Abstract
Global resource limits and increasing demand for non-fossil energy sources have expanded the research on alternative fuels. Among them, algal biomass is designated as a third-generation feedstock with promising opportunities and the capability to be utilized for energy production in the long term. The paper presents the potential for converting beach wrack containing macroalgal biomass into gaseous fuel as a sustainable option for energy production, simultaneously improving the organic waste management that the coastline is facing. Beach wrack collected in the northern Baltic Sea region was converted by gasification technology applicable for carbon-based feedstock thermal recovery, resulting in syngas production as the main product and by-product biochar. Proximate and ultimate analysis, trace and major element quantification, detection of calorific values for macroalgal biomass, and derived biochar and syngas analysis were carried out. A higher heating value for beach wrack was estimated to be relatively low, 5.38 MJ/kg as received (or 14.70 MJ/kg on dry basis), but produced syngas that contained enough high content of CH4 (42%). Due to macroalgal biomass specifics (e.g., high moisture content and sand admixture), an adjusted gasification process, i.e., the combination of thermochemical procedures, such as mild combustion and pyrolytic biomass conversion, might be a better choice for the greater economic value of biowaste valorization.
1. Introduction
It is foreseen that, by 2040, global energy consumption will increase by more than 40–50% [1,2]. The use of fossil fuels is reducing for various reasons, such as regional geopolitical changes in supply chains and policies implemented to mitigate climate change globally. Increasing demand for energy leads to an elevated need for biofuel production. However, the exploitation of agricultural and forestry sectors as an energy source conflicts with land use necessities for food and feed production, limited access to fresh water and new farmlands, and elevated land requirements with demand for soil quality improvement. Global resource limits have escalated research on alternative materials. Among them, algal biomass is designated as a third-generation feedstock with estimated long-term availability and capability to be utilized for energy production [3,4,5,6,7]. The utilization of biomass and biowaste, including various cultivated or wild algae, for bioenergy production in the near future will be applied extensively to keep up with the development of new technologies [8,9,10,11]. Despite the apparent potential of diverse algal biomass, until now, most of the research has focused on the investigation of microalgae rather than macroalgae [12,13]. Moreover, technical and engineering difficulties must be resolved to develop a commercial system that could produce economically viable algal biofuel [14,15].
The term ‘beach wrack’ (also known as seaweed cast ashore in masses, storm-cast beach wrack, beach strand, wrack band, or beach-cast sea wrack) is attributed to a material washed from the sea and accumulated on beaches due to natural marine movements induced by area-specific hydrodynamic and climatic conditions. The bulk of beach wrack masses usually consists of organic material compiled of aquatic vegetation biomass and remains of marine organisms at different stages of decomposition. Another part, the mineral material in beach wrack, is variable and mainly comprised of sand and rock particles. Waves, tides, currents, and storms are the most significant factors affecting beach wrack composition and accumulation amount, time, and place [16,17,18]. Furthermore, the anthropogenic impact on beaches and beach wrack masses is inevitable, and litter admixture is always present to a greater or lesser extent [19,20,21].
Increasing eutrophication induced by excessive nutrient loads due to anthropogenic activities is a severe environmental concern affecting offshore, onshore, and coastline ecology. General conditions of the Baltic Sea, such as languid water exchange and low water salinity, promote eutrophication much more than in other seas. In addition, temperature increase in step with climate change has a significant impact on the sea’s ecosystems [16,22,23,24,25]. Therefore, many areas of the Baltic Sea are subjected to excessive growth of marine vegetation that, at the end of its lifespan, generates an accumulation of beach wrack masses on the coastline, causing, from season to season repeating and from year to year increasing, inconveniences for coastal areas [7,26,27,28]. If not removed, the beach wrack is exposed to on-site decomposition at aerobic conditions influenced by local environmental factors such as humidity, solar radiation intensity, and temperature. The on-site decomposition of algal biomass is accompanied by environmental and social consequences. For example, social inconveniences are related to the unpleasant odor, untidiness, and unattractiveness of sandy beaches, escalated presence of insects finding beach wrack as a habitat, decreased bathing water quality due to increased microbiological contamination adversely affecting the life in coastal municipalities, coastline nature and beaches as recreation areas, and subsequently having a direct impact on such branches of activities as the tourism-related industry and water sports. Thus, beach wrack can be assessed as organic waste [17,22,29,30,31]. At the same time, environmental consequences are attributed to excessive nutrient output from beach wrack decomposition that may result in reinforcement of eutrophication due to the entailed dominance of opportunistic species in marine vegetation [25,32]. Aerobic decomposition of macroalgal biomass may release potentially toxic compounds adversely affecting littoral ecosystems, as well as have an impact on greenhouse gas (GHG) emissions [27,28,31,33]. For instance, a study measuring CO2 and CH4 emissions from the breakdown of macroalgae estimated that CO2–C flux from beach wrack globally might reach 19.04 TgC/yr (equal to the same value of megatons of carbon (MtC) [31]. Other research emphasizes that about a billion tons of GHG are emitted into the atmosphere annually, released from the decomposition of macroalgal biomass [28]. Furthermore, beach wrack that remained dry on higher coastal lines released by 72% fewer emissions than repeatedly wetted algal masses accumulated in lower intertidal zones [31]. On the other hand, beach wrack is a material of natural occurrence. It has a particular role in coastal and marine ecology, providing a range of ecosystem services such as ensuring habitat to the sea and coastline animal communities, binding drifting sands, taking part in nutrient cycling, and playing a role in coastline fortification [23,30,31,32,33,34,35]. A study based on nutrient content estimation in marine macrophytes revealed that the removal of macroalgal biomass from the coastline may have a more significant impact on P content than N in the sea and could decrease the N/P ratio towards the natural so-called Redfield ratio; and the state of the coastal environment is not adversely affected [36]. However, removing macroalgal biomass from beaches should be assessed in every individual case to consider the balance between environmental and social benefits and drawbacks.
Gasification is a thermochemical process converting carbonaceous materials into gaseous fuel, applicable for further utilization in energy production. Gasification implements the breakdown of C, H, and O present in biomass and its char into basic volatile compounds such as CO, H2, CO2, CH4, and H2O [6]. It belongs to developing energy extraction methods applied for dried biomass (Figure 1) and is considered a quite convenient thermochemical process for bioenergy production from algal biomass and agricultural and forestry residues [11,37,38,39].
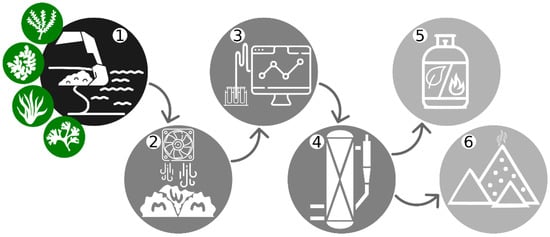
Figure 1.
Schematic representation of the technological process: onshore/offshore (handpick or mechanized) collection of beach wrack (1), preprocessing such as dewatering, drying (2), analysis (3), gasification procedure (4), its final products such as syngas (5), and by-produced residuals—chars, tars, and ash (6).
Macroalgal biomass is assessed as rich in carbohydrates, contains a smaller amount of proteins and lipids, and the content of lignin in it is tiny. Therefore, it is more eligible as a feedstock for biofuel production than other biomass rich in lignin which is a reluctant material if biological conversion is applied [7].
Gaseous fuel derived by gasification is known as producer gas, synthesis gas, or syngas and usually contains prevailing gases such as CO2, H2, CO, CH4, N2, and a minor amount of other gaseous substances [11,40]. The calorific character of syngas usually varies from 4 to 15 MJ/m3, being approximately 3–8 times lower than for natural gas (35–40 MJ/m3) [41].
The advantages of gasification can be listed as follows: (1) various biomasses or biowaste are always available (primarily considering waste material), (2) technology is relatively cheap, not demanding high capital investments, and (3) technological application is relatively uncomplicated. Furthermore, the advantage of gasification is that biomass refining is not urgently necessary; however, the specific character of biomass composition may significantly impact its calorific character and output products [12,41]. Depending on local amounts of biomass availability, various sizes of gasification devices may be classified from small to industrial scale [6,42,43].
Gasification basically involves four biomass processing phases: the drying phase occurring at 100–150 °C; the pyrolysis phase occurring at 150–700 °C; the oxidation phase or combustion at 700–1500 °C; and the reduction phase or gasification at 800–1100 °C. Various temperatures and thermochemical reactions may occur at different stages. The process is consistent with the biomass or biowaste specifics (moisture content, chemical composition, ash content), feedstock rate, and gasifying agent [11,41,44]. More intensive cracking of formed tars occurs in downdraft gasifiers, and produced syngas contains low contents of tars or might even be tar-free; therefore, it can be applicable for direct use to drive combustion engines [6,45].
Besides syngas production, a gasification by-product of particular economic value is biochar that can be further utilized as a soil amendment to improve functional redundancy of soil biota, increase organic carbon stability, reduce N2O emissions, facilitate soil formation, adsorb contaminants, as well as favor water circulation, and support maintaining the soil pH buffering capacity. Biochars derived from algal biomass have a relatively lower C content, low specific surface area (<70 g/m2), and cation exchange capacity, but a higher content of N, P, K, Ca, and Mg, as well as higher acidity [44,46,47,48]. Biochar can have several other application options, such as fuel in gasifiers or combustors, domestic charcoal, activated carbon, manure treatment, feed additive, or even tar-reforming catalyst [49,50,51,52,53].
Other by-products of gasification are tars, which, if not removed periodically, may adversely affect the process by causing fouling, corrosion, emissions, erosion, efficiency losses, and eventually, plant shutdown. The presence and amount of such elements as Ca, Cl, Fe, K, Mg, Na, P, S, and Si in biomass may play a significant role in tar occurrence [54]; therefore, studying biomass composition is of great importance for better results of thermochemical processes.
This study aims to evaluate the applicability of locally available beach wrack accumulating seasonally in the Baltic Sea coastal areas and to demonstrate the potential of using gasification for processing macroalgae-containing biomass into syngas as a primary product and biochar as a by-product.
2. Materials and Methods
2.1. Origin of Beach Wrack
The beach wrack material applied for the investigation was obtained from the central to northern Baltic Sea region at 17 sampling points (sandy beaches) located in southern Sweden, western Estonia, and Latvia, by the coastal towns and villages, as shown in Figure 2.
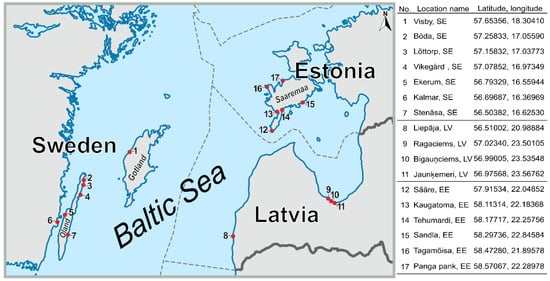
Figure 2.
Schematic map of locations and coordinates of beach wrack sampling points in the Baltic Sea coastal areas (SE—Sweden, LV—Latvia, EE—Estonia).
Samples of beach wrack were collected as they were found on-site—containing macroalgal biomass in a mixture of sand and litter; the collection was performed in the summer season, during June and July 2019. At each sampling point, the sampling material was gathered in the beach section between the water’s edge and the beach’s back (vegetation) in a selected sampling sector (Figure 3).

Figure 3.
Typical beach wrack sampling sector at the seaside coastline: fixed 100 m length (A) in relation to variable site-specific beach width (B) (photo of the Baltic Sea coast in Poland taken by Radek Kucharski (137294100@N08/51380554569) and retrieved from flickr.com, San Francisco, CA, USA).
2.2. Beach Wrack Composition and Quantitative Analysis
After collecting the beach wrack, it was cleaned of sizeable inert impurities that might adversely affect the gasification device. Then, fresh material was examined to detect dominant species of macroalgae by a hand-sorting identification procedure. Afterwards, the amount of sand and litter admixture in macroalgal biomass was detected in separated and weighed fractions. Macroalgal biomass was then dried to constant weight and processed for further research.
Beach wrack samples and their produced biochar were subjected to quantitative multi-element analysis performed by acid digestion [55,56]. For quantitative analysis, 1 g of a sample was weighed into a Teflon tube, followed by the addition of 8 mL 65% HNO3 (≥65% w/v, analytical grade, Sigma-Aldrich, St. Louis, MO, USA) and 2 mL 30% H2O2 (30% w/v, analytical grade, Enola, Riga, Latvia). High-pressure-assisted acid digestion was implemented using a microwave digestion system (ETHOS Easy, Milestone, Sorisole, Italy) at 200 °C for 30 min. Each sample was subjected to triplicate analysis, and blank samples were prepared to ensure quality assurance. The resulting sample solutions were diluted to 50 mL with deionized water (<0.1 µS/cm, 18 MΩ/cm, ELIX-3, Merck Millipore, Darmstadt, Germany). The concentration of major and minor elements was determined by inductively coupled plasma mass spectrometry with optical emission (ICP-OES) detection (iCAP 700, Thermo Fisher Scientific, Waltham, MA, USA). The elements quantified were Al, As, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Se, Sr, Ti, Tl, V, and Zn. The detection limit for certain elements was approved by ICP-OES calibration solutions. Quantified concentrations were expressed on a dry basis (DB) of the sample.
2.3. Proximate and Ultimate Analysis
The parameters to perform proximate analysis were determined for consolidated samples and involved the detection of moisture content according to ISO 18134-1:2015, ash content—ISO 18122:2015, volatile matter—ISO 18123:2015, and bulk density—ISO 17828:2015 [57,58,59,60]. The content of fixed carbon was calculated by subtracting the content of volatile matter and ash from the initial mass of the sample [61].
The ultimate analysis involved the determination of C, H, N, O, S, and Cl content. The content of C, H, and N was determined according to ISO 16948:2015 standard method applicable for solid biofuels [62]. The content of Cl and S was determined by applying ISO 16994:2016 [63]. O content was estimated according to ISO 16993:2016 [64].
Detected parameters were expressed for samples as received (AR) and on a dry basis (DB).
2.4. Calorific Character and Thermogravimetric Analysis
Characteristic calorific values of beach wrack samples were estimated according to ISO 18125:2017 standard method performed in a bomb calorimeter (AC-350, LECO, St. Joseph, MI, USA) [65].
The thermal decomposition character of beach wrack was studied by thermogravimetric analysis (TGA), which provides simulation and modelling options for operational gasification conditions [6]. TGA was performed using a thermogravimetric device Q600 SDT (TA Instruments, New Castle, DE, USA), applying a heating rate of 20 °C/min under the dynamic flow of N (100 mL/min), using about 10 mg of a solid powder sample until complete decomposition of organic matter (at 900 °C). During the analysis, differential thermal gravimetric curves were recorded.
2.5. Experimental Gasification and Syngas Analysis
Before the gasification, dewatering and air-drying by spreading macroalgal biomass on metallic mesh in a 100–150 mm layer (Figure 4) were implemented, ventilation was applied at a temperature of 30–40 °C for about 72 h [44].

Figure 4.
Drying of beach wrack.
Derived air-dried macroalgal biomass compiled about 20% of the fresh material. Gasification was applied to consolidated beach wrack samples (n = 3).
Gasification was implemented in an experimentally developed extruder-type gasification device with a stainless-steel reactor tube under an N2 stream at a temperature of 300–600 °C. The allothermal gasification process was applied using an external heat source. Samples were placed into the reactor tube and then heated to the desired temperature, applying a heating rate of 5 °C/min and maintaining the final temperature for 30 min. Derived volatile products passed through the vertical column heated with an inductive heater up to 1200 °C with the following gas cooling down in a water–ice mixture. At elevated temperatures, tar cracking occurred, and heavy organic gaseous substances passed breakdown into the syngas components [44].
To analyze the obtained syngas composition, a multigas analyzer (ETG-MCA 100 Syn, ETG, Chivasso, Italy) was exploited. For the measurements of gases such as CH4, CO2, CO, and O2, a non-dispersive infrared (NDIR) gas detector with a single optical path platform was used, but H2 was measured using a principle of thermal conductivity detector. Internal calibration for gas concentration analysis was performed, and the measurement accuracy was confirmed using synthetic gas mixtures such as H2:CH4 and N2:CO (GC purity, Linde, Dublin, Ireland). After the procedure, the reactor was cooled down by an N2 stream. Solid gasification by-product biochar was collected and stored in sealed containers for further analysis.
3. Results and Discussion
3.1. Beach Wrack Mass Composition
The mass composition of beach wrack samples mainly consisted of reeds (~50%), various algae (~36%), and sand (~14%), indicated on average by dry mass (Figure 5). The studied samples mainly contained algae species dominant in the coastal areas of the Baltic Sea, such as filamentous algae Fucus vesiculosus (L.), green algae Cladophora glomerata (L.), and red algae Furcellaria lumbricalis (Hudson), with small amounts of marine eelgrass Zostera sp. in some samples, as similarly observed by other studies related to Baltic beach wrack investigation [16,17].

Figure 5.
Diversity of beach wrack deposits observed at the Baltic Sea coastline of Latvia (A–C) and Estonia (D–F) in July 2019.
The undesirable admixture in beach wrack mass was identified, consisting mainly of sand, plastic waste, and wood debris. In general, beach wrack may contain a significant content of inert substance admixtures, such as sand and rocks, depending on site-specific environmental conditions and the collection specifics of beach wrack masses [44]. To achieve relative purity of algal biomass, during the sampling and preprocessing, sizeable pieces of plastic waste (bottles, packaging remains, etc.) were removed. Admixture of the microplastic amount was expected to be less than 1% [16]; however, the amount was estimated at 3.0 ± 0.5%. Admixture of wood debris was found to be in similar amounts. Nevertheless, the main impurity to beach wrack was mineral material (sand), detected in variable amounts (from 5 up to 33%), which is strongly dependent on the influence of climatic events, such as storms, resulting in an increased sand blend to macroalgal biomass, and rain leading to the opposite effect by improved washing out of sand from beach wrack [16,17].
3.2. Characterization of Beach Wrack
3.2.1. Quantification of Major and Minor Elements
In the composition of beach wrack, major elements such as Ca (16.95 g/kg), S (16.11 g/kg), K (9.75 g/kg), Mg (8.63 g/kg), and Na (7.56 g/kg, indicating total mean values, respectively) prevailed, followed by Fe (2.35 g/kg), Al (1.77 g/kg), P (1.39 g/kg), and Mn (0.33 g/kg) (Figure 6); they are listed among inorganic-forming elements [4]. The range of elements varied quite widely, but the most compact ranges were addressed to Mg (3.99–12.02 g/kg) and P (1.10–3.13 g/kg), indicating their more or less constant concentration in the samples of beach wrack.
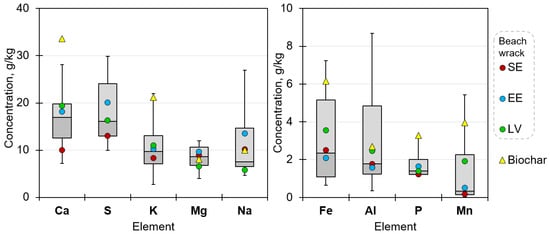
Figure 6.
Major element concentrations (grams per kg DB) in beach wrack collected in Sweden (SE), Estonia (EE), and Latvia (LV), excluding statistically relevant outliers and indicating a range between the 25th and 75th percentile, total mean, and mean by countries. In addition, element concentrations in biochar derived as a gasification by-product from consolidated beach wrack samples are shown.
The distribution of major elements according to the country of sample origin was variable. A considerably lower concentration of Ca (10.00 g/kg) was detected in samples from Sweden; also in addition, concentrations of S, K, P, and Mn were lower than in samples of other origins. A higher than the total mean concentration of S and Na was detected in samples from Estonia, but Fe and Mn in beach wrack collected in Latvia. It could be possible that particular traits are estimated if the investigation of beach wrack is performed over several seasons. For the majority of elements, mean concentration by country was various, while for some elements, such as K, Mg, and P, mean values ranged quite similarly. It should be noted that Si quantification was encumbered due to high sand admixture in the samples; therefore, obtained results were not verifiable to be presented.
Evaluation of minor element concentrations revealed that the prevailing elements were Sr (266.81 mg/kg), B (167.67 mg/kg), Ba (79.07 mg/kg), Ti (55.52 mg/kg), and Zn (46.13 mg/kg, indicating total mean values, respectively), followed by Ni (10.41 mg/kg), Cr (9.31 mg/kg), Cu (7.76 mg/kg), As (6.37 mg/kg), Li (6.32 mg/kg), V (4.31 mg/kg), Pb (2.15 mg/kg), but Co (1.41 mg/kg), Cd (0.98 mg/kg), and Mo (0.97 mg/kg, indicating total mean values, respectively) were detected at tiny concentrations (Figure 7). Some elements, such as Sb, Se, and Tl, were not quantified as their concentration was below the detection limit.
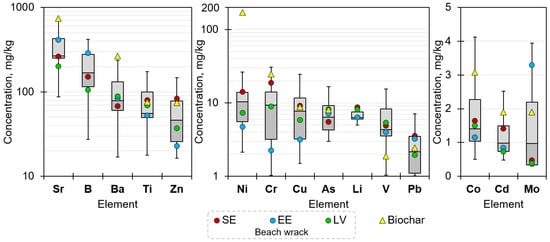
Figure 7.
Minor element concentrations (milligrams per kg DB) in beach wrack collected in Sweden (SE), Estonia (EE), and Latvia (LV), excluding statistically relevant outliers and indicating a range between the 25th and 75th percentile, total mean, and mean by countries. In addition, element concentrations in biochar derived as a gasification by-product from consolidated beach wrack samples are shown.
Differences among the sample origin by country indicated that samples from Estonia contained considerably lower than mean concentrations of Zn, Ni, Cr, and Cu, but higher Sr, B, and Mo. Considerably higher than mean concentrations of Zn, Ni, Cr, Li, and Pb were detected in beach wrack from Sweden. The concentrations of minor elements in samples from Latvia were closer to the total mean, except for Li, which exceeded it, and for Sr, Ba, Cd, and Mo, which were lower.
Particular attention should be paid to potentially harmful elements referred to as heavy metals, such as As, Cd, Cr, Cu, Mn, Ni, Pb, and Zn, being among the most concerning environmental contaminants and undoubted human and animal health hazard inducers. The presence and elevated concentrations of these elements may significantly affect the use of biomass, but their volatility indicates element transfer potential to energy production by-products [56]. Table 1 presents the total and statistically relevant concentration ranges of these elements, indicating outliers if any were observed. The statistical data reveal that most excluded outliers were detected for beach wrack samples collected in Sweden, possibly indicating a greater environmental contamination impact than in other sampling sites.

Table 1.
Concentration ranges in beach wrack (indicating excluded outliers) and biochar for the elements of environmental and health concern.
Identifying outliers could recognize the elements of concern whose concentration was elevated in individual samples, which might be essential to assess the environmental safety of biomass. However, long-term (over several seasons) monitoring of beach wrack composition could provide a better insight into whether the detected outliers were just occasional cases or whether some influencing factors might affect the potentially harmful element content. Generally, in practice, it would be recommended to use rapid testing (e.g., using a handheld XRF analyzer) before beach wrack utilization, such as in direct applications as a fertilizer or composting material [66].
Previous research has shown that contamination of macroalgae with potentially toxic metals such as Hg and Cd is quite common [17,26]. Due to the specifics of quantification technical aspects, Hg was not determined in this study. A study on beach wrack chemical composition revealed that Cd content in macroalgae could vary by species, e.g., Furcellaria lumbricalis contained Cd 1.1–3.2 mg/kg, but Polysiphonia 4.3–4.7 mg/kg, expressed on DB [26,67], which can be assessed as a relatively higher content than that detected in the present study. A better characterization of Cd content in beach wrack can be described by estimating the Cd/P mass ratio, ranging from 513 to 1161 mg Cd per kg of P for Estonian and Swedish samples, respectively (Table 2).

Table 2.
The mean concentration of Cd and P, and Cd/P ratio for beach wrack by sample origin.
A study performed using beach wrack collected in the Burgsviken Bay area of Gotland in the Baltic Sea revealed that the Cd/P mass ratio ranged from 39 to 647 [26], suggesting that beach wrack analyzed during the current study could not be suitable for soil fertilization without implementing preprocessing, especially if a maximum permissible total concentration of 60 mg Cd per 1 kg of P were applied [26], which might affect the quality estimation of derived biochar.
3.2.2. Proximate and Ultimate Analysis Outcomes
The proximate analysis indicated that the moisture level for beach wrack samples after air-drying was considerably high—on average 63.4% AR (Table 3). If fresh terrestrial biomass is usually characterized by 10–63% moisture, then for fresh algal biomass, the values are much more impressive at 70–90% [4], indicating that de-watering and drying of beach wrack is a non-negotiable step in preprocessing and choice of the conversion procedure. However, it should be noted that complete drying to gain a dry basis of biomass for commercial purposes of gasification would be not economically feasible.

Table 3.
Results of proximate analysis of consolidated beach wrack samples, expressed on a dry basis (DB) and as received (AR); n/a – not analyzed.
The detected ash content for beach wrack was on average 8.1% AR (22.1% DB), revealing comparability with general observations, as the content of ash for algae may usually vary from 13 to 43% DB, being one of the limiting factors for beach wrack utilization in energy production [4].
The volatile matter for the studied Baltic beach wrack was detected on average as 21.3% AR (58.2% DB), which is a little higher than compiled from related research (mean 52.4% DB) [4]. A higher value of volatile matter can be considered an advantage of any biomass or biowaste regarding its applicability for thermochemical conversion; however, terrestrial biomass is characterized by considerably higher volatile matter (mean 75.4% DB) than algal biomass, suggesting its better use for blended biofuels than alone [4].
The fixed carbon content in the beach wrack was, on average, 7.2% AR (15.4% DB), while in particular marine macroalgae, the value can reach 24.4% DB [4].
The bulk density was detected to be, on average, 210 kg/m3 for fresh macroalgal material. Usually, bulk density for algal biomass is higher than for terrestrial biomass and may reach up to 954 kg/m3; a higher density can have a more adverse effect on the conversion process [4].
In a study on beach wrack derived on the Swedish coastline of the Baltic Sea, the proximate analysis revealed comparable values: a relatively low content of volatile matter (20.4%), a significant content of fixed carbon (11.8%), and a considerably high content of ash (33.9%) [44]. Another study indicated proximate data for macroalgae species Sargassum horneri as follows: moisture content (10.46%), ash content (29.29%), volatile matter (48.85%), and fixed carbon content (11.60%) [6], revealing that the proximate composition of beach wrack can be very variable and could be diverse not only regarding algae species, but seasonal peculiarities, thus leading to problematic standardization relevant for biomass to be applied as feedstock in gasification for commercial purposes.
The values of ash content, volatile matter, and fixed carbon of Baltic beach wrack are quite comparable to those detected for wheat straw biomass, i.e., 16.09%, 64.42%, and 19.49%, respectively [61]. If compared to algal biomass, data on various microalgae have been compiled and a reveal moisture content of 0–10% (mean 5.04%), a volatile matter content range of 54.45–82.70% (71.50%), an ash content of 2.40–37.80% (8.72%), and a fixed carbon content of 0.69–36.90% (13.37%) [68]; it can be assessed that macroalgal biomass may contain a higher amount of ash and fixed carbon, but a lower content of volatile matter. Proximate analysis parameters are significant indicators for the best choice of applicable waste-to-energy technology affecting thermal decomposition. For example, if the volatile matter is at a higher content, it may promote material ignition at a lower temperature, thus positively affecting combustion efficiency. If the moisture content of the biomass is low, thermochemical conversion is recommended as the most optimal process [61]; therefore, beach wrack drying is a significant biomass preprocessing step if gasification is applied as a conversion method.
The ultimate analysis revealed that, on average, on a dry basis, the beach wrack contained 37.33% C and 30.52% O. The content of H, N, S, and Cl was considerably lower, being, on average, 4.66%, 3.33%, 1.23%, and 0.87%, respectively (Table 4).

Table 4.
Elemental composition ranges of consolidated beach wrack samples, expressed on a dry basis (DB) and as received (AR).
If taking into account available data on different microalgae, then the ultimate parameters were detected as follows: 25.46–53.01% (mean 45.20%) of C, 2.79–8.90% (6.80%) of H, 19.30–55.02% (31.95%) of O, 1.01–11.26% (7.20%) of N, and 0.20–2.42% (0.72%) of S [68]. The comparison of data suggests that macroalgal biomass might contain a lower content of C, H, O, and N, but a higher content of S. In another study that presented an ultimate analysis for wheat straw biomass, it was indicated that C and H content (5.57% and 37.20% for wheat straw, respectively) was very similar to beach wrack. However, N and S content (1.14% and 0.20% for wheat straw, respectively) was higher in beach wrack [61].
The concentration of Cl was detected to be the lowest for beach wrack samples obtained in Latvia, but higher for Sweden (Table 5).

Table 5.
Mean concentration of Cl in beach wrack by sample origin.
Considering proximate, ultimate, and chemical quantification analyses, it is possible to select the most appropriate biomass conversion method among the processes usually applied for biomass conversion into valuables of economic and environmental importance, such as fermentation, anaerobic digestion, combustion, or gasification [61].
3.3. Estimated Calorific Values and Thermogravimetric Analysis
For beach wrack, the gross calorific value, known as higher heating value (HHV), was detected to be 5.38 MJ/kg AR (14.70 MJ/kg DB) and the net calorific value—lower heating value (LHV)—was 3.45 MJ/kg AR (13.69 MJ/kg DB). If the values for the dry basis are compared, they are comparable to those estimated for other biomass, such as wheat straw, for which the HHV and LHV were detected to be 14.86 and 14.50 MJ/kg, respectively [61]; however, for algae pellets, experimentally detected HHV was higher at 18.82 MJ/kg [69]. However, it should be noted that drying of beach wrack is an energy-consuming step that may lead to unprofitable biomass utilization. A study on microalgae provided an overview of the HHV for various microalgae, ranging from 11.10 to 23.52 MJ/kg depending on species; this study also provided a suggestion to predict the HHV taking into account the results of the ultimate and proximate analysis of microalgal biomass [68]. Recalculation using current data for macroalgal biomass and suggested formulas for microalgal biomass [68] resulted in a comparable HHV for beach wrack, ranging from 13.98 to 16.71 MJ/kg and a mean of 14.87 MJ/kg, which matches well with an experimentally estimated HHV for beach wrack, indicating that the calorific values of algal biomass are quite easily predictable. Another study provided the HHV for various macroalgae species, ranging from 15.00 MJ/kg for Fucus vesiculosus to 17.60 MJ/kg for Laminaria digitata [70], indicating that for separated algae and, therefore, possibly cleaner macroalgal biomass, a higher HHV can be reached. Estimating the predicted HHV is a significant step in managing biomass conversion choice, as the HHV is considered a significant indicator of biomass utility for conversion into energy. The efforts to predict the HHV by calculations have been applied to various types of biomasses. However, a uniform approach according to the type and character of the material is of importance [68,69,71].
More attention has to be paid to the HHV for samples as received, and the value was not expected to be high for Baltic beach wrack due to high moisture content and a considerable amount of mineral admixture. The HHV for samples as received reveals a more realistic calorific character of beach wrack that is necessary for gasification implementation with a commercial purpose. The HHV for Baltic Sea beach wrack (5.38 MJ/kg AR) is comparable with the HHV for pine needles (5.70 MJ/kg) and municipality solid waste fraction (5.63 MJ/kg) [69].
The thermal decomposition of beach wrack demonstrated that the greatest part of the macroalgal biomass decomposed in the interval of 180–380 °C (Figure 8). The process is expressed as endothermic and exothermic reactions and begins with the initial combustion of volatiles, followed by slower burning and biochar formation [70].
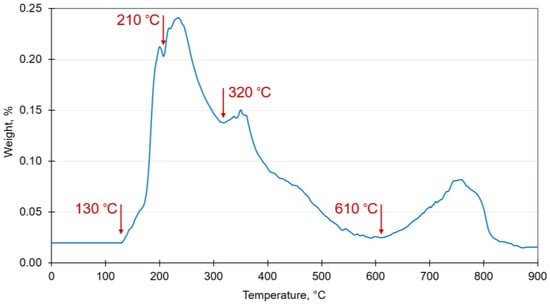
Figure 8.
Thermal decomposition character of a consolidated beach wrack sample.
The first decomposition maximum for the beach wrack was reached at 130 °C, which is related to the loss of adsorbed water molecules and also the volatilization of light organic compounds. The following maximums occurred at 180–250 °C, 320–380 °C, and 610–770 °C, implementing thermal conversion of carbohydrates. The thermal behavior of the beach wrack was in agreement with similar findings reported in the literature where algal biomass was used for energy production [44,70,72].
3.4. Characterization of Gasification and Its Outcomes
Marine waste is generally characterized by a significantly high content of inert substances. Sand and rocks can compile a remarkable part of beach wrack mass, depending on the beach wrack formation conditions, collection method, and preprocessing such as cleaning, washing, dewatering, and drying [17]. In addition, bulk density and thermal conductivity of dried marine waste biomass are expected to be low. These factors may influence the performance of the gasification process, as well as syngas and by-product formation. The thermal conductivity of biomass or biowaste used as a fuel is significant in the initial phase of the gasification process during the pyrolytic combustion stage [4,70,73]. Limiting the fugitive process is a major challenge for conventional gasifiers. Therefore, choosing the most appropriate gasification technology is quite restricted [43].
The beach wrack gasification was performed in a facility developed for pyrolytic decomposition of various wastes by applying thermal cracking of pyrolysis-derived products. Gasification is a process that implements the transformation of solid carbonaceous materials into gaseous products, as well as solid by-products such as biochar, ash, and tars [74]. Applied gasification design is conceptually divided into two thermal transformation processes: starting with thermal treatment of biomass into biochar at a lower temperature (torrefaction), which affects biomass properties to improve the quality of gasification outcomes, then followed by thermal treatment at a high temperature to gain syngas and to eliminate or reduce the formation of tars. The gasification facility consisted of an extruder-type gasifier (where the biomass is compacted in a continuous 42 mm diameter briquette blast pressed into a heated extruder using a hydraulic piston), a chamber for separation of gasification-derived products, and a thermal cracker for gaseous by-products, functioning in a semi-continuous operation mode due to periodical off-loading of by-produced biochar [43,44]. The process does not require an air or oxygen flow inlet but applies allothermal gasification using an external heat source. However, nitrogen flow is used to maintain process safety. The operating temperature of the extruder, where the primary reforming of beach wrack into syngas and biochar is carried out, can be set and automatically adjusted to the desired temperature from 300 to 600 °C. In the chamber for separation of pyrolysis-derived products, obtained syngas is separated from biochar, which is then collected in an airtight container and can be unloaded after cooling. Then, the gas is fed to the secondary high-temperature reformer, where the gas is heated to 800–1200 °C. At elevated temperatures, high-turbulence thermal cracking of tar occurs, but heavy organic gaseous substances are reformed into the syngas components such as H2, CO, CO2, and CH4 [74]. In the output, the gas is cooled and collected.
Obtained syngas from the applied gasification procedure for consolidated Baltic beach wrack samples contained the following gaseous compounds: 42% CH4, 34% CO2, 12% CO, 8% H2, 2% O2, and 2% other hydrocarbons. Generally, its composition can be comparable partly to syngas obtained from dry biomass and biogas obtained from wet biomass (Table 6) [39]. However, the particular biomass composition and character are of great importance.

Table 6.
Comparison of biogas and syngas composition depending on the conversion method [39].
Although the HHV for the beach wrack was estimated to be low, the produced syngas contained a promising amount of CH4, thus indicating syngas applicability for further use in heat production. Impure and tar-rich syngas can only be used as fuel for combustion in boilers, whereas high-purity syngas is usable as fuel for power and heat generation in internal combustion engine power plants; moreover, it can be utilized as a feedstock for chemicals and second-generation biofuels [72].
Around 1.36 m3 of syngas was generated from 1 kg of macroalgal biomass at an overall gasification efficiency of 70%. The syngas yield was limited due to the specifics of the beach wrack (particularly, high moisture content and sand admixture). The yield and composition of syngas depend on various factors such as the character of the biomass, the gasification agent, gasifier type, and operation procedure [5,73]. Depending on the feedstock character, the yield of CH4 may vary from 100 to 340 L from 1 kg of biomass [5,7].
The by-product of biomass gasification of economic value is biochar. The content of fixed carbon in beach wrack, if it exceeds 10%, can be considered high enough for the production of biochar. Generally, almost 50% of the total carbon in feedstock biomass may be retained as fixed carbon by biochar production [44]. A noteworthy indication is that biochar derived from the biomass as received can be assessed as a carbon-neutral product compared to fossil char [75]. The chemical composition of derived biochar (Figure 6 and Figure 7) indicated a considerably high concentration of the major element Ca (33,597.90 ± 20.29 mg/kg) compared to Ca content in the beach wrack. Other concentrations of major elements in decreasing sequence—K (21,221.56 ± 13.55 mg/kg), Na (10,168.73 ± 17.60 mg/kg), Mg (8059.73 ± 20.25 mg/kg), Fe (6156.03 ± 18.57 mg/kg), Mn (3948.72 ± 4.82 mg/kg), P (3281.32 ± 29.49 mg/kg), and Al (2689.43 ± 19.50 mg/kg)—corresponded to the total relevant concentration ranges of elements detected in the beach wrack. The concentration of minor elements in the biochar was the highest for Ni (171.13 ± 1.89 mg/kg), much above the total relevant concentration ranges of elements detected in the beach wrack. The reason for such a difference in Ni concentration could be related to technical equipment used for the gasification, e.g., a Ni-containing catalyst or char-receiving container [5]. Therefore, more profound research on biochar composition in relation to the biomass conversion procedure is needed. Minor elements in the biochar can be ranked by concentration from largest to smallest concentration, as follows: Sr, Ba, Ni, Zn, Ti, Cr, Cu, As, Co, Pb, Cd, Mo, and V. Regarding the elements of concern, potentially toxic elements in biochar can be detected at a higher concentration than in beach wrack if assessing mean values (Table 1), but they fit into the total relevant concentration range. Some elements, e.g., Cd, Cr, Mn, and Ni, exceeded the statistically relevant concentration range detected for beach wrack, affecting the biochar’s quality and further utility options [43]. Before biochar’s practical use, it is necessary to follow normative limitations for potentially toxic elements such as heavy metals. It is suggested that if pure biochar has limited application possibilities in agriculture, its utility can perform better in a mixture with other ingredients; furthermore, biochar can also be used for energy production purposes if it is not applicable to agricultural needs.
4. Conclusions
The research revealed the benefits and drawbacks of using beach wrack containing macroalgal biomass for gasification to obtain syngas as a primary product, and biochar as a by-product applicable as a prospective biofuel for further energy and heat production or other economic value. The main benefit is finding that Baltic beach wrack masses are relatively suitable for thermal conversion implemented by the gasification process. However, some obstacles such as moisture, mineral matter content, and excess of salts may adversely affect the quality and yield of out-coming products. Still, they can be overcome by optimizing the technology and feedstock preprocessing.
The main limiting factor to beach wrack’s successful utilization is the high amount of minerals, i.e., sand, present in macroalgal biomass if it is not subjected to the specific extra cleaning procedure before the gasification procedure. However, implementing such a step is cumbersome in cost-effective algal waste management. Another limiting factor is the very high moisture content. Both these factors adversely affect the calorific character of beach wrack if it is utilized as received. At the same time, the presence of municipal waste in macroalgal biomass was not assessed as a limiting obstacle.
The detected higher heating value for the Baltic Sea beach wrack was relatively low (5.38 MJ/kg AR or 14.70 MJ/kg DB), but it should be noted that it can be improved by combining various biomass and biowaste types. A promising amount of CH4 in the produced syngas suggests its further applicability in heat production.
Other concerns can be attributed to the chemical composition of beach wrack regarding the concentration of certain chemical elements; for example, a high content of alkali metals may adversely affect the process of gasification, but a high content of potentially toxic elements such as heavy metals may have a negative impact on the quality of the derived biochar, limiting its applicability potential in agriculture. Furthermore, the macroalgal biowaste of beach wrack is characterized by a relatively higher ash content than other biomasses due to a high concentration of inorganic compounds, which require the correct choice of conversion technology. The adjusted gasification process, i.e., the combination of thermochemical procedures such as mild combustion and pyrolytic conversion, might be a better choice.
It is suggested that further investigation on macroalgal biomass blended with other organic waste as a feedstock for thermochemical conversion is implemented to achieve a better calorific character and a higher biofuel yield. If adjusted to the character and properties of the initial biomass, gasification technologies can meet the market needs and contribute to zero waste management.
Author Contributions
Conceptualization, V.B. and J.B.; methodology, V.B., I.G., D.P. and K.-M.P.; software, M.A.I. and A.C.; validation, O.A., W.H., Y.J. and M.S.; formal analysis, E.H., M.S., E.S., R.H.S. and I.Z.; investigation, V.S., V.B., V.R., M.Z., J.P.-U. and H.A.A.; resources, M.K. (Maris Klavins), I.G. and M.W.; data curation, Z.V.-G., V.R. and A.E.K.; writing—original draft preparation, Z.V.-G., V.B. and J.B.; writing—review and editing, Z.V.-G., J.P.-U., A.C. and J.B.; visualization, Z.V.-G.; supervision, M.K. (Maris Klavins), M.K. (Mait Kriipsalu) and M.W.; project administration, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
J. Burlakovs acknowledges the support received from the PASIFIC program “GeoReco” project funding from the European Union’s Horizon 2020 research and innovation program within the Marie Sklodowska-Curie grant agreement No.847639 and the Ministry of Education and Science (Poland). The study was based upon the targets supported by COST (European Cooperation in Science and Technology) Action No.CA18238 (Ocean4Biotech). On behalf of I. Zekker, acknowledgments are addressed to the collaborative project “Identifying best available technologies for decentralized wastewater treatment and resource recovery for India (SARASWATI 2.0)”, co-funded by the European Commission within the Horizon 2020 Framework Programme (grant agreement No.821427) and from the Government of India (Department for Science and Technology), as well as project No.SLTKT20427 “Sewage sludge treatment from heavy metals, emerging pollutants and recovery of metals by fungi” and the University of Tartu Development fund PLTKT ARENG53. A.E. Krauklis acknowledges the support provided by project No.lzp-2021/1-0090 “CircleP” funded by the Latvian Council of Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; US EIA: Washington, DC, USA, 2016; 276p. [CrossRef]
- Van Asselt, H. Governing fossil fuel production in the age of climate disruption: Towards an international law of ‘leaving it in the ground’. Earth Syst. Gov. 2021, 9, 100118. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Ahmed, A.; Lam, S.S.; Lee, J.; Show, P.L.; Park, Y.-K. Sustainable valorization of algae biomass via thermochemical processing route: An overview. Bioresour. Technol. 2022, 344, 126399. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.B.; Chen, W.-H.; Ubando, A.T.; Park, Y.-K.; Lin, K.-Y.A.; Pugazhendhi, A.; Nguyen, T.-B.; Dong, C.-D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Lymperatou, A.; Engelsen, T.K.; Skiadas, I.V.; Gavala, H.N. Different pretreatments of beach-cast seaweed for biogas production. J. Clean. Prod. 2022, 362, 132277. [Google Scholar] [CrossRef]
- Milledge, J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Proced. 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Nandhini, R.; Berslin, D.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Thermochemical conversion of municipal solid waste into energy and hydrogen: A review. Environ. Chem. Lett. 2022, 20, 1645–1669. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Anaerobic digestion and gasification of seaweed. In Grand Challenges in Biology and Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 237–258. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.B.H.J.M.; Chew, K.W.; Munawaroh, H.S.H.; Shamsuddin, A.H.; Show, P.L. Algae as potential feedstock for various bioenergy production. Chemosphere 2022, 287, 131944. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Begum, R.A.; Abdolrasol, M.G.; Hossain Lipu, M.S.; Mohamed, A.; Rashid, M.M. Review of baseline studies on energy policies and indicators in Malaysia for future sustainable energy development. Renew. Sust. Energ. Rev. 2018, 94, 551–564. [Google Scholar] [CrossRef]
- Suursaar, Ü.; Torn, K.; Martin, G.; Herkül, K.; Tiit, K. Formation and species composition of stormcast beach wrack in the Gulf of Riga, Baltic Sea. Oceanologia 2014, 56, 673–695. [Google Scholar] [CrossRef]
- Chubarenko, B.; Woelfel, J.; Hofmann, J.; Aldag, S.; Beldowski, J.; Burlakovs, J.; Garrels, T.; Gorbunova, J.; Guizani, S.; Kupczyk, A.; et al. Converting beach wrack into a resource as a challenge for the Baltic Sea (an overview). Ocean Coast. Manag. 2021, 200, 105413. [Google Scholar] [CrossRef]
- Pardilhó, S.; Boaventura, R.; Almeida, M.; Dias, J.M. Marine macroalgae waste: A potential feedstock for biogas production. J. Environ. Manag. 2022, 304, 114309. [Google Scholar] [CrossRef]
- Esiukova, E. Plastic pollution on the Baltic beaches of Kaliningrad region, Russia. Mar. Pollut. Bull. 2017, 114, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Haseler, M.; Weder, C.; Buschbeck, L.; Wesnigk, S.; Schernewski, G. Cost-effective monitoring of large micro- and meso-litter in tidal and flood accumulation zones at south-western Baltic Sea beaches. Mar. Pollut. Bull. 2019, 149, 110544. [Google Scholar] [CrossRef] [PubMed]
- Fetisov, S.; Chubarenko, I. Marine litter stormy wash-outs: Developing the neural network to predict them. Pollutants 2021, 1, 156–168. [Google Scholar] [CrossRef]
- Risén, E.; Nordström, J.; Malmström, M.E.; Gröndahl, F. Non-market values of algae beach-cast management—Study site Trelleborg, Sweden. Ocean Coast. Manag. 2017, 140, 59–67. [Google Scholar] [CrossRef]
- Takolander, A.; Cabeza, M.; Leskinen, E. Climate change can cause complex responses in Baltic Sea macroalgae: A systematic review. J. Sea Res. 2017, 123, 16–29. [Google Scholar] [CrossRef]
- Liiv, J.; Zekker, I.; Tämm, K.; Rikmann, E. Greenhouse Gases Emissions and Climate Change—Beyond Mainstream. MOJ Bioorg. Org. Chem. 2020, 4, 10–16. Available online: https://medcraveonline.com/MOJBOC/MOJBOC-04-00104.pdf (accessed on 3 August 2022).
- Vigouroux, G.; Destouni, G. Gap identification in coastal eutrophication research—Scoping review for the Baltic system case. Sci. Total Environ. 2022, 839, 156240. [Google Scholar] [CrossRef] [PubMed]
- Franzén, D.; Infantes, E.; Gröndahl, F. Beach-cast as biofertiliser in the Baltic Sea region-potential limitations due to cadmium-content. Ocean Coast. Manag. 2019, 169, 20–26. [Google Scholar] [CrossRef]
- Domnin, D.; Chubarenko, B.; Grave, A. Baseline assessment of beach cast appearance in the South-Eastern Baltic by video monitoring at a pilot site in the Kaliningrad Oblast (Russia). Mar. Pollut. Bull. 2021, 173, 112994. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, Y.; Sukhikh, S.; Kalashnikova, O.; Chupakhin, E.; Ivanova, I.; Chubarenko, B.; Gorbunova, J.; Babich, O. Assessment of the resource potential of Baltic Sea macroalgae. Appl. Sci. 2022, 12, 3599. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Trevathan-Tackett, S.M.; Baldock, J.A.; Kelleway, J.J. Converting beach-cast sea-grass wrack into biochar: A climate-friendly solution to a coastal problem. Sci. Total Environ. 2017, 574, 90–94. [Google Scholar] [CrossRef]
- Kupczyk, A.; Kołecka, K.; Gajewska, M. Solving the beach wrack problems by on-site treatment with reed beds towards fertilizer amendments. J. Ecol. Eng. 2019, 20, 252–261. [Google Scholar] [CrossRef]
- Liu, S.; Trevathan-Tackett, S.M.; Ewers Lewis, C.J.; Ollivier, Q.R.; Jiang, Z.; Huang, X.; Macreadie, P.I. Beach-cast sea-grass wrack contributes substantially to global greenhouse gas emissions. J. Environ. Manag. 2019, 231, 329–335. [Google Scholar] [CrossRef]
- Malm, T.; Råberg, S.; Fell, S.; Carlsson, P. Effects of beach cast cleaning on beach quality, microbial food web, and littoral macrofaunal biodiversity. Estuar. Coast. Shelf Sci. 2004, 60, 339–347. [Google Scholar] [CrossRef]
- Eklund, B.; Svensson, A.P.; Jonsson, C.; Malm, T. Toxic effects of decomposing red algae on littoral organisms. Estuar. Coast. Shelf Sci. 2005, 62, 621–626. [Google Scholar] [CrossRef]
- Inácio, M.; Karnauskaitė, D.; Baltranaitė, E.; Kalinauskas, M.; Bogdzevič, K.; Gomes, E.; Pereira, P. Ecosystem services of the Baltic Sea: An assessment and mapping perspective. Geogr. Sustain. 2020, 1, 256–265. [Google Scholar] [CrossRef]
- Heckwolf, M.J.; Peterson, A.; Jänes, H.; Horne, P.; Künne, J.; Liversage, K.; Sajeva, M.; Reusch, T.B.H.; Kott, J. From ecosystems to socio-economic benefits: A systematic review of coastal ecosystem services in the Baltic Sea. Sci. Total Environ. 2021, 755, 142565. [Google Scholar] [CrossRef] [PubMed]
- Bucholc, K.; Szymczak-Żyła, M.; Lubecki, L.; Zamojska, A.; Hapter, P.; Tjernström, E.; Kowalewska, G. Nutrient content in macrophyta collected from southern Baltic Sea beaches in relation to eutrophication and biogas production. Sci. Total Environ. 2014, 473–474, 298–307. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sust. Energ. Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Ebadi, A.G.; Hisoriev, H. Bio-Oil Production from Fast Pyrolysis of Cladophora glomerata in a Fluidized Bed Reactor. Bulg. Chem. Commun. 2017, 49, 504–508. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_49_Number_2_2017/49-2-2017-Ebadi-504-508.pdf (accessed on 3 August 2022).
- Ardolino, F.; Arena, U. Biowaste-to-biomethane: An LCA study on biogas and syngas roads. Waste Manag. 2019, 87, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Bolívar Caballero, J.J.; Zaini, I.N.; Yang, W. Reforming processes for syngas production: A mini-review on the current status, challenges, and prospects for biomass conversion to fuels. Appl. Energy Combust. Sci. 2022, 10, 100064. [Google Scholar] [CrossRef]
- Indrawan, N.; Kumar, A.; Moliere, M.; Sallam, K.A.; Huhnke, R.L. Distributed power generation via gasification of biomass and municipal solid waste: A review. J. Energy Inst. 2020, 93, 2293–2313. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Klavins, M.; Bisters, V.; Burlakovs, J. Small scale gasification application and perspectives in circular economy. Environ. Clim. Technol. 2018, 22, 42–54. [Google Scholar] [CrossRef]
- Katrantsiotis, C.; Sachpazidou, V.; Ibrahim, A.; Bisters, V.; Burlakovs, J.; Hogland, W. Case Study 5: The Baltic Beach Wrack Thermal Recovery and Relevant Analytical Performances (ALREA). In Case Studies for Innovative Solutions of Beach Wrack Use: Report of the Interreg Project CONTRA; Chubarenko, B., Schubert, H., Woelfel, J., Eds.; Interreg Baltic Sea Region Project CONTRA: Rostock, Germany, 2021; pp. 52–62. Available online: https://www.beachwrack-contra.eu/wp-content/uploads/2021/05/CONTRA-Output05-WEB.pdf (accessed on 3 August 2022).
- Sheth, P.N.; Babu, B.V. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresour. Technol. 2009, 100, 3127–3133. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. The trends in research on the effects of biochar on soil. Sustainability 2020, 12, 7810. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; de Nys, R. Algal biochar—Production and properties. Bioresour. Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef]
- Galhetas, M.; Lopes, H.; Freire, M.; Abelha, P.; Pinto, F.; Gulyurtlu, I. Characterization, leachability and valorization through combustion of residual chars from gasification of coals with pine. Waste Manag. 2012, 32, 769–779. [Google Scholar] [CrossRef]
- García-García, A.; Gregório, A.; Franco, C.; Pinto, F.; Boavida, D.; Gulyurtlu, I. Unconverted chars obtained during biomass gasification on a pilot-scale gasifier as a source of activated carbon production. Bioresour. Technol. 2003, 88, 27–32. [Google Scholar] [CrossRef]
- Boateng, A.A.; Cooke, P.H.; Hicks, K.B. Microstructure development of chars derived from high-temperature pyrolysis of barley (Hordeum vulgare L.) hulls. Fuel 2007, 86, 735–742. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Sánchez, M.E.; Mora, M.; Barrón, V. Slow pyrolysis of relevant biomasses in the Mediterranean basin. Part 2. Char characterisation for carbon sequestration and agricultural uses. J. Clean. Prod. 2016, 120, 191–197. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Hernández, J.J.; Ballesteros, R.; Aranda, G. Characterisation of tars from biomass gasification: Effect of the operating conditions. Energy 2013, 50, 333–342. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Stankevica, K. Impact of micro- and macroelement content on potential use of freshwater sediments (gyttja) derived from lakes of eastern Latvia. Environ. Geochem. Health 2018, 40, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Jagustyn, B.; Kmiec, M.; Smedowski, L.; Sajdak, M. The content and emission factors of heavy metals in biomass used for energy purposes in the context of the requirements of international standards. J. Energy Inst. 2017, 90, 704–714. [Google Scholar] [CrossRef]
- ISO 18134-1:2015; Solid Biofuels. Determination of Moisture Content. Oven Dry Method. Part 1: Total Moisture. ISO: Geneva, Switzerland, 2015; 5p.
- ISO 18122:2015; Solid Biofuels. Determination of Ash Content. ISO: Geneva, Switzerland, 2015; 6p.
- ISO 18123:2015; Solid Biofuels. Determination of the Content of Volatile Matter. ISO: Geneva, Switzerland, 2015; 8p.
- ISO 17828:2015; Solid Biofuels. Determination of Bulk Density. ISO: Geneva, Switzerland, 2015; 8p.
- Montero, G.; Coronado, M.A.; Torres, R.; Jaramillo, B.E.; García, C.; Stoytcheva, M.; Valenzuela, E. Higher heating value determination of wheat straw from Baja California, Mexico. Energy 2016, 109, 612–619. [Google Scholar] [CrossRef]
- ISO 16948:2015; Solid Biofuels. Determination of Total Content of Carbon, Hydrogen and Nitrogen. ISO: Geneva, Switzerland, 2015; 20p.
- ISO 16994:2016; Solid Biofuels. Determination of Total Content of Sulfur and Chlorine. ISO: Geneva, Switzerland, 2016; 11p.
- ISO 16993:2016; Solid Biofuels. Conversion of Analytical Results from One Basis to Another. ISO: Geneva, Switzerland, 2016; 10p.
- ISO 18125:2017; Solid Biofuels. Determination of Calorific Value. ISO: Geneva, Switzerland, 2017; 56p.
- Burlakovs, J. Contamination Remediation with Soil Amendments by Immobilization of Heavy Metals. Ph.D. Thesis, University of Latvia, Riga, Latvia, 2015; 146p. [Google Scholar]
- Greger, M.; Malm, T.; Kautsky, L. Heavy metal transfer from composted macroalgae to crops. Eur. J. Agron. 2007, 26, 257–265. [Google Scholar] [CrossRef]
- Magalhaes, I.B.; de Paula Pereira, A.S.A.; Silva, T.A.; dos Santos Renato, N. Predicting the higher heating value of microalgae biomass based on proximate and ultimate analysis. Algal Res. 2022, 64, 102677. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Salam, P.A. Estimation of higher heating value of biomass from proximate analysis: A new approach. Fuel 2012, 99, 55–63. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of higher heating value (HHV) based on the structural composition for biomass. Fuel 2021, 299, 120860. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Amadeo, N.; Laborde, M.; Alemany, L.J. Hydrogen-rich gas production from algae-biomass by low temperature catalytic gasification. Catal. Today 2015, 257 Pt 2, 177–184. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Larina, O.M.; Sytchev, G.A. Manufacturing gaseous products by pyrolysis of microalgal biomass. Int. J. Hydrogen Energy 2020, 45, 1569–1577. [Google Scholar] [CrossRef]
- Tezer, O.; Karabag, N.; Ongen, A.; Colpan, C.O.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Garrels, T. Case Study 2: Bio-Coal from Beach Wrack (BWC). In Case Studies for Innovative Solutions of Beach Wrack Use: Report of the Interreg Project CONTRA; Chubarenko, B., Schubert, H., Woelfel, J., Eds.; Interreg Baltic Sea Region Project CONTRA: Rostock, Germany, 2021; pp. 16–27. Available online: https://www.beachwrack-contra.eu/wp-content/uploads/2021/05/CONTRA-Output05-WEB.pdf (accessed on 3 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).