vegetation, control: Tap water (without CIP) having planted mat,
- T1: CIP-contaminated water having planted mat,
- T2: CIP-contaminated water (having mat without vegetation) with bacterial consortium,
- T3: CIP-contaminated water having the planted mat and the bacterial consortium (suspension),
- T4: CIP-contaminated water having the planted mat and the bacterial consortium (immobilized).
A sample of the water was taken every five days from each tank. The samples were kept cool until further analysis. The evapotranspiration losses were recovered by filling tanks with fresh water to a capacity of 20 L per tank. No rain was observed during the experimental period.
2.6. Water Quality Parameter Analysis
APHA standard methods were used measure water quality parameters such as pH (4500-H), EC (2510), turbidity (2130), chemical oxygen demand (5220), biochemical oxygen demand (5210B), and total organic carbon (5310) [51].
2.7. Determination of Ciprofloxacin
All reagents and stock solutions required were prepared in 0.02 M HCl and deionized water. Approximately 1 g of CIP was dissolved in 0.02 M HCl. The contents were then dissolved and swirled well. The stock solution (1000 ppm) was filtered and further dilutions were prepared. This solution was kept in the dark in an airtight container. Then, 8 g of ferric chloride was dissolved in 100 mL deionized water as a complexing agent. CIP tablets were ground to a fine powder using a pestle and mortar. Approximately 1 g of CIP was then dissolved in 0.02 M HCl.
CIP has two functional groups, namely, an ionizable carboxylic group (pka: 6.05) and a piperanyzyl group (pka: 8.22). The drug shows absorption maxima in the visible region at 530 nm. The fluorine atom present in the quinolone group acts as an electron withdrawing group, while the benzene ring in the CIP drug has lower electron density than the terminal nitrogen atom in the piperazinyl moiety. Therefore, CIP serves as an n-electron donor to form complexes with metals. This finding supports that the interaction of the CIP drug and reagent takes place at only one site which is the N-atom of the piperazinyl ring considering the steric and electron donating factors shown by [52]. Under standard conditions, CIP reacts with ferric chloride to produce a red-colored complex. This reaction is specific for fluoroquinolone hydrochlorides, and is used for spectrophotometric determination of fluoroquinolones. Briefly, a 3 mL aliquot of CIP containing 0.5 to 9.0 μg/mL was added to 3.0 mL of ferric chloride solution. A red-colored complex was instantly formed which was further diluted with distilled water (10 mL). The complex was then kept at room temperature (25 ± 0.5 °C) for 0.5 h to check its stability. Finally, the absorbance was recorded at 530 nm.
2.8. Bacterial Persistence
During the experiment, the persistence of the inoculated bacteria in water, root, and shoot was evaluated using the cultivation-dependent plate count method [37]. Briefly, the CIP-contaminated water sample was directly spread on LB agar plates. The plant’s roots and shoots were cleaned with distilled water for 2 min before being washed with 70% ethanol for 10 and 5 min, respectively. Thereafter, the tissues were soaked in a 2% solution of sodium hypochlorite for about 1 min. Lastly, sterile distilled water was used to wash the surface sterilized roots and shoots three times. The suspension was prepared by using a pestle and mortar to grind 5 g of the roots and shoots in normal saline (0.9%, w/v). The suspension was then plated on LB agar medium having 50 mg L−1 CIP. All of the Petri plates were put in the incubator for 48 h (37 °C) for CFU enumeration. Restriction fragment length polymorphism (RFLP) was measured to compare the isolates to the inoculated strains [46].
2.9. Plant Biomass
To evaluate the toxicity of CIP and the impact of inoculated bacteria on plant growth, the length of the roots/shoots and plant’s dry biomass were recorded at the end of the experiment. A measuring scale was used to manually measure the root and shoot lengths, and the roots and shoots were oven-dried for 72 h at 80 °C for dried biomass estimation.
2.10. Toxicity Analysis
The toxicity of raw and treated wastewater was studied in vitro using wheat (Triticum aestivum L.) seeds [53]. Briefly, five seeds were put in a dish with filter paper saturated with distilled water, or treated and untreated CIP-contaminated water. Seeds were allowed to germinate at room temperature (25 °C) in darkness. The test was conducted in triplicate. After five days, germinated seeds were viewed. Seeds were believed to have germinated when the combined length of the plumule and radicle was more than 2 mm. Plantlet growth (root length and total length) was measured.
2.11. Data Analysis
The SPSS software package was used to examine the results of physicochemical parameters such as pH, EC, COD, BOD, TOC, and CIP concentrations, bacterial persistence, and plant biomass. An analysis of variance was used to evaluate the treatments, followed by a post hoc Tukey test (p ≤ 0.05).
3. Results and Discussion
3.1. Performance Evaluation
Performance of established FTWs with free and immobilized bacteria was evaluated by analyzing physicochemical parameters of the raw and treated water. All the treatments having FTWs significantly reduced the pH, EC, turbidity, COD, BOD, TOC, and CIP concentrations of the water (Figure 3 and Figure 4). The treatments having both vegetation and bacterial inoculation (T3 and T4) exhibited a significantly greater reduction in the level of all the tested water quality parameters than the treatments having only vegetation (T1), bacterial inoculation (T2), and non-planted mat (C). This may be linked to the fact that these bacteria had previously been isolated from the roots and shoots of plants planted in contaminated soil, and may have evolved mechanisms for the degradation of organic pollutants, including CIP, and also supported the host plant’s health in a symbiotic way. Moreover, the bacteria having pollutant degradation and plant growth-enhancing activities enhance the efficiency of wetlands to clean water [25,53]. More CIP removal was observed in the treatments comprising immobilized cells than the treatments having free bacteria. This could be due to the fact that immobilized bacterial cells could efficiently proliferate on the polystyrene sheet as compared to the suspension form, and ultimately exhibited more degradation (from 100 to 10 mg L−1) of CIP. It is well established that bacteria on fixed beds perform better than bacteria in suspension [54]. Similar studies were carried out earlier in which immobilized bacteria exhibited more efficient degradation of organic contaminants in water compared to free cells [55,56]. Similarly, the treatment (T4) having immobilized bacteria exhibited maximum reduction in EC (from 3.50 to 1.00 mS cm−1), pH (from 7.8 to 5.9), turbidity (from 45 to 6.2 FAU), COD (from 220 to 30 mg L−1), and BOD (from 95.2 to 18.8 mg L−1).
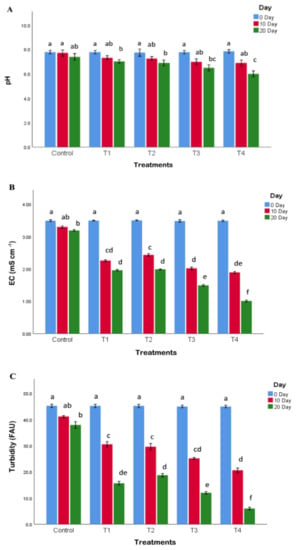
Figure 3.
Effect on pH (A), electrical conductivity (EC) (B), and turbidity (C). Only CIP-contaminated water (control), CIP-contaminated water treated by FTWs planted with P. australis (T1), CIP-contaminated water having only bacterial consortium (T2), CIP-contaminated water treated by FTWs planted with P. australis and consortium (T3), and CIP-contaminated water treated by FTWs planted with P. australis, and augmented with bacterial consortium immobilized on polystyrene sheet (T4). Different letters indicate significant differences tested at p ≤ 0.05.
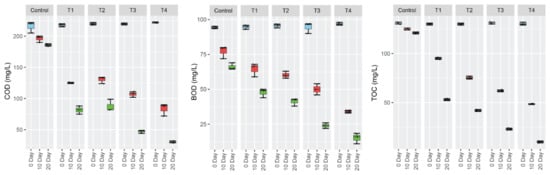
Figure 4.
Remediation of ciprofloxacin (CIP)-contaminated water treated by floating treatment wetlands. Effect on chemical oxygen demand (COD) (D), biochemical oxygen demand (BOD) (E), and total organic carbon (TOC) (F). Only CIP-contaminated water (control), CIP-contaminated water treated by FTWs planted with P. australis (T1), CIP-contaminated water having only bacterial consortium (T2), CIP-contaminated water treated by FTWs planted with P. australis and bacterial consortium (T3), and CIP-contaminated water treated by FTWs planted with P. australis and augmented with bacterial consortium immobilized on polystyrene sheet (T4). Statistical significance is tested at p ≤ 0.05.
The pH may be lowered as a result of the degradation of organics and/or discharge of organic acids by plant roots [53]. Plant nutrient intake and chemical and biological nutrients adhering to roots and soil particles could all be contributing to the reduction in EC [37]. In all the treatments, a similar pattern of turbidity removal was found. This shows that plants and bacteria play an important role in decreasing pH, EC, and turbidity of the water. Similar results have been documented earlier [45,53]. Moreover, plant roots act as adsorbents and biosorbents, and aid in the removal of suspended and dissolved particles from water [25].
The decrease in BOD, COD, and TOC could be attributed to bacterial enzymatic activity that degrades CIP and converts it into simpler substances, which are later consumed as nutrients [57]. The reduction in COD and TOC indicates that there was efficient degradation of CIP in the water. Furthermore, in such an environment, a high oxygen concentration is crucial, and the availability of oxygen is responsible for effective interactions between the plant’s roots and their associated bacteria. Helophytic plants diffuse oxygen in the rhizosphere in a well-growing FTW system, allowing microorganisms to proliferate and eventually lead to the breakdown of pollutants [53,57]. Plant–microbe interactions increased oxidation and reduction processes, which are critical for the elimination and breakdown of a wide range of pollutants [58].
3.2. Ciprofloxacin Removal
The reduction in CIP concentration in the water exposed to all the treatments was significantly greater as compared to the control (Figure 5). The elimination of CIP was quick in the first five days followed by a slow reduction up to 20 days. The maximum (97%) removal of CIP from the water was seen in the treatment inoculated with the bacteria immobilized on polystyrene and it was significantly greater than other treatments. However, the minimum removal (48%) of CIP from the water was recorded for the treatment (T1) planted without bacterial consortium; this was followed (58%) by the treatment (T2) inoculated with bacterial consortium only. The plant-associated bacteria degrade the organic chemicals in the very close vicinity of roots [59]. The biodegradation of CIP could have occurred by the cleavage of isoxazole/piperazinyl rings by sulfite reductase and CYP450 enzyme systems, respectively [38]. Earlier, it was shown that 100% removal of CIP may be achieved by effective microbial degradation [60]. The accumulation of CIP in the root and shoot of the plant was not observed in this investigation, but antibiotics have been reported to be transported to the stems and leaves of plants [61,62,63].
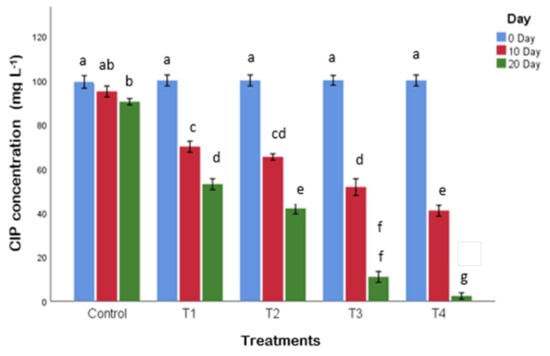
Figure 5.
Remediation of ciprofloxacin (CIP)-contaminated water treated with FTWs. Only CIP-contaminated water (control), CIP-contaminated water treated by FTWs planted with P. australis (T1), CIP-contaminated water having only bacterial consortium (T2), CIP-contaminated water treated with FTWs planted with P. australis and bacterial consortium (T3), and CIP-contaminated water treated by FTWs planted with P. australis and augmented with bacterial consortium immobilized on polystyrene sheet (T4). Different letters indicate significant differences tested at p ≤ 0.05.
3.3. Inoculated Bacteria in FTWs
Plant–bacteria synergism is important in the breakdown of organic pollutants in phytoremediation. The population of pollutant-degrading bacteria in various plant compartments has been found to be closely related to the plant’s ability to remediate water [25,57]. The current study examined the persistence/existence of the inoculated bacteria in the water, roots, and shoots of the plant. The water from the unplanted treatment (T2) contained fewer inoculated bacteria than the water from the planted treatments (T3 and T4) (Table 1). This could be due to a lack of symbiotic relationship between the bacteria and the plant. Similarly, the plant provides microbes with nutrition, oxygen, and a haven, allowing them to thrive, multiply, and reproduce [25].

Table 1.
Total bacterial population (CFU mL−1) in the ciprofloxacin-contaminated water treated by floating treatment wetlands (FTWs) planted with Phragmites australis.
Survival and colonization of the augmented bacteria are critical for successful pollution degradation in the FTWs [46]. The bacteria survived in the root and shoot interiors of the plant in our investigation. The bacterial population was likewise much higher in the root interior of P. australis than in the shoot interior. Previous research has also shown that augmented bacteria colonized the root more than the shoot, suggesting that the root environment has a higher colonization potential than that of the shoot [47]. In several previous investigations, the number of inoculated bacteria in the root interior was shown to be higher than in the shoot interior [47,64]. Among the treatments, the maximum bacterial population was found in water, shoot, and root of the FTWs (T4) inoculated with the bacterial consortium immobilized on polystyrene sheets (Table 2).

Table 2.
Population of the inoculated bacteria in the roots and shoots of Phragmites australis.
3.4. Plant Growth
Toxic pollutants in water have long been known to limit plant growth [46,65]. The root and shoot lengths, as well as root and shoot biomass of the plant (Table 3), were measured in this investigation. It was seen that the augmented plants (T2 and T3) grew faster than the plants without augmentation. This is because of the plant–bacteria symbiotic relationship, in which bacteria can encourage plant growth by lowering the abiotic stress in contaminated water, such as the presence of CIP in water [66]. Bacteria also promote plant growth by producing phytohormones and allowing essential nutrients to be bioavailable [67,68]. Previous studies demonstrated that the inoculated bacteria increased plant development by lowering pollutant-induced toxicity [53].

Table 3.
Effect of ciprofloxacin and bacterial inoculation on the growth of Phragmites australis.
3.5. Phytotoxicity Analysis
Seed germination toxicity assay was conducted at the end of the study to assess the quality of treated water. The length of roots was taken as an indicator for toxicity analysis, an approach consistent with the previous studies performed to assess the toxicity of organic contaminants [69]. The relative data of root length (RL) and total length (TL) of plantlets are presented in Table 4. The plantlets produced from seeds exposed to water treated by FTWs showed greater RL and TL than the seeds exposed to the water without treatment. It might be due to the reduction in the concentration of CIP in the water by the CIP-degrading bacteria. However, the plantlets produced from the seeds irrigated with the water treated by FTWs having a bacterial consortium immobilized on a polystyrene sheet showed the maximum RL (24 mm) and TL (40 mm). The seeds exposed to untreated water developed minor radicles and no plumule. This is due to the toxic effects of CIP-contaminated water [70,71]. In an earlier study, authors [32] evaluated five major veterinary antibiotics, namely tetracycline, sulfamethazine, norfloxacin, erythromycin, and chloramphenicol, for their phytotoxic effects on the germination of seeds of lettuce, tomato, carrot, and cucumber. According to their results, these antibiotics significantly reduced root elongation, which is the most sensitive endpoint for phytotoxicity studies. They also found that TC was the most toxic, and lettuce was the most susceptible to the tested antibiotics.

Table 4.
Effect of water treated by floating treatment wetlands on the growth of plantlets of wheat.
3.6. Practical Applications
This study confirms that attached growth systems can help achieve maximum remediation in a short time period. This is mainly due to low shear force and better biofilm formation on the fixed bed which is generally followed by effective catabolic activities by the inoculated microbial communities. Authors [54] recently argued that remediation efficiency can be significantly enhanced in the presence of attached growth systems as compared to the suspended growth systems. In addition, attached growth systems support high biomass formation, retaining extracellular polymeric substances, with a rich microbial community to drive pollutant transformation effectively. Compared to the suspended biological treatment processes, immobilized and fixed bed treatment systems also require less aeration without any need for additional organic carbon. The high biomass can further enhance flocculation and sedimentation of suspended particles in highly turbid wastewater often present in field-based systems. The overall approach appears to be scalable, sustainable, and cost-effective as observed in our earlier field-scale floating wetlands studies [64].
4. Conclusions
The application of immobilized bacteria onto polystyrene sheets significantly increased CIP remediation (97%) via FTW. To this end, helophytic grasses such as Phragmites australis could be a suitable option due to its metabolic abilities to support microbial proliferation and enhance organic mineralization in the root zone (German: Wurzelraumverfahren). The bacterial strains A. lwoffii ACRH76, B. pumulis C2A1, and Acinetobacter sp. HN3 were able to develop successful partnership with the plant, even in the presence of CIP, indicating abilities to survive antibiotic stress. FTW supported by immobilized bacteria offers sustainable and scalable solutions to treat wastewater contaminated with antibiotics. Owing to near-natural means of remediation and comparably low energy requirements, the technology is particularly attractive for countries with economic constraints such as Pakistan. Further studies are, however, encouraged to explore the metabolic activity of the bacteria during the degradation of CIP.
Author Contributions
Conceptualization, M.A. (Muhammad Afzal) and E.I.; methodology, S.W.A.S., A.H. and M.u.R.; software, S.W.A.S., M.T.; validation, S.I., M.A. (Muhammad Arslan), S.I., E.I. and M.A. (Muhammad Afzal); formal analysis, S.W.A.S.; investigation, S.W.A.S.; resources, E.I. and M.A. (Muhammad Afzal); data curation, M.T., S.I. and M.A. (Muhammad Arslan); writing—S.W.A.S.; writing—review and editing, M.T., M.A. (Muhammad Arslan), M.A. (Muhammad Afzal); visualization, S.W.A.S., M.T.; supervision, E.I., S.I. and M.A. (Muhammad Afzal); project administration, M.A. (Muhammad Afzal); funding acquisition, M.A. (Muhammad Afzal). All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to the Higher Education Commission (HEC) for the grant no. TTSF-77.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Worku, F.; Tewahido, D. Retrospective assessment of antibiotics prescribing at public primary healthcare facilities in Addis Ababa, Ethiopia. Interdiscip. Perspect. Infect. Dis. 2018, 2018, 4323769. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.; Kolpin, D.W.; Leung, K.M.; Lai, R.W.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Abimbola, S.O.; Otieno, M.A.; Cole, J. Reducing the use of antimicrobials as a solution to the challenge of antimicrobial resistance (AMR): Approaching an ethical dilemma through the lens of plan-etary health. Challenges 2021, 12, 23. [Google Scholar] [CrossRef]
- Arslan, M.; El-Din, M.G. Bacterial diversity in petroleum coke based biofilters treating oil sands process water. Sci. Total Environ. 2021, 782, 146742. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Ismail, N. Ciprofloxacin removal from non-clinical environment: A critical review of current methods and future trend prospects. J. Water Process Eng. 2022, 47, 102725. [Google Scholar] [CrossRef]
- Brar, R.K.; Jyoti, U.; Patil, R.K.; Patil, C.H. Fluoroquinolone antibiotics: An overview. Adesh Univ. J. Med. Sci. Res. 2020, 2, 26–30. [Google Scholar] [CrossRef]
- Yuan, X.-L.; Wu, X.-Y.; He, M.; Lai, J.-P.; Sun, H. A Ratiometric Fiber Optic Sensor Based on CdTe QDs Functionalized with Glutathione and Mercaptopropionic Acid for On-Site Monitoring of Antibiotic Ciprofloxacin in Aquaculture Water. Nanomaterials 2022, 12, 829. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Truong, Q.-M.; Chen, C.-W.; Chen, W.-H.; Dong, C.-D. Pyrolysis of marine algae for biochar production for adsorption of Ciprofloxacin from aqueous solutions. Bioresour. Technol. 2022, 351, 127043. [Google Scholar] [CrossRef]
- Aziz, H.A.; El-Saghier, A.M.; Badr, M.; Abuo-Rahma, G.E.D.A.; Shoman, M.E. Thiazolidine-2, 4-dione-linked ciprofloxacin derivatives with broad-spectrum antibacterial, MRSA and topoisomerase inhibitory activities. Mol. Divers. 2022, 26, 1743–1759. [Google Scholar] [CrossRef] [PubMed]
- Kergaravat, S.V.; Hernandez, S.R.; Gagneten, A.M. Second-, third-and fourth-generation quinolones: Ecotoxicity effects on Daphnia and Ceriodaphnia species. Chemosphere 2021, 262, 127823. [Google Scholar] [CrossRef] [PubMed]
- Shehu Imam, S.; Adnan, R.; Kaus, N.H.M. Photocatalytic degradation of ciprofloxacin in aqueous media: A short review. Toxicol. Environ. Chem. 2018, 100, 518–539. [Google Scholar] [CrossRef]
- Falyouna, O.; Maamoun, I.; Bensaida, K.; Tahara, A.; Sugihara, Y.; Eljamal, O. Chemical deposition of iron nanoparticles (Fe0) on titanium nanowires for efficient adsorption of ciprofloxacin from water. Water Pract. Technol. 2022, 17, 75–83. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, X.; Zheng, X.; Feng, X.; Yu, A. Adsorption and fenton-like degradation of ciprofloxacin using corncob biochar-based magnetic iron–copper bimetallic nanomaterial in aqueous solutions. Nanomaterials 2022, 12, 579. [Google Scholar] [CrossRef]
- Antonelli, R.; Malpass, G.R.P.; da Silva, M.G.C.; Vieira, M.G.A. Fixed-bed adsorption of ciprof-loxacin onto bentonite clay: Characterization, mathematical modeling, and DFT-based calculations. Ind. Eng. Chem. Res. 2021, 60, 4030–4040. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Zubair, M.; Shaida, M.A.; Manzar, M.S.; Abutaleb, A.; Naushad, M.; Iqbal, J. Sustainable green nanoadsorbents for remediation of pharmaceuticals from water and wastewater: A critical review. Environ. Res. 2022, 204, 112243. [Google Scholar] [CrossRef]
- Bizi, M.; El Bachra, F.E. Evaluation of the ciprofloxacin adsorption capacity of common industri-al minerals and application to tap water treatment. Powder Technol. 2020, 362, 323–333. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photo-catalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Wajahat, R.; Yasar, A.; Khan, A.M.; Tabinda, A.B.; Bhatti, S.G. Ozonation and photo-driven oxidation of ciprofloxacin in pharmaceutical wastewater: Degradation kinetics and energy require-ments. Pol. J. Environ. Stud. 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Del Álamo, A.C.; Pariente, M.; Martínez, F.; Molina, R. Trametes versicolor immobilized on rotating biological contactors as alternative biological treatment for the removal of emerging concern micropollutants. Water Res. 2020, 170, 115313. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, M.; Feng, Z.; Qin, Y.; Wang, Y.; Tan, L.; Sun, T. Ultra-high adsorption of tetracycline antibiotics on garlic skin-derived porous biomass carbon with high surface area. New J. Chem. 2020, 44, 1097–1106. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Liu, C.; Chang, B.V. Biodegradation of amoxicillin, tetracyclines and sulfonamides in wastewater sludge. Water 2020, 12, 2147. [Google Scholar] [CrossRef]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Benvenuti, T.; Hamerski, F.; Giacobbo, A.; Bernardes, A.M.; Zoppas-Ferreira, J.; Rodrigues, M.A. Constructed floating wetland for the treatment of domestic sewage: A real-scale study. J. Environ. Chem. Eng. 2018, 6, 5706–5711. [Google Scholar] [CrossRef]
- Russo, N.; Pino, A.; Toscano, A.; Cirelli, G.L.; Caggia, C.; Arioli, S.; Randazzo, C.L. Occurrence, diversity, and persistence of antibiotic resistant enterococci in full-scale constructed wetlands treating urban wastewater in Sicily. Bioresour. Technol. 2019, 274, 468–478. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. Phytoremediation for the elimination of metals, pesticides, PAHs, and other pollutants from wastewater and soil. In Phytobiont and Ecosystem Restitution; Springer: Singapore, 2018; pp. 101–136. [Google Scholar]
- Franchi, E.; Fusini, D. Plant Growth-Promoting Rhizobacteria (PGPR) Assisted Phytoremediation of Inorganic and Organic Contaminants Including Amelioration of Perturbed Marginal Soils. In Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 477–500. [Google Scholar]
- Feng, N.-X.; Yu, J.; Xiang, L.; Yu, L.-Y.; Zhao, H.-M.; Mo, C.-H.; Li, Y.-W.; Cai, Q.-Y.; Wong, M.-H.; Li, Q.X. Co-metabolic degradation of the antibiotic ciprofloxacin by the enriched bacterial consortium XG and its bacterial community composition. Sci. Total Environ. 2019, 665, 41–51. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nghiem, L.D.; Oh, S. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge. Chemosphere 2018, 211, 600–607. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Li, C.; Yu, G.; Wang, Y. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge. J. Hazard. Mater. 2018, 343, 59–67. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Colares, G.S.; Lutterbeck, C.A.; Dell’Osbel, N.; Machado, Ê.L.; Rodrigues, L.R. Floating treatment wetlands in domestic wastewater treatment as a decentralized sanitation alternative. Sci. Total Environ. 2021, 773, 145609. [Google Scholar] [CrossRef] [PubMed]
- Benrahmane, L.; Mouhir, L.; Kabbour, A.; Laaouan, M.; El Hafidi, M. Effectiveness of floating treatment wetlands with Cyperus papyrus used in sub-humid climate to treat urban wastewater: A case study. J. Ecol. Eng. 2022, 23, 157–168. [Google Scholar] [CrossRef]
- Sánchez-Galván, G.; Olguín, E.J.; Melo, F.J.; Jiménez-Moreno, D.; Hernández, V.J. Pontederia sagittata and Cyperus papyrus contribution to carbon storage in floating treatment wetlands estab-lished in subtropical urban ponds. Sci. Total Environ. 2022, 832, 154990. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Andreotti, F.; Barros, L.; Almeida, T.; Mucha, A.P. Response of microbial communities colonizing salt marsh plants rhizosphere to copper oxide nanoparticles contamination and its implications for phytoremediation processes. Sci. Total Environ. 2017, 581, 801–810. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean–Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Jia, Y.; Khanal, S.K.; Shu, H.; Zhang, H.; Chen, G.-H.; Lu, H. Ciprofloxacin degradation in anaerobic sulfate-reducing bacteria (SRB) sludge system: Mechanism and pathways. Water Res. 2018, 136, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hou, D.; Jiang, D.; Chen, W. Bioremediation of marine oil spills by immobilized oil-degrading bacteria and nutrition emulsion. Biodegradation 2021, 32, 165–177. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Guo, S.; Liu, X.; Tang, J. Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 2022, 286, 131663. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for bio-transformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Zafar, R.; Bashir, S.; Nabi, D.; Arshad, M. Occurrence and quantification of prevalent antibiotics in wastewater samples from Rawalpindi and Islamabad, Pakistan. Sci. Total Environ. 2021, 764, 142596. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Arslan, M.; Rehman, K.; Tahseen, R.; Afzal, M. Phragmites australis—A helophytic grass—Can establish successful partnership with phenol-degrading bacteria in a floating treatment wetland. Saudi J. Biol. Sci. 2019, 26, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Fahid, M.; Arslan, M.; Shabir, G.; Younus, S.; Yasmeen, T.; Rizwan, M.; Siddique, K.; Ahmad, S.R.; Tahseen, R.; Iqbal, S.; et al. Phragmites australis in combination with hydrocarbons degrading bacteria is a suitable option for remediation of diesel-contaminated water in floating wetlands. Chemosphere 2020, 240, 124890. [Google Scholar] [CrossRef]
- Fatima, K.; Afzal, M.; Imran, A.; Khan, Q.M. Bacterial rhizosphere and en-dosphere populations associated with grasses and trees to be used for phytoremediation of crude oil contaminated soil. Bull. Environ. Contam. Toxicol. 2015, 94, 314–320. [Google Scholar] [CrossRef]
- Anwar, S.; Liaquat, F.; Khan, Q.M.; Khalid, Z.M.; Iqbal, S. Biodegradation of chlorpyrifos and its hydrolysis product 3, 5, 6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J. Hazard. Mater. 2009, 168, 400–405. [Google Scholar] [CrossRef]
- Jabeen, H.; Iqbal, S.; Anwar, S. Biodegradation of chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol by a novel rhizobial strain M esorhizobium sp. HN3. Water Environ. J. 2015, 29, 151–160. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Alexandria, VA, USA, 2012. [Google Scholar]
- Akram, M.; Anwar, J.; Alshemarya, A.Z.; Goh, Y.-F.; Awan, A.S.; Farooqi, Q.H. Quantitative determination of ciprofloxacin and levofloxacin antibacterials by Spec-trophotometeric and high-performance liquid chromatography. Malays. J. Fundam. Appl. Sci. 2015, 11, 329. [Google Scholar]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Christova, N.; Kabaivanova, L.; Nacheva, L.; Petrov, P.; Stoineva, I. Bio-degradation of crude oil hydrocarbons by a newly isolated biosurfactant producing strain. Biotechnol. Biotechnol. Equip. 2019, 33, 863–872. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Treatment of the textile industry effluent in a pilot-scale vertical flow con-structed wetland system augmented with bacterial endophytes. Sci. Total Environ. 2018, 645, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Skrzypiec, K.; Gajewska, M.H. The use of constructed wetlands for the treatment of industrial wastewater. J. Water Land Dev. 2017, 34, 233–240. [Google Scholar] [CrossRef]
- Sauvêtre, A.; May, R.; Harpaintner, R.; Poschenrieder, C.; Schröder, P. Metabolism of carbamazepine in plant roots and endophytic rhizobacteria isolated from Phragmites australis. J. Hazard. Mater. 2018, 342, 85–95. [Google Scholar] [CrossRef]
- Sauvêtre, A.; W, A.; Y, L.; Vestergaard, G.; Miksch, K.; Schröder, P.; Radl, V. Enrichment of endophytic Actinobacteria in roots and rhizomes of Miscanthus× giganteus plants exposed to diclofenac and sulfamethoxazole. Environ. Sci. Pollut. Res. 2020, 27, 11892–11904. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Zeng, J.; Li, J.; Liu, Y.; Sun, X.; Xu, L.; Li, L. Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain. Nanomaterials 2021, 11, 1660. [Google Scholar] [CrossRef]
- Guo, X.; Wang, P.; Li, Y.; Zhong, H.; Li, P.; Zhang, C.; Zhao, T. Effect of copper on the removal of tetracycline from water by Myriophyllum aquaticum: Performance and mechanisms. Bioresour. Technol. 2019, 291, 121916. [Google Scholar] [CrossRef]
- Yan, Y.; Pengmao, Y.; Xu, X.; Zhang, L.; Wang, G.; Jin, Q.; Chen, L. Migration of antibiotic ciprofloxacin during phytoremediation of contaminated water and identification of transformation products. Aquat. Toxicol. 2020, 219, 105374. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Yasin, M.; Tauseef, M.; Zafar, Z.; Rahman, M.; Islam, E.; Iqbal, S.; Afzal, M. Plant-Microbe synergism in floating treatment wetlands for the enhanced removal of Sodium Dodecyl Sulphate from water. Sustainability 2021, 13, 2883. [Google Scholar] [CrossRef]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef]
- Phenrat, T.; Teeratitayangkul, P.; Prasertsung, I.; Parichatprecha, R.; Jitsangiam, P.; Chomchalow, N.; Wichai, S. Vetiver plantlets in aerated system de-grade phenol in illegally dumped industrial wastewater by phytochemical and rhizomicrobial degra-dation. Environ. Sci. Pollut. Res. 2017, 24, 13235–13246. [Google Scholar] [CrossRef] [PubMed]
- Afridi, S.M.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; Sultan, T. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Yadav, S.; Chandra, R. Detection and assessment of the phytotoxicity of residual organic pollutants in sediment contaminated with pulp and paper mill effluent. Environ. Monit. Assess. 2018, 190, 581. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).