Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Experimental Procedure
2.3. Characterization of Samples
3. Results and Discussion
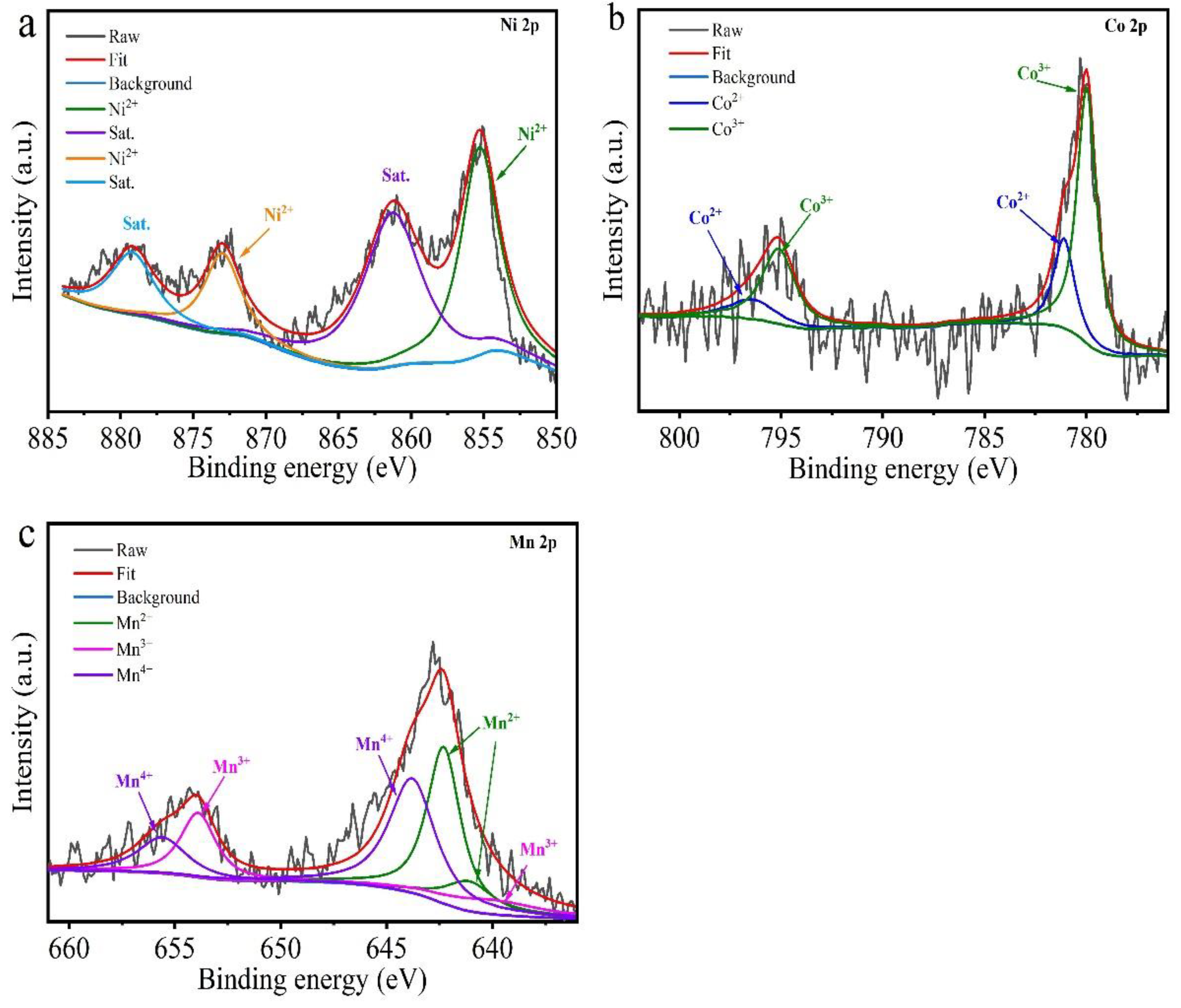
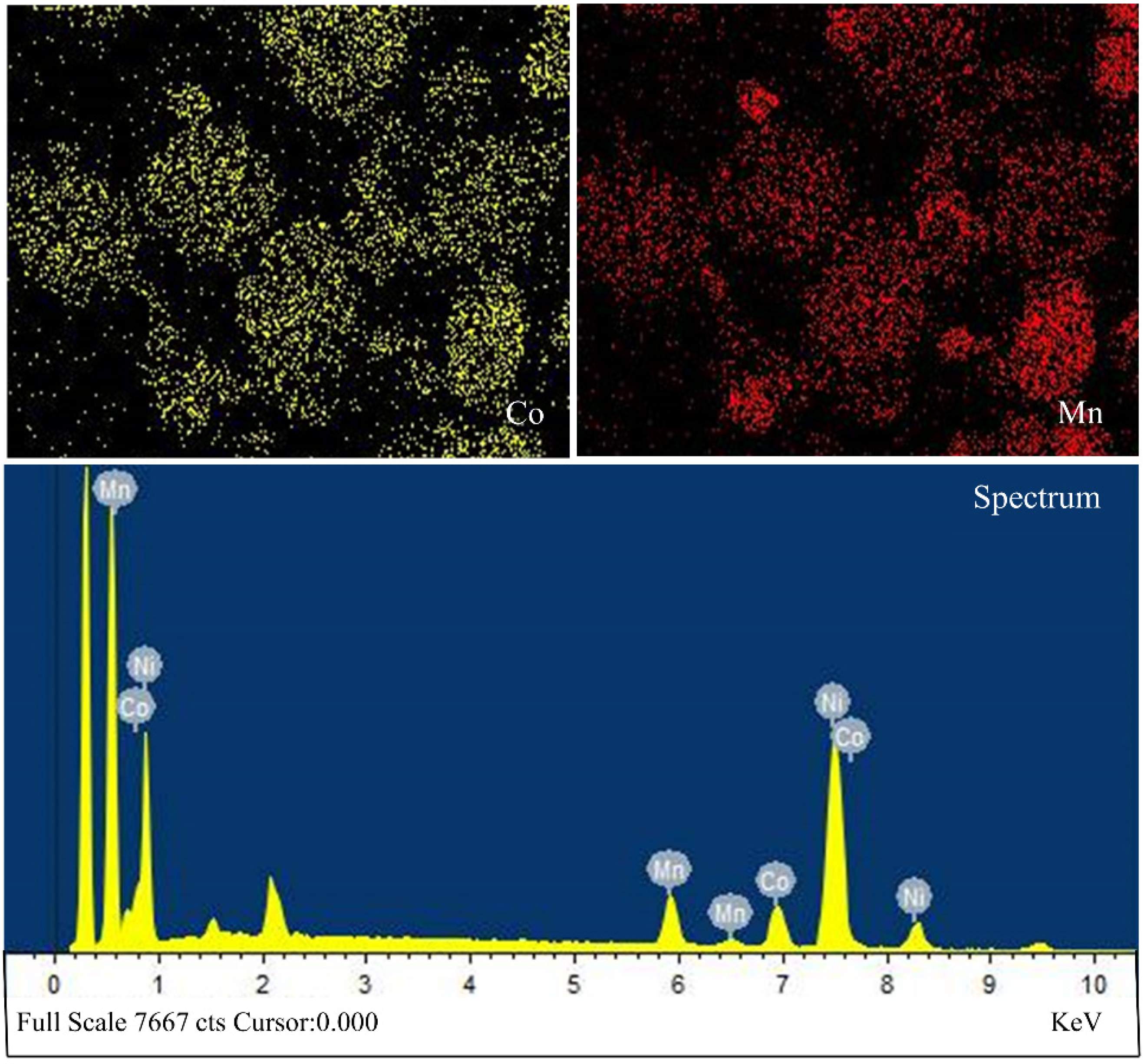
3.1. Analysis of Original Cathode Powder
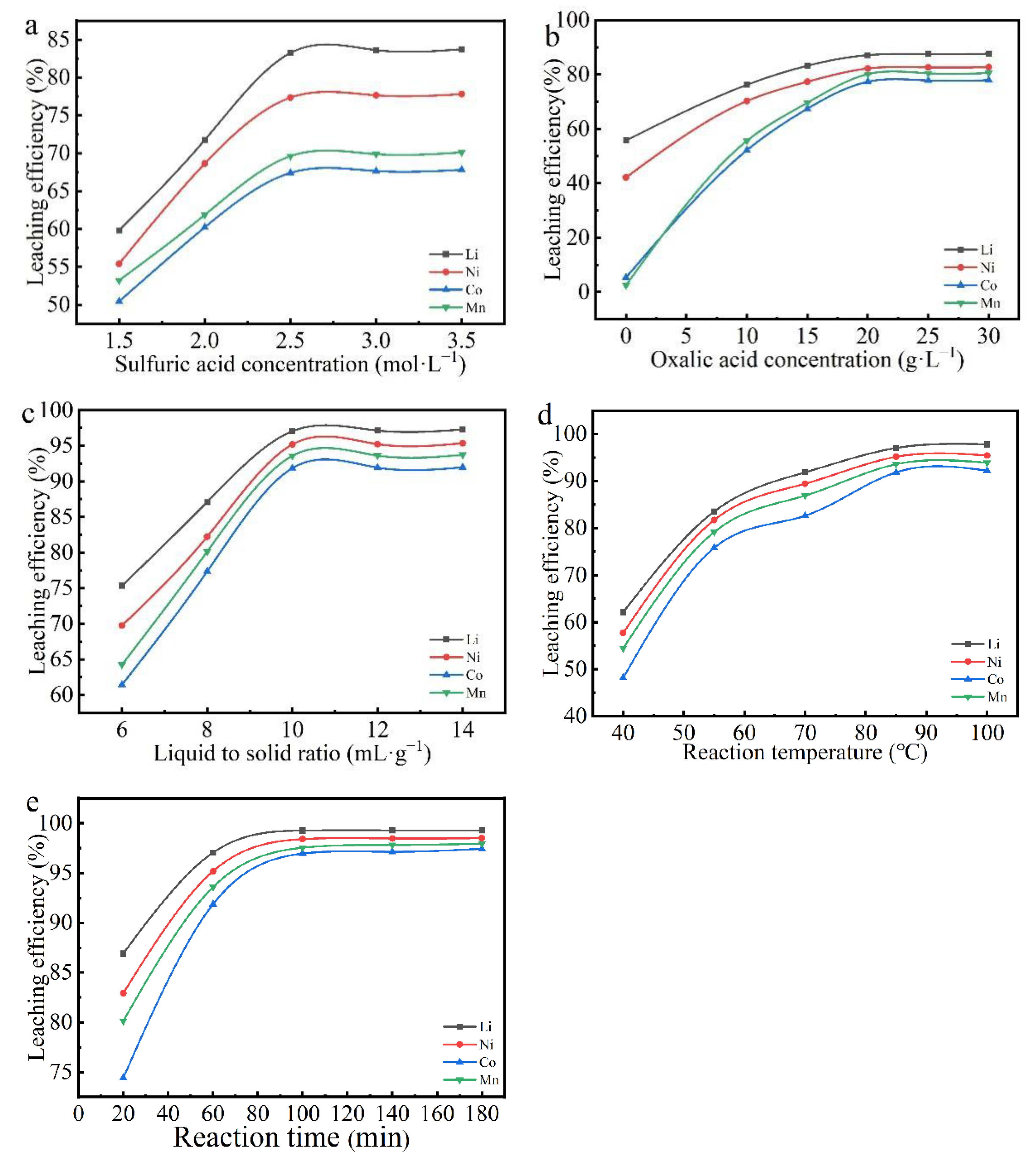
3.2. Leaching Conditions
3.2.1. The Effect of Sulfuric Acid Concentration on Leaching Rate
3.2.2. The Effect of Oxalic Acid Concentration on Leaching Rate
3.2.3. The Effect of Liquid-to-Solid Ratio on Leaching Rate
3.2.4. The Effect of Reaction Temperature on Leaching Rate
3.2.5. The Effect of Reaction Time on Leaching Rate
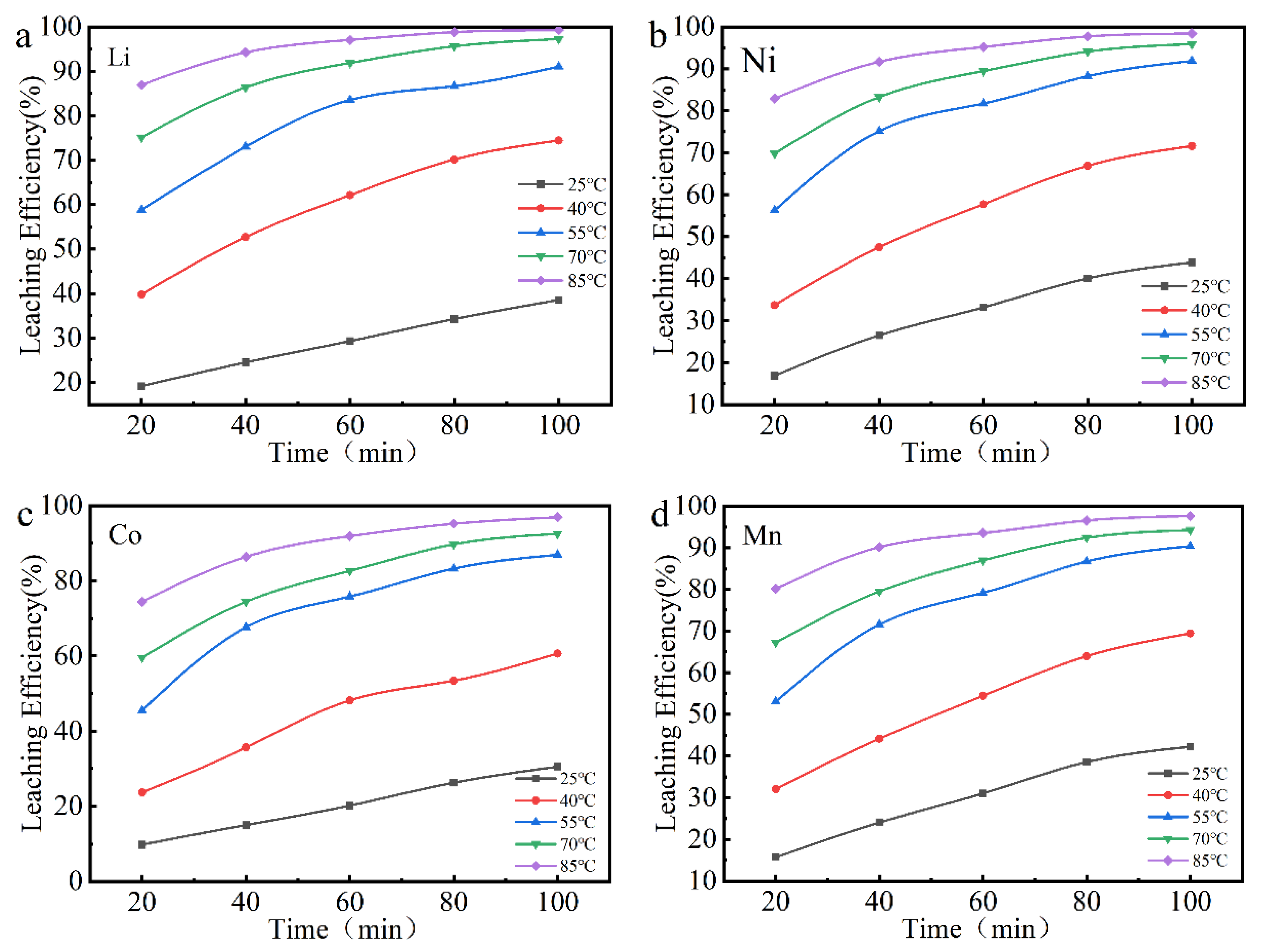
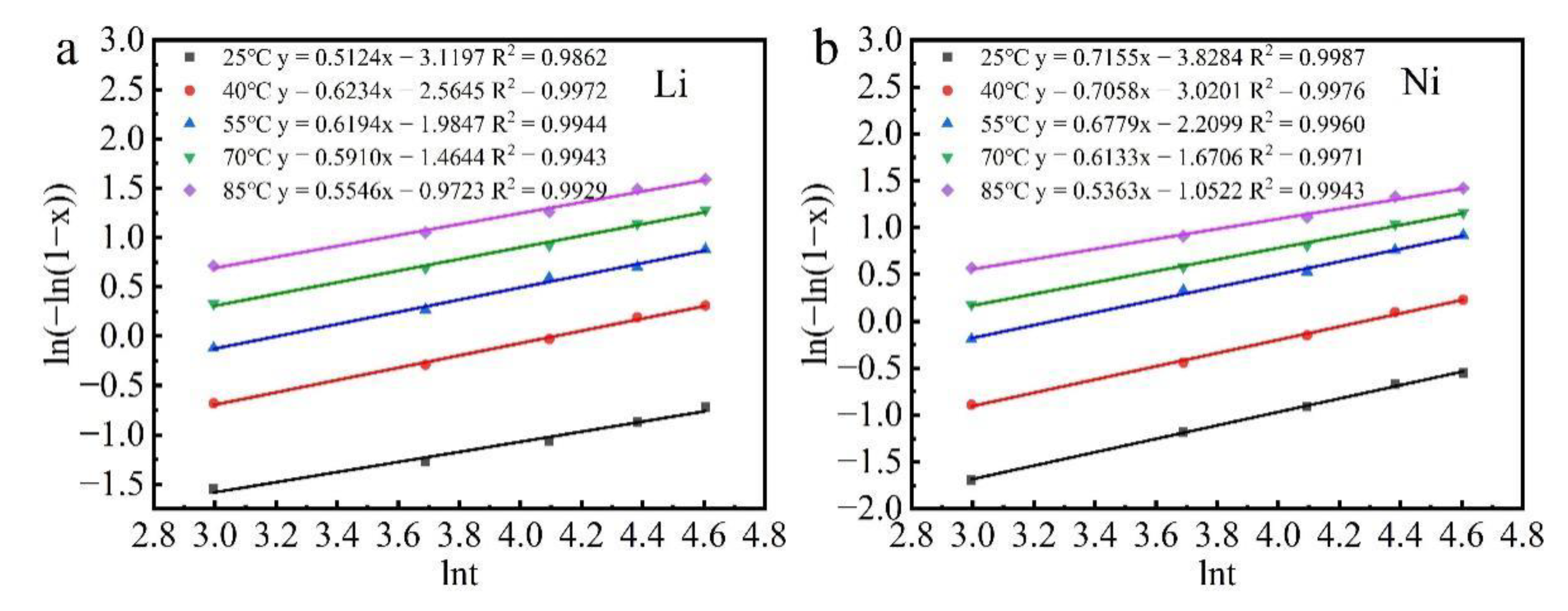
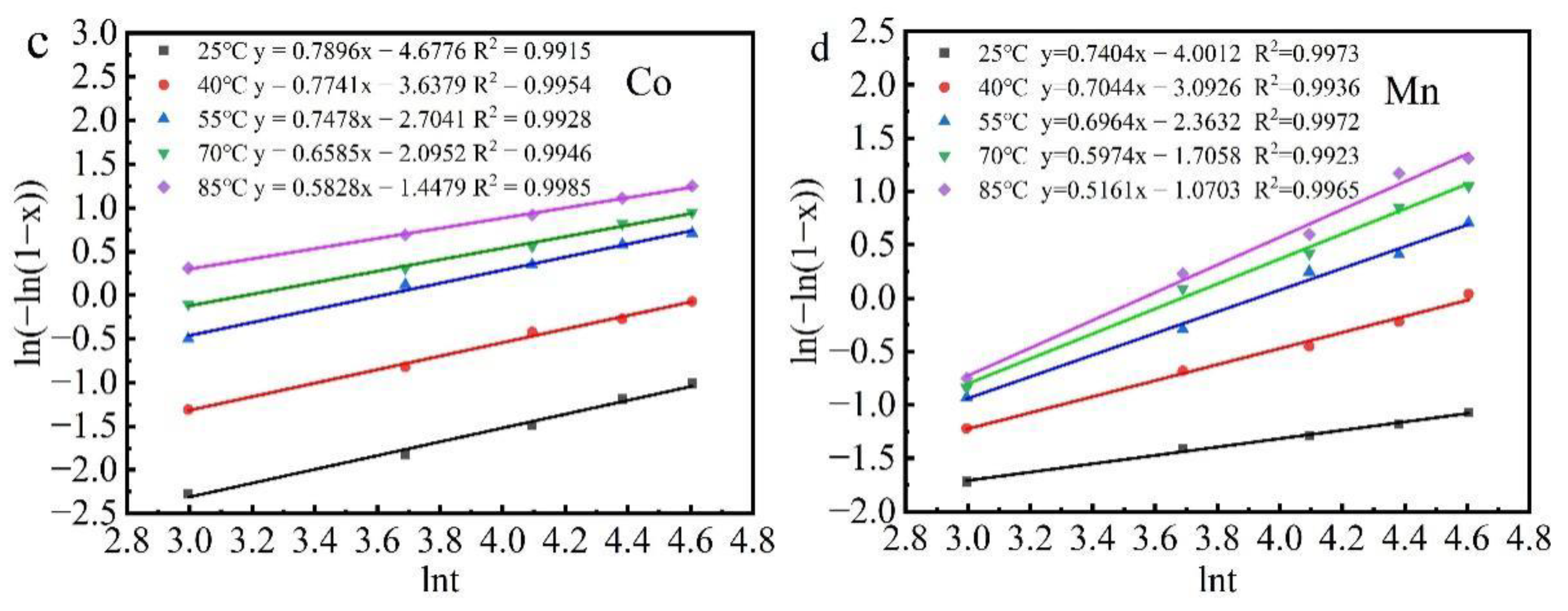
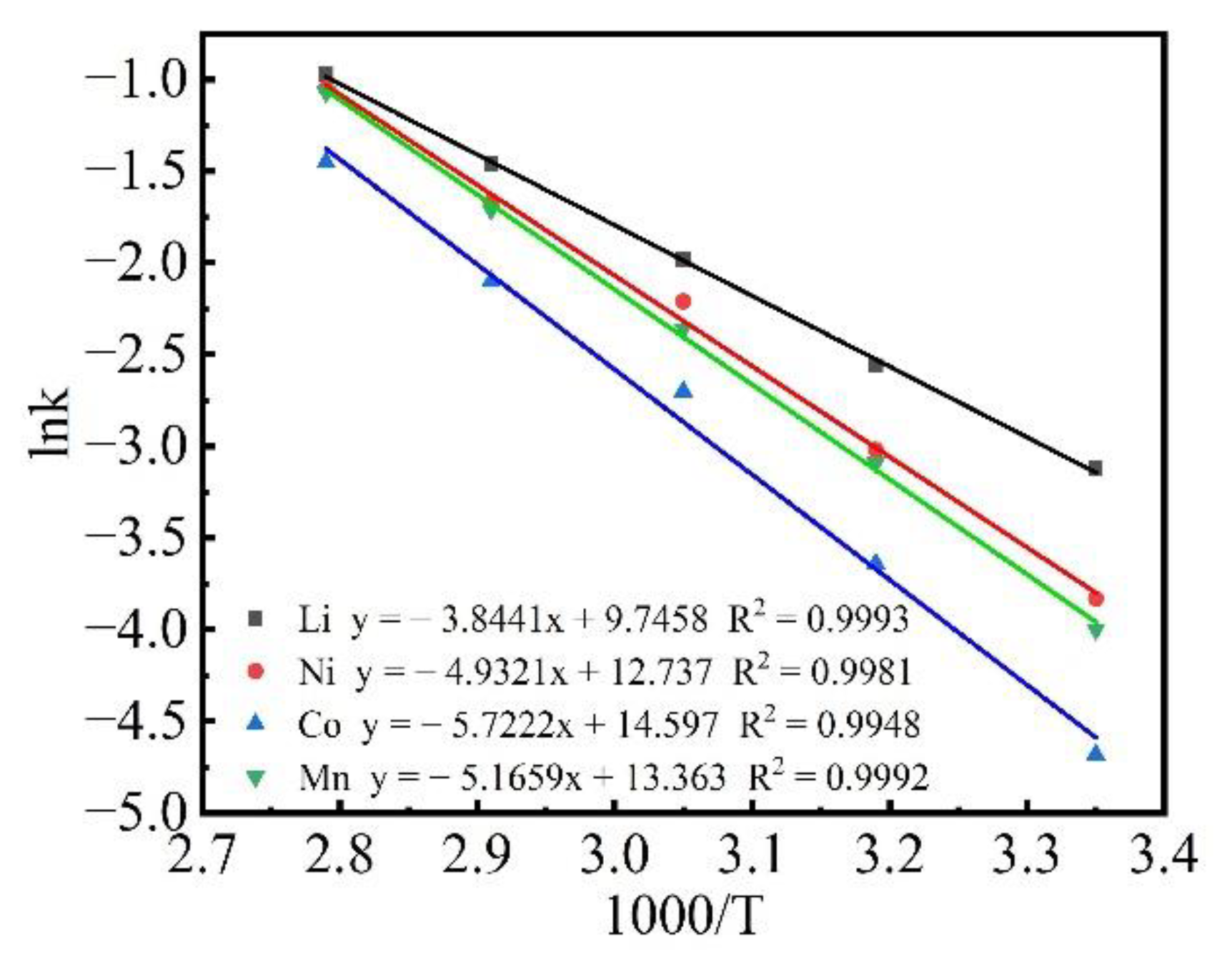
3.3. Kinetic Analysis
3.4. Characterization of Spent Lithium-Ion Cathode Powder before and after Leaching
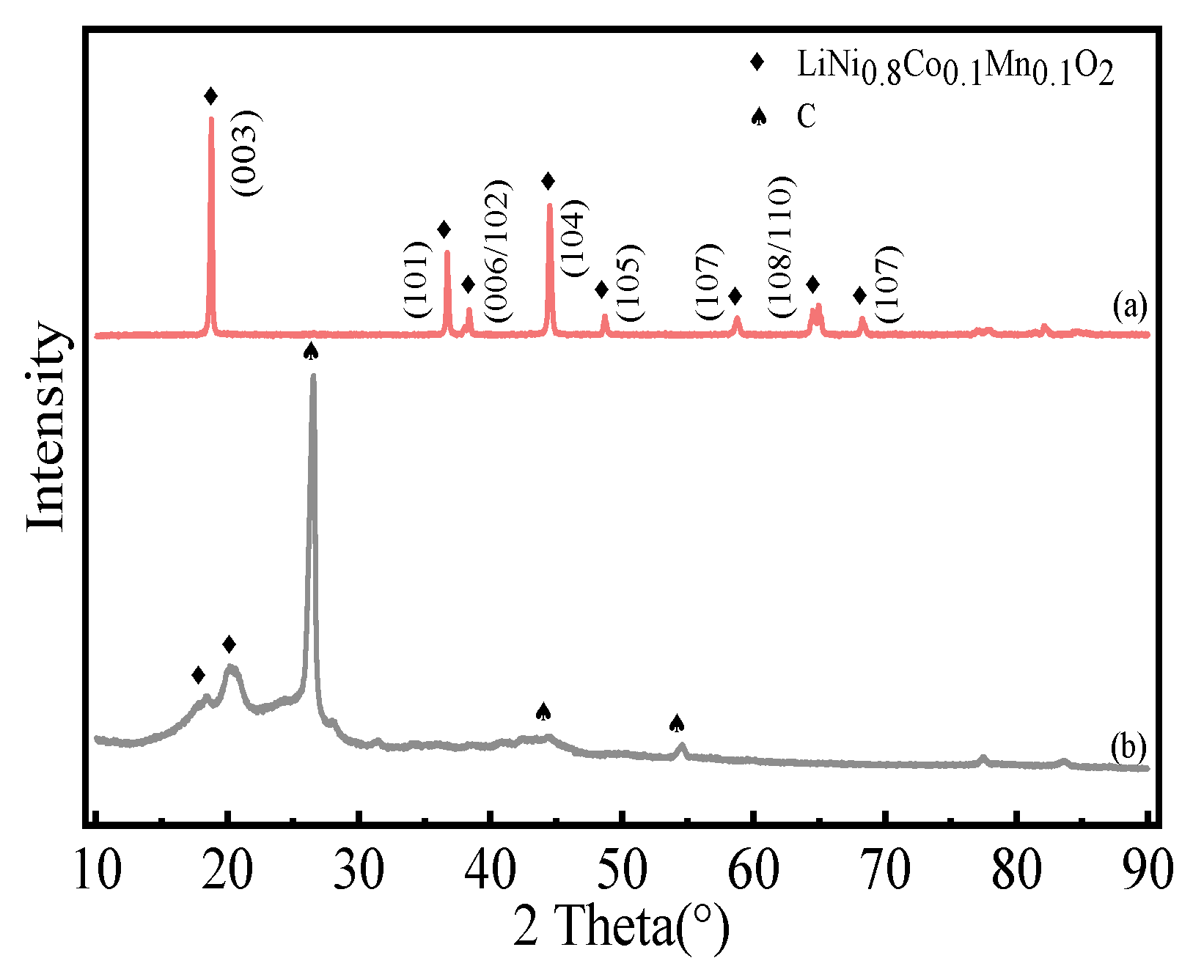
3.4.1. XRD Analysis
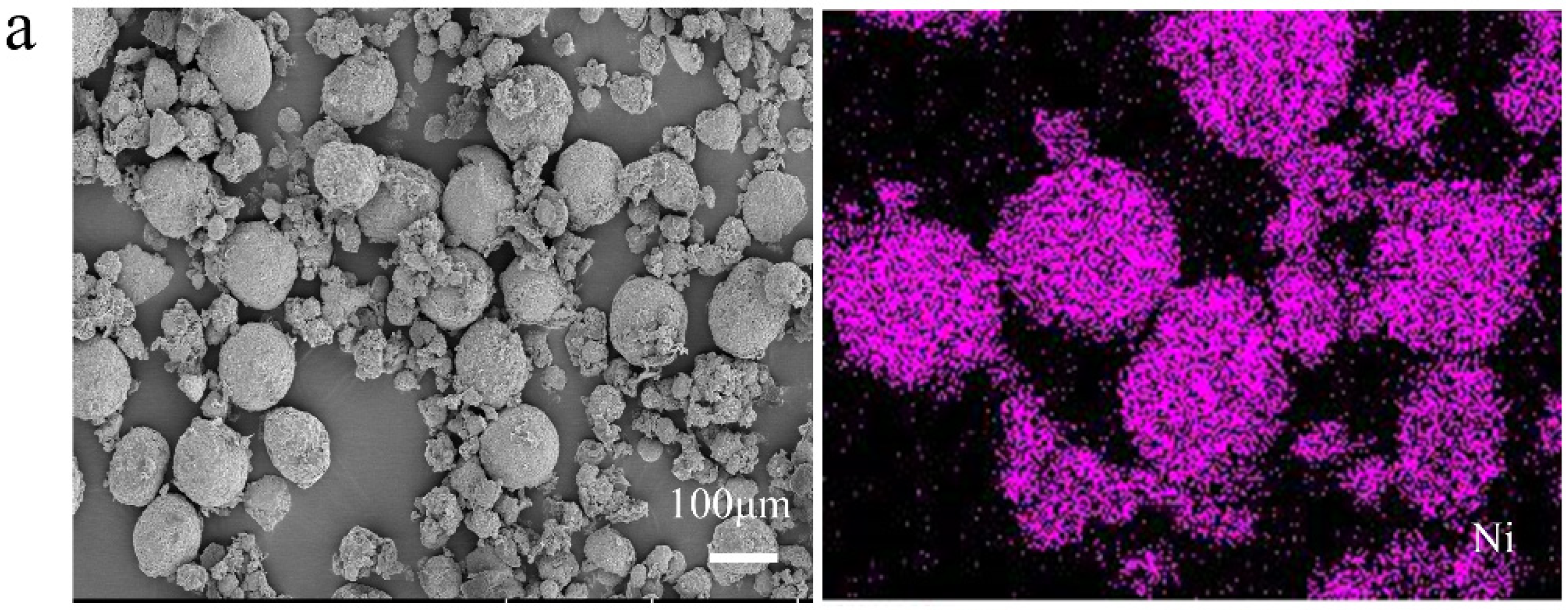
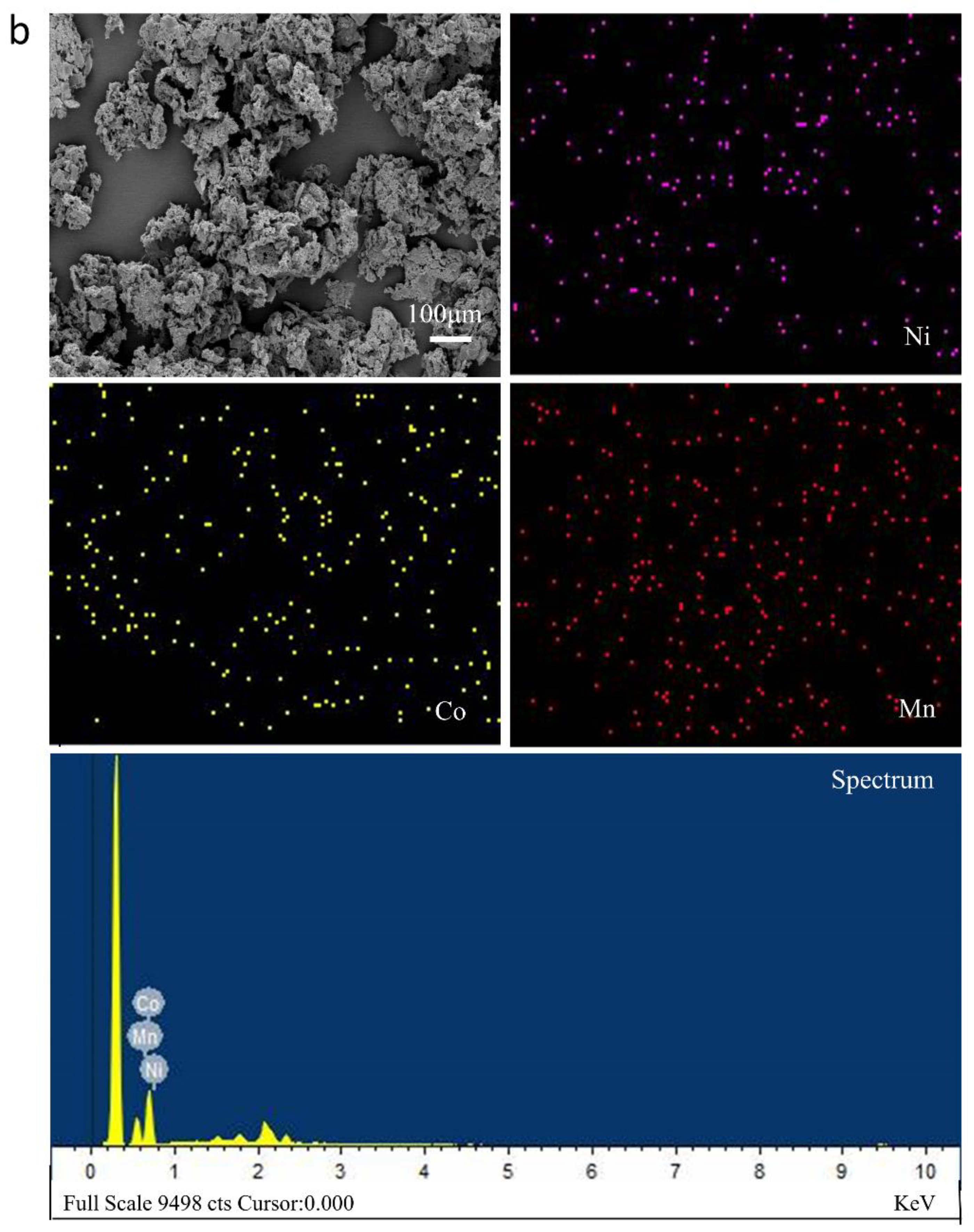
3.4.2. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Tang, J.; Wu, F.; Dai, X.; Zhou, J.; Pang, H.; Duan, X.; Xiao, B.; Li, D.; Long, J. Robust MXene adding enables the stable interface of silicon anodes for high-performance Li-ion batteries. Chem. Eng. J. 2022, 452, 139139. [Google Scholar] [CrossRef]
- Gandoman, F.H.; Jaguemont, J.; Goutam, S.; Gopalakrishnan, R.; Firouz, Y.; Kalogiannis, T.; Omar, N.; Van Mierlo, J. Concept of reliability and safety assessment of lithium-ion batteries in electric vehicles: Basics, progress, and challenges. Appl. Energy 2019, 251, 113343. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarboniza-tion. Chem. Rev. 2020, 121, 1623–1669. [Google Scholar] [CrossRef]
- Zhao, T.; Yao, Y.; Wang, M.; Chen, R.; Yu, Y.; Wu, F.; Zhang, C. Preparation of MnO2-Modified Graphite Sorbents from Spent Li-Ion Batteries for the Treatment of Water Contaminated by Lead, Cadmium, and Silver. Acs. Appl. Mater. Inter. 2017, 9, 25369–25376. [Google Scholar] [CrossRef]
- Lin, J.; Li, L.; Fan, E.; Liu, C.; Zhang, X.; Cao, H.; Sun, Z.H.; Chen, R. Conversion Mechanisms of Selective Extraction of Lithium from Spent Lithium-Ion Batteries by Sulfation Roasting. ACS Appl. Mater. Interfaces 2020, 12, 18482–18489. [Google Scholar] [CrossRef]
- Fujita, T.; Chen, H.; Wang, K.-T.; He, C.-L.; Wang, Y.-B.; Dodbiba, G.; Wei, Y.-Z. Reduction, reuse and recycle of spent Li-ion batteries for automobiles: A review. Int. J. Miner. Met. Mater. 2021, 28, 179–192. [Google Scholar] [CrossRef]
- Xia, X.; Li, P. A review of the life cycle assessment of electric vehicles: Considering the influence of batteries. Sci. Total Environ. 2022, 814, 152870. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhang, X.; Yang, E.; Tu, Y. A green process to recover valuable metals from the spent ternary lithium-ion batteries. Sep. Purif. Technol. 2022, 299, 121782. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2021, 406, 124332. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, J.; Wanaldi, R.; Zhou, X.; Wang, H.; Zhou, C.; Yang, J. A promising selective recovery process of valuable metals from spent lithium ion batteries via reduction roasting and ammonia leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef]
- Tian, G.; Yuan, G.; Aleksandrov, A.; Zhang, T.; Li, Z.; FathollahiFard, A.M.; Ivanov, M. Recycling of Spent Lithium-Ion Bat-teries: A Comprehensive Review for Identification of Main Challenges and Future Research Trends. Sustain. Energy. Techn. 2022, 53, 102447. [Google Scholar]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.; Shojaosadati, S. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Hu, Q.; Wang, Z.; Zheng, J.; Wu, L.; Zhang, L. Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 2009, 99, 7–12. [Google Scholar] [CrossRef]
- Chen, H.; Gu, S.; Guo, Y.; Dai, X.; Zeng, L.; Wang, K.; He, C.; Dodbiba, G.; Wei, Y.; Fujita, T. Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3. Hydrometallurgy 2021, 205, 105746. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Xie, H.; Qu, J.; Qu, X.; Xing, P.; Yin, H. Hydrometallurgical recovery of spent cobalt-based lithium-ion battery cathodes using ethanol as the reducing agent. Environ. Res. 2020, 181, 108803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, J.; Xu, Z. Challenges to Future Development of Spent Lithium Ion Batteries Recovery from Environmental and Technological Perspectives. Environ. Sci. Technol. 2019, 54, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Shi, P. The Study of Recovery Technology of Ternary Cathode Materials from Spent Lithium-Ion Batteries. Master’s Thesis, Beijing Institute of Technology, Beijing, China, 2015. [Google Scholar]
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Fan, X.; Song, C.; Lu, X.; Shi, Y.; Yang, S.; Zheng, F.; Huang, Y.; Liu, K.; Wang, H.; Li, Q. Separation and Recovery of Valuable Metals from Spent Lithium-Ion Batteries via Concentrated Sulfuric Acid Leaching and Regeneration of LiNi1/3Co1/3Mn1/3O2. J. Alloys Compd. 2021, 863, 158775. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Zhai, L.; Zhang, X.; Curtiss, L.; Jin, Y.; Wu, F.; Chen, R.; Amine, K.; China Electric Power Research Institute; et al. A facile recovery process for cathodes from spent lithium iron phosphate batteries by using oxalic acid. CSEE J. Power Energy Syst. 2018, 4, 219–225. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective Reduction Leaching of Vanadium and Iron by Oxalic Acid from Spent V2O5-WO3/TiO2 Catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Maddah, F.; Alitabar, M.; Yoozbashizadeh, H. Reductive leaching of indium from the neutral leaching residue using oxalic acid in sulfuric acid solution. Int. J. Miner. Met. Mater. 2021, 28, 373–379. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Sun, H.; Huang, Y.; Han, G. Selective extraction and recovery of tin from hazardous zinc-leaching residue by oxalic acid/sulfuric acid mixture leaching and hydrolytic precipitation. J. Clean. Prod. 2022, 342, 130955. [Google Scholar] [CrossRef]
- Binder, J.O.; Culver, S.P.; Zeier, W.G.; Janek, J. A Rapid and Facile Approach for the Recycling of High-Performance LiNi1−x−yCoxMnyO2 Active Materials. ChemSusChem 2021, 14, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, C.; Liu, R.; Xie, Z.; Liu, Z.; Cen, S.; Tao, C.; Guo, S. Leaching kinetics of manganese from pyrolusite using pyrite as a reductant under microwave heating. Sep. Purif. Technol. 2021, 277, 119472. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Y.; Zou, Y.; Yang, Z.; Zhang, B.; Zhao, Y.; Zhang, M.; Meng, Q.; Dong, P. Leaching kinetics and interface reaction of LiNi0.6Co0.2Mn0.2O2 materials from spent LIBs using GKB as reductant. J. Environ. Manag. 2021, 300, 113710. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- George, R.; Sugunan, S. Kinetics of adsorption of lipase onto different mesoporous materials: Evaluation of Avrami model and leaching studies. J. Mol. Catal. B Enzym. 2014, 105, 26–32. [Google Scholar] [CrossRef]
- Ebrahimzade, H.; Khayati, G.R.; Schaffie, M. Leaching kinetics of valuable metals from waste Li-ion batteries using neural network approach. J. Mater. Cycles Waste Manag. 2018, 20, 2117–2129. [Google Scholar] [CrossRef]
- Ajiboye, E.A.; Panda, P.K.; Adebayo, A.O.; Ajayi, O.O.; Tripathy, B.C.; Ghosh, M.K.; Basu, S. Leaching Kinetics of Cu, Ni and Zn from Waste Silica Rich Integrated Circuits Using Mild Nitric Acid. Hydrometallurgy 2019, 188, 161–168. [Google Scholar] [CrossRef]
| Cathode Material | Acid | Reductant | Other Leaching Conditions | Leaching Efficiency | Cost ($) | Ref. |
|---|---|---|---|---|---|---|
| NCM | H2SO4 = 2.5 mol·L−1 | oxalic acid = 20 g·L−1 | temperature = 85 °C, time = 100 min, S/L 10 g·L−1 | 99.26% Li, 98.41% Ni, 96.95% Co, 97.54% Mn | 3.14 | This work |
| NCM | H2SO4 = 2.5 mol·L−1 | H2O2 = 2 vol % | temperature = 85 °C, time = 2 h, S/L ratio = 5 g·L−1 | Nearly 100% Li, 98% Ni, 97% Co, 96% Mn | 46.69 | [19] |
| NCM | HCl = 6 mol·L−1 | H2O2 = 5 vol % | temperature = 85 °C, time = 2 h, S/L ratio = 12.5 g·L−1 | 99% Li, 98% Ni, 97% Co, 98% Mn | 59.12 | [20] |
| NCM523 | HNO3 = 0.5 mol·L−1 | C6H8O6 = 0.5 mol·L−1 | temperature = 85 °C, time = 10 min, S/L ratio = 20 g·L−1 | nearly 100%, Li, Ni, Co, and Mn | 37.21 | [21] |
| NCM111 | C6H8O7 = 2.0 mol·L−1 | H2O2 = 2 vol % | temperature = 80 °C, time = 90 min, S/L ratio = 30 g·L−1 | 99% Li, 97% Ni, 95% Co, 94% Mn | 45.33 | [22] |
| NCM | C3H6O3 = 1.5 mol·L−1 | H2O2 = 0.5 vol % | temperature = 70 °C, time = 20 min, S/L ratio = 20 g·L−1 | 97.7% Li, 98.2% Ni, 98.8% Co, 98.4% Mn | 1.8 × 104 | [23] |
| NCM111 | C6H12O7 = 1.0 mol·L−1 | H2O2 = 1 mol·L−1 | temperature = 70 °C, time = 80 min, S/L ratio = 30 g·L−1 | 99% Li, 99% Ni, 97% Co, 96% Mn | 9.1 × 106 | [24] |
| NCM622 | H2SO4 = 1.8 mol·L−1 | ginkgo biloba = 9 g·L−1 | temperature = 80 °C, time = 40 min, S/L ratio = 15 g·L−1 | 99.99 % Li, 98.65% Ni, 98.41 % Co, 98.25% Mn | 64.8 | [25] |
| Metal Element | Li | Ni | Co | Mn | Fe | Al | Others |
|---|---|---|---|---|---|---|---|
| Content (wt%) | 6.87 | 41.13 | 5.05 | 4.71 | 0.21 | 0.28 | trace |
| T/°C | Surface Chemical Control Model | Diffusion Control Model | Log Rate Law Model | Avrami Model |
|---|---|---|---|---|
| 25 | 0.9680 | 0.9247 | 0.9263 | 0.9915 |
| 40 | 0.9563 | 0.9622 | 0.9528 | 0.9954 |
| 55 | 0.9117 | 0.9329 | 0.9653 | 0.9928 |
| 70 | 0.9558 | 0.9605 | 0.9340 | 0.9946 |
| 85 | 0.9444 | 0.9406 | 0.9641 | 0.9985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Wang, J.; Yang, P.; He, Y.; Wang, S.; Zhao, P.; Wang, H. Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid. Sustainability 2022, 14, 14169. https://doi.org/10.3390/su142114169
Yang C, Wang J, Yang P, He Y, Wang S, Zhao P, Wang H. Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid. Sustainability. 2022; 14(21):14169. https://doi.org/10.3390/su142114169
Chicago/Turabian StyleYang, Chunyuan, Jiawei Wang, Pan Yang, Yue He, Song Wang, Pingyuan Zhao, and Haifeng Wang. 2022. "Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid" Sustainability 14, no. 21: 14169. https://doi.org/10.3390/su142114169
APA StyleYang, C., Wang, J., Yang, P., He, Y., Wang, S., Zhao, P., & Wang, H. (2022). Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid. Sustainability, 14(21), 14169. https://doi.org/10.3390/su142114169







