Abstract
Sugarcane grown under a wide range of agro-climatic conditions accounts for ~80% of the sugar production worldwide. Since sugarcane productivity is severely affected by abiotic stresses and hence, an experiment was conducted for two consecutive years during 2020 and 2021 on popular sub-tropical sugarcane varieties. The experiment was laid out in two-factorial RBD consisting of nine sugarcane genotypes (Co 98014, Co 0118, Co 0238, Co 05011, Co 06034, Co 09022, Co 12029, Co 15023 and Co 15027) and salinity treatments (Control, ECiw ~ 4, 8 and 12 dS m−1) in 5 replications. Two budded setts were planted in pots and irrigated with saline water of respective levels till formative phase and observed the build-up in electrical conductivity of soil extract (ECse) from 0.48 (control) to 2.99, 4.81 and 7.08; while further saline irrigation increased the ECse values to 4.48, 6.24 and 9.33 dS m−1 in treatments ECiw ~ 4, 8 and 12 dS m−1, respectively. Increase in soil EC decreased plant survival by 24.1, 47.0 and 79.6% under continued irrigation of ECiw ~ 4, 8, 12 dS m−1 with respect to control. Continued saline irrigation caused significant reduction in growth, which was associated with reduction in relative water content (RWC) and gas exchange traits. RWC decreased by 4.91 to 21.9%, chlorophyll content by 8.46 to 32.75%, photosynthetic rate (Pn) by 16.85 to 91.44%, stomatal conductance by 14.96 to 84.25%, transpiration rate by 14.13% to 89.8% and chlorophyll fluorescence by 5.33 to 42.67% from ECiw ~ 4 to 12 dS m−1, respectively. Significant variations in Na+ and K+ ion content was observed under elevated saline condition in roots, leaves and juice extract of genotypes. Na+/K+ ratio, an important trait for screening salinity tolerance, increased in all genotypes as compared to control, the increase was predominant in susceptible varieties. Single cane weight (SCW) was drastically affected by saline irrigation, with a reduction of 36.4, 68.5 and 83.5% at ECiw ~ 4, 8 and 12 dS m−1, respectively as compared to control, with similar declining trend in juice quality. Based on our results, Co 0238, Co 0118 and Co 98014 were tolerant to salinity stress by maintaining higher Pn, lower leaf Na+/K+ ratio, higher SCW and higher juice sucrose content.
1. Introduction
Sugarcane (Saccharum officinarum L.) is an important cash crop cultivated for sugar and bio-energy production worldwide. India is the second largest producer and consumer of sugarcane with cultivation over 5.06 M ha with an average productivity of 80.10 t ha−1 [1,2]. Recent years have seen a rapid development in the cane and sugar yield in Indian sugarcane cultivation. Estimates revealed that by the year 2030, India will require a productivity of 110 t ha−1 with average sugar recovery of 10.75% to match the production demand [3]. Despite being a C4 crop, it does well in tropical environments and is grown all over the world in a variety of tropical and subtropical climates. Climate change induced frequent occurrence of abiotic stresses particularly drought, cold and salinity hampers growth, productivity, and juice quality of sugarcane. Natural and anthropological factors, drainage problems and increasing use of marginal quality water is further accelerating the process of soil salinization with an estimated projection of nearly 10 M ha of fertile land succumbing to salinization every year [4]. In India, if the current trend continues unabated, nearly 16.2 M ha area will be prone to salinity stress by the year 2050 [5]. Increasing soil salinity and poor-quality irrigation water coupled with moisture stress during high water demand period (formative phase), largely coinciding with grand growth phase resulted in low cane yields.
Sugarcane is considered as highly salt sensitive and earlier studies on sugarcane reported that EC of soil greater than 1.7 dS m−1 at critical growth stages significantly hastens cane length, girth, NMC and yield [6], every unit increase beyond 1.7 dS m−1 limits yield to the tune of 5.9% [7]. Whereas salt water of >8 dS m−1 caused significant reduction in growth and physiological traits, hampering yield by 50% [8,9]. In sugarcane, salinity at critical stages particularly at formative phase which is high water demanding stage (550 mm water) leads to physiological disorders due to the increase of toxic salts in the root zone, reducing the osmotic potential of the soil and water uptake [10,11], which consequently affects its normal physiology and entire metabolic balance even at cellular levels through osmotic and ionic adjustments [12,13,14]. Reclamation of saline soils is time consuming and tedious due to the expanding area under salinity and scarcity of good quality water. Keeping these facts in mind, identification of moderately tolerant/tolerant sugarcane varieties would greatly help to reduce the loss in cane productivity under marginal saline soils. ICAR-Sugarcane Breeding Institute, Coimbatore has developed several promising sugarcane genotypes that perform well under harsh conditions with sustained growth, physiological traits and ionic balance. The present study was aimed to evaluate nine promising sub-tropical sugarcane varieties under increasing salinity stress regimes to identify tolerant genotypes that might break the existing plateau of cane productivity and counteract salinization effects by improving productivity of marginal lands.
2. Materials and Methods
An experiment was conducted at ICAR-Sugarcane Breeding Institute, Regional Center, Karnal during 2020 and 2021 to evaluate salinity tolerance in nine promising sub-tropical sugarcane viz., Co 98014, Co 0118, Co 0238, Co 05011, Co 06034, Co 09022, Co 12029, Co 15023 and Co 15027 in randomized complete block design with five replications. The two-budded setts (3 each in single pot) were planted during first fortnight of April and allowed to germinate by providing normal irrigation water during the first month at an interval of 7 to 10 days. Porcelain pots were filled with 40 kg soil (field capacity 28% v/v) of ECe ~ 0.45 dS m−1 collected from experimental farm, SBI-RC, Karnal having bulk density of 1.45 g/cc and porosity approximately 39% (Table 1). Initially ~13.2 litre water (up to field capacity) was given in the pots and evaporation was noted through pan. Afterwards 7.5 L water given at weekly interval during summer season and fortnight interval during winter season. During the entire study average period pan evaporation was 3 mm day−1, i.e., 21 mm week−1. After germination, salinity stress was imposed in the pots continuously till harvest by irrigating with saline water (Chloride dominated water made by mixing NaCl, Na2SO4, CaCl2, MgCl2) of ECiw ~ 4, 8 and 12 dS m−1 and good quality water (ECiw ~ 0.43 dS m−1) to serve as control. Soil sampling and leachate collection was carried out before sowing, at formative phase and after crop harvest to record the build-up of salts in terms of ECe.

Table 1.
Physico-chemical properties of the soil before conducting the experiment.
Different physiological traits at formative stage as well as cane length (with measuring tape) and yield were recorded at harvest to evaluate the performance of different genotypes. Top fully expanded leaf with visible dewlap (TVD) was sampled for recording the physiological traits such as chlorophyll content (using chlorophyll multi-meter) and gas exchange traits. Randomly tagged TVD leaves were used to record gas exchange attributes such as photosynthetic rate (Pn), stomatal conductance (gS) and transpiration rate (Tr) using Infra-Red Gas Analyzer (LICOR 6800), relative water content (RWC) [15] and chlorophyll a fluorescence (Fv/Fm). For analysing Na+ and K+ content in different plant parts, samples were collected at formative phase and oven dried at 60 °C for 72 hrs or till attaining constant weight. 100 mg of oven dried and well ground plant material (roots and leaves) and 10 mL of juice extract was digested with 10 mL of HNO3:HClO4 (3:1) di-acid mixture. The digested residue (1 mL) was diluted to 50 mL with double distilled water and readings were taken with AAS (Systronics Flame Photometer 128). Recorded observations structured thematically and entered into the spread sheet (Microsoft office-excel). Before analysis observations under each variable were tested for normality (Q-Q plot of residuals) through Shapiro-Wilk (W) test. Violated variables were transformed through appropriate transformation method. Two-way ANOVA was used to dissect the genotype (G), salinity (S) and G x S effects on each variable using the General Linear Model in SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA). Multiple comparison analysis was performed using Tukey’s HSD test to determine the significant differences between genotypes or treatments at 5% level of significance. Important morpho-physiological traits were prioritized in sugarcane genotypes, through traits modeling using stepwise regression (backward elimination) approach in STAR statistical software [16]. Pearson correlation coefficients were estimated to determine the association between combined data across the gradient salinity stress morphological and physiological traits.
3. Results
3.1. Build-Up of Salinity in Soil
Continued irrigation with saline water of ECiw ~ 4, 8 and 12 dS m−1 resulted in the build-up of soil ECe compared to the initial soil ECe (0.36 dS m−1) (Figure 1), that shows increasing irrigation water salinity caused higher deposition of toxic ions at formative phase, which is one of the most critical stage for sugarcane growth. ECe values (dS m−1) increased from 0.48 (control) to 2.99, 4.81 and 7.08 with irrigation of 4, 8 and 12 dS m−1, respectively. Further irrigations increased ECe values to 4.48, 6.24 and 9.33 dS m−1 at under ECiw ~ 4, 8 and 12, respectively (Figure 1) at harvest. It was also observed that 12 irrigations with saline water showed injurious symptoms in most of the tested genotypes (Figure 2).
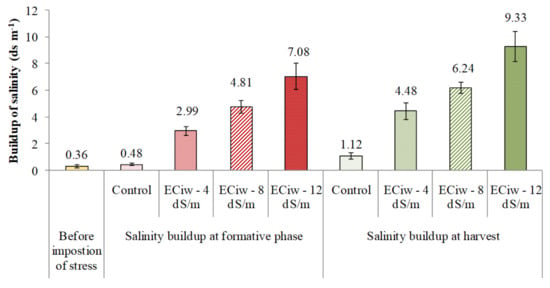
Figure 1.
Effect of irrigation water salinity on soil ECe at formative stage and after harvest.
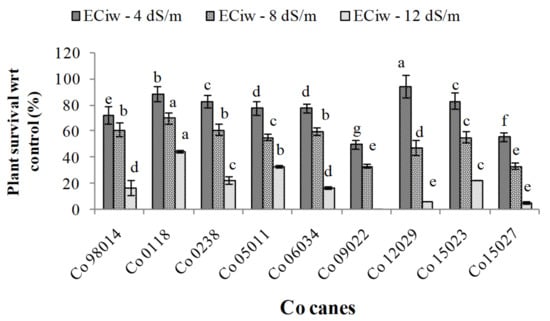
Figure 2.
Effect of irrigation water salinity on genotypes survival rate (%). [Different alphabets denote they are significantly different compared to others].
3.2. Plant Survival under Different Levels of Salinity
In sugarcane, higher stress at formative stage severely affected survival rate (compared to initial germination rate) and plant growth. The results noted (Figure 2) in genotypes showed 75.9%, 53.0% and 20.4% mean germination percent under continued irrigation of 4, 8, 12 dS m−1, respectively (Figure 2). Among the genotypes, Co 0118 and Co 05011 showed more than 33.3% survival at ECiw ~ 12 dS m−1, whereas Co 12029 and Co 15027 showed only 5% survival and Co 09022 didn’t survive (Figure 2). Therefore, stress intensity and timing play a crucial role in survival and plant growth. The effects of salinity stress on plant phenotype were characterized by stunted slow growth, leaf area reduction and decrease in associated physiological traits especially gas exchange attributes along with RWC. The present results showed statistically significant variation for cane length and physiological traits among different treatments and genotypes (Table 1). Two-way ANOVA revealed that mean cane length was 184.57 cm under control conditions, which decreased by 26.9%, 48.7% and 59.3% under continued irrigation of 4, 8, 12 dS m−1, respectively.
3.3. Effect of Salinity on Cane Length and RWC
Among different genotypes, maximum cane length was observed for Co 98014 (153.58 cm) followed by Co 06034 (141.0 cm), Co 0118 (131.67 cm) and Co 0238 (131.21 cm). It was also observed that at highest level of salinity (ECiw–12 dS m−1), higher decrease was noted in Co 15023 (72.16%) and Co 06034 (67.72%), whereas Co 0238 and Co 0118 showed the least decrease of less than 50%. Reduction in growth is mostly associated with alterations in plant water status and gas exchange traits. Present investigation also signifies the same results for RWC, which decreased due to increasing salinity showing 4.91%, 14.93% and 21.90% reduction in RWC under continued irrigation of 4, 8, 12 dS m−1, respectively (Table 2). Significant variability was noted among genotypes, with varietal mean of RWC ranging from 72.8% to 78.89%. The highest RWC was observed in Co 12029 and lowest in Co 0118 (Table 2), with least reduction of 3.0%, 8.0% and 14.2% under ECiw ~ 4, 8, 12 dS m−1, respectively, was observed in the latter, whereas Co 15027 and Co 06034 showed the highest reductions compared to control.

Table 2.
Effect of irrigation water salinity on morpho-physiological traits in sub-tropical sugarcane varieties.
3.4. Effect of Salinity on Gas Exchange Attributes
Similar to RWC, gas exchange traits showed significant decline due to increasing salinity. Chlorophyll, an important molecule that participates in photosynthetic process declined by 8.46%, 21.73% and 32.75% under 4, 8 and 12 dS m−1, respectively (Table 2). Co 0238 retained higher chlorophyll (29.67 µg/cm2) than other genotypes. Among the genotypes, Co 12029 and Co 15027 showed maximum reduction of 17.71%, 32.30%, 55.06% and 19.83%, 44.5%, 57.21% under 4, 8 and 12 dS m−1, respectively, while Co 0118 showed the lowest. Photosynthetic rate showed drastic reduction of 16.85%, 54.93% and 91.44% with 4, 8 and 12 dS m−1 (Table 2). Genotypes showed significant variability for Pn, being highest in Co 0238 (15.11 µmol m−2 s−1) and lowest in Co 15023 (12.8 µmol m−2 s−1) and Co 15027 (12.76 µmol m−2 s−1). Stomatal conductance was 0.127 mmol m−2 s−1 at control, while salinity stress led to 14.96%, 55.91% and 84.25% reduction in gS at 4, 8 and 12 dS m−1, respectively, in comparison to control. Under highest salinity of ECiw ~ 12 dS m−1, Co 0238, Co 98014 and Co 0118 showed higher stomatal conductance more than 0.031 mmol H2O m−2 s−1 (Table 2). Similarly, salinity stress led to 14.13%, 55.43% and 89.8% reduction in the transpiration rate at ECiw ~ 4, 8 and 12 dS m−1, respectively, in comparison to control. Under highest salinity of ECiw ~ 12 dS m−1, Co 0238 and Co 98014 showed higher transpiration rate of 0.95 mmol H2O m−2 s−1 (Table 2). Another important trait, photochemical efficiency (Fv/Fm) measured from chlorophyll a fluorescence to study the effect of salinity stress being reduced by 5.33%, 17.33% and 42.67% at ECiw ~ 4, 8 and 12 dS m−1, respectively, in comparison to control (Table 2). All genotypes showed statistically at par values for Fv/Fm except Co 06034. The results revealed that saline irrigation of ECiw ~ 12 dS m−1 caused drastic reduction in the observed gas exchange traits.
3.5. Effect of Salinity on Ion Dynamics
Another important aspect of salinity stress is the ionic toxicity or accumulation of toxic ions. The present study deals with salt dynamics of Na+ and K+ ions in roots, leaves and juice extract (Table 3), wherein it was observed that leaves retained maximum Na+ (1.81%) in comparison to roots (0.18%) and juice extract (0.31%). Salinity stress enhanced Na+ in roots, leaves and juice. Roots of Co 15027 (0.22%) had the highest Na+ accumulation followed by Co 12029 (0.21%), while lowest value was noted in Co 98014 (0.15%). Similar to roots, genotypes leaves showed statistically significant variability for leaf Na+, being lowest in Co 06034 (1.35%) and highest in Co 15027 (2.68%). genotypes leaves had mean Na+ of 0.97% under control condition which increased to 1.43% under ECiw ~ 4 dS m−1, 1.98% under ECiw ~ 8 dS m−1 and 2.88% under ECiw ~ 12 dS m−1 (Table 2). Na+ accumulation also increased in juice extract by two to eight-fold with increasing salinity stress (Table 3). Juice extract of Co 06034 had highest Na+ (0.61%) and lowest in Co 98014 (0.17%). Antagonistic to Na+, K+ displayed decreasing trends in roots, leaves and juice extract. Roots K+ decreased by 23.4% to 68.75%, leaves K+ by 29.0% to 68.1% and juice K+ by 25.7% to 59.3% in the tested varieties (Table 3). Non-significant differences were noted for root K+ among genotypes. Leaf K+ had significant variability with maximum accumulation in Co 0238 (3.98%) followed by Co 05011 (2.64%) and Co 0118 (2.57%), while minimum in Co 12029 (1.51%) and Co 09022 (1.57%).

Table 3.
Effect of irrigation water salinity on Na+ (%DW) and K+ (%DW) content of roots, leaves and juice in genotypes.
With regard to juice extract, Co 0238 (2.38%) and Co 98014 (2.33%) showed highest K+, while lowest (1.25%) was observed in Co 12029. Na+/K+ ratio an important indicator of salinity stress increased with increase in salinity, predominant increase was noticed in the leaf tissues (Figure 3). Roots represented mean Na+/K+ of 0.20 under control condition which increased to 0.43, 0.90 and 1.34 with progressive increase of salinity and genotypes displayed mean Na+/K+ ratio of 0.50 to 1.43, being lowest in Co 0238 and highest in Co 09022. Leaves had the highest values for Na+/K+ ratio comprising roots and juice (Figure 3). Under control condition, mean leaf Na+/K+ was 0.30, which increased by 2.2, 4.07 and 10.5 folds under ECiw ~ 4, 8 and 12 dS m−1, respectively. Among genotypes, Co 0238 had the lowest leaf Na+/K+ of 0.59, while Co 05011, Co 0118, Co 98014 and Co 06034 displayed Na+/K+ equivalent to 1.0 and maximum Na+/K+ of more than 2.0 in Co 15027 and Co 12029 (Figure 3). It was interesting to note that juice extract had minimum Na+/K+ (0.03–0.67), being maximum in Co 06034 and minimum in Co 0238.
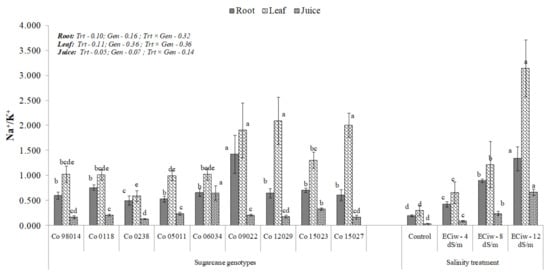
Figure 3.
Na+/K+ dynamics in root, leaves and juice under salinity stress in genotypes. [Different alphabets denote they are significantly different compared to others].
3.6. Effect of Salinity on SCW
Single cane weight (SCW) was drastically affected by continued saline irrigation, with 36.4%, 68.5% and 83.5% mean reduction in SCW at ECiw ~ 4, 8 and 12 dS m−1, respectively, in comparison to control (Table 4). Under control conditions, SCW varied from 0.399 g plant−1 (Co 98014) to 0.765 g plant−1 (Co 15027) with mean value of 0.552 g plant−1. Continued irrigation of ECiw ~ 4 dS m−1 reduced cane weight by 12.0% (Co 06034) to 62.4% (Co 15023), ECiw ~ 8 dS m−1 by 55.1% (Co 98014)–83.2% (Co 15023) and ECiw ~ 12 dS m−1 caused reduction of 61.2% (Co 98014)–89.4% (Co 15023), while Co 09022 didn’t show growth (Table 4). These results suggested that continued irrigation of ECiw ~ 12 dS m−1 was not appropriate for sugarcane production as it caused average reduction of 83.5% in SCW among the tested genotypes.

Table 4.
Effect of irrigation water salinity on single cane weight (g/plant) in genotypes.
3.7. Effect of Salinity on Sucrose Content
Sucrose content in juice, measured as Pol (%), declined with increasing salinity stress, with a reduction of 4.19%, 6.28% and 8.90% under irrigation water salinity of ECiw ~ 4, 8 and 12 dS m−1, respectively, in comparison to control (Figure 4A). Genotypes also displayed significant variation in mean sucrose content and higher value for sucrose content were recorded for Co 0118 and Co 0238; while Co 12027 showed lowest sucrose content. Figure 4B represented the data on per cent reduction among different genotypes at different salinity regimes and noted reductions in the range of 0.47% (Co 98014)–6.18% (Co 0238) at ECiw ~ 4 dS m−1, 2.50% (Co 98014)–9.82% (Co 12029) at ECiw ~ 8 dS m−1 and 4.58% (Co 09022)–13.69% (Co 05011) at ECiw ~ 12 dS m−1.
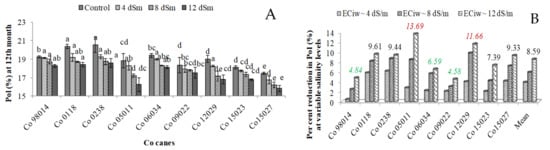
Figure 4.
Effect of irrigation water salinity on pol (%) in genotypes (A) and (B) showed per cent reduction in pol (%) at variable irrigation water salinity in genotypes (the values given in figure representing percent reduction at ECiw ~ 12 dS m−1).
Correlation was drawn to analyze the association of different traits with cane weight under saline environment and found that all traits showed significant positive correlation with cane weight except Na+ and Na+/K+ in different plant parts (Figure 5). Highest significant positive correlation was noted with gas exchange traits that have significant role in contributing yield.
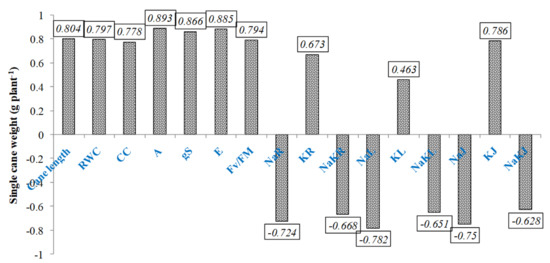
Figure 5.
Correlation of SCW with other studied traits under saline irrigation. RWC—Relative water content, CC-Chlorophyll content; Pn—net photosynthetic rate; gS—Stomatal conductance; E-Transpiration rate; Fv/Fm—Chlorophyll fluorescence; NaR—Root sodium content; KR—Root potassium content; NaKR—Root Na/K; NaL—Leaf sodium content; KL—Leaf potassium content; NaKL—Leaf Na/K; NaJ—Juice sodium content; KJ—Juice potassium content; NaKJ—Juice Na/K.
3.8. Biplot Analysis Showing Differential Response of Sugarcane Varieties
Different association matrix was developed to identify salt tolerance in varieties (Figure 6) and found that based on association of two important yield governing traits (Pn and SCW), genotypes Co 12029, Co 0238 and Co 0118 were found salt tolerant that maintained higher Pn with high SCW (Figure 6A). While Figure 6B showed association between SCW and leaves Na+/K+ and based on these two traits, Co 0238, Co 0118, Co 98014 and Co 06034 that had higher cane weight with low leaf Na+/K+ (Figure 6B). Other traits based on quality, i.e., sucrose content (Pol %) and juice Na+/K+ revealed five genotypes, i.e., Co 0238, Co 0118, Co 98014, Co 09022 and Co 12029 showed better tolerance than others (Figure 6C).
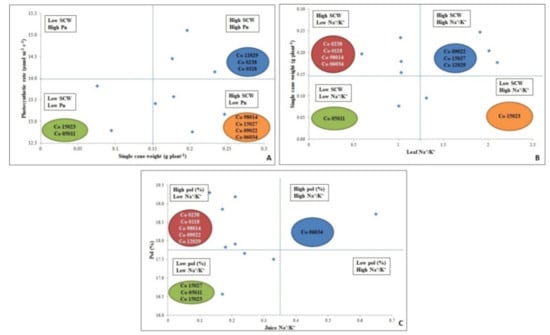
Figure 6.
Association among different traits to identify salt tolerance in different genotypes; (A) Pn vs. SCW; (B) SCW vs. Leaf Na+/K+ and (C) Pol(%) vs. Juice Na+/K+.
3.9. Traits Modeling for Cane Yield under Salinity Stress
To select the model physiological traits contributing to cane yield at higher salinity stress (~9ECe), a stepwise regression approach was applied. Physiological traits that played a significant role in inducing variations in the cane yield of sugarcane genotypes were selected through all possible regression analysis (Supplementary Table S1). The results of regression analysis indicated that 6 traits (Pn, E, NaR, NaJ, KJ and Juice Na/K) were able to induce significant variation in the yield. Therefore, these traits were removed and further stepwise regression analysis was done (backward selection). Analytical results of traits modelling indicated that Pn, E, NaR, NaJ, KJ and Na/KJ contributed significantly to cane yield of sugarcane under salinity stress. The remaining traits, i.e., CCS, gS, Fv/Fm, SL, KR, Na/KR, NaL, KL, Na/KL and RWC had non-significant contribution and therefore, were eliminated during stepwise regression (Supplementary Table S1). The results of Stepwise regression indicated that Pn, E, NaR, NaJ, KJ and Na/KJ with cumulative R2 = 80.85 contributed significantly to total variation in cane yield (Supplementary Table S2). Based on regression coefficients value of component traits, the following equation for the prediction of cane yield under salt stress was computed (Supplementary Table S3).
Model Fitted: SCW ~−23.38+ 0.86 Pn + 2.84 E+ 4.62 NaR + (−21.67) NaJ + 12.85 KJ + 20.90 NaKJ.
This model could be best fit for the estimation of cane yield, because it reflects the smallest Mallows’ Cp criterion. In addition, ranks were given to each sugarcane genotype based on predicted yield. Three genotypes, Co 0238, Co15027 and Co 0118 had relatively higher ranking and were identified as tolerant under salt stress (Supplementary Table S4). Conversely, genotypes Co 05011, Co 06034 and Co 15023 had relatively lower ranking and thus, were found as sensitive to salt stress. The salt tolerant genotypes identified based on predictor traits of this model were similar to those which were identified based on actual cane yield.
4. Discussion
Higher concentration of salts in the soil adversely affects the growth and development of plants. It is generally believed that salt tolerance potential differs inherently from crop to crop and genotype to genotype, as well as the stage of plant growth, stress duration, as higher salt uptake inhibits growth and various physico-biochemical processes [17,18,19,20]. During the initial phase, saline irrigation imposed osmotic stress in plants by restricting water absorption which hampered most of the physiological processes and later on toxic ion (Na+) accumulation led to nutrient imbalance in plants through ionic stress which ultimately caused poor growth and yield [21]. Earlier studies conducted on sugarcane reported that EC of soil greater than 1.7 dS m−1 at critical growth stages significantly reduced cane length, girth, NMC and yield [6] and every unit increase beyond 1.7 dS m−1 limits yield to the tune of 5.9% [7].
4.1. Effect of Salinity on Survival (%) and Cane Length
It is difficult for terrestrial plants to grow well in salty environments. High amounts of sodium and chloride ions are toxic if they accumulate in the cytoplasm of plant cells, which is made more difficult by the high osmotic pressure around the roots that might reduce the survivability percent. Additionally, energy that may be employed for growth is spent for the ion transport required for minimising toxicity and maintaining ion homeostasis under salinity. Our results also showed that higher EC during formative phase significantly affected the plant survivability and cane length in all the studied genotypes and this might be due to restricted water and nutrient availability or associated with alterations in gas exchange attributes [22]. Literature also reported that salt water of >8 dS m−1 caused significant reduction in growth, physiological traits and hampered yield by 50% [8,9].
4.2. Effect of Salinity on Physiological Traits
Relative water content (RWC), an important physiological trait that measures tissue hydration significantly reduced (4.91–29.1%) due to continued saline irrigation, which might be attributed to the presence of salts that caused disturbance in turgor by lowering the water uptake efficiency [23]. Sugarcane crop is high-water demanding crop (1200 to 1800 mm in the subtropical zone and 1600–2700 mm in tropical zone) and the formative stage only requires (550 mm water). Genotypes represents significant variability’s in RWC (72.8–78.89%) as water is an important component of cellular metabolism as well as helped in maintaining osmotic balance and sequestration of toxic ions [24]. Chlorophyll content also decreased with increasing salinity and directly associated with plant photosynthetic ability and plays a vital role in the absorption and transmission of light energy. Therefore, genotypes that had higher chlorophyll showed higher gas exchange properties. Present results revealed that varieties Co 0238 and Co 06034 retained higher chlorophyll content possibly due to the higher activity of ALA synthase enzyme or lower activity of chlorophyllase enzyme [25]. In addition, irrigation water salinity caused excessive accumulation of Na+ and Cl− in the leaf tissues that might cause destruction in the chlorophyll pigment or restricted synthesis, resulting in loss of greenness. Salinity stress also affected gas exchange attributes particularly photosynthesis, an important physiological process that provides 90% of the plant dry matter [26]. Salt stress exhibited significant reduction in photosynthetic rate (Pn)-16.85%, 54.93% and 91.44%, stomatal conductance (gS)-14.96%, 55.91% and 84.25% and transpiration rate (Tr)-14.13, 55.43 and 89.8% at ECiw ~ 4, 8 and 12 dS m−1, respectively. These reductions in Pn could be primarily attributed to stress induced partial stomatal closure to prevent the loss of water, accompanied by a reduction in stomatal conductance, lowered transpiration and consequently, stomatal limitation of intercellular CO2 concentration [10,27,28], but non-stomatal limitations (chlorophyll degradation or reduced activity of photosynthetic enzymes) also played major role [21,29]. It is also confirmed from the results that saline irrigation of ECiw ~ 8 dS m−1 or excess salinity in soil during formative phase critically hampered these traits because more than 85% reduction was noted in Pn, gS and E at ECiw ~ 12 dS m−1. Among genotypes, Co 0238 and Co 05011 maintained higher gas exchange traits by maintaining higher chlorophyll and considering differential response of genotypes, higher photosynthetic rates may be an indicative of an adaptive response to salt stress. Fv/Fm is a very sensitive tool to study stress-induced photo-inhibitory effects on plants, as we noted reductions of 5.33%, 17.33% and 42.67% at ECiw ~ 4, 8 and 12 dS m−1, respectively, in comparison to the control. Studies revealed that salinity induced decrease in Fv/Fm might be due to limited water uptake that reduces the electrochemical potential of ATP synthase and photo-system I, which further limits ATP and NADPH formation by negatively affecting the photosynthetic apparatus [30,31].
4.3. Effect of Salinity on Ion Dynamics
Other important aspect to study the response of salinity is ionic balance particularly Na+ and K+ in different plant parts and noted that sugarcane leaves retained maximum Na+ (1.81%) in comparison to roots (0.18%) and juice extract (0.31%) while K+ was maximum in juice and leaves (Table 3). The excessive accumulation of Na+ in the leaves could lead to detrimental effects on the availability of water in root medium causing cellular dehydration, reduced turgor and disturbed plant metabolism [32,33]. Generally, plants face a dilemma with regard to sodium metabolism, i.e., minimal uptake of Na+ is desirable to build osmotic potential, to absorb water and maintain turgor, whereas excess Na+ is toxic for plant [34,35]. Na+ and K+ ions compete for entry into plant root cells due to electrophysiological similarity and thus replacement of K+ by Na+ often leads to nutritional imbalances [36,37]. It is also found that higher K+ in leaves might acts as organic osmolyte, helping in stomatal opening and phloem sugar loading, since high external Na+ often competitively inhibits its uptake that generally leads to growth impairment in plants under salt stress [21,38]. Maintenance of adequate levels of Na+/K+ in different plant parts is important for sugarcane survival in saline conditions, which was observed in our study where in the roots, leaves and juice extract had mean Na+/K+ of 0.2, 0.3 and 0.03 under control condition which increased to 0.43, 0.9 and 1.34 in roots; 0.66, 1.22 and 3.15 in leaves and 0.09, 0.24 and 0.67 in juice extract with progressive increase of salinity (Figure 4). Under saline conditions, plants accumulated Na+ at the expense of K+ and an optimal Na+/K+ ratio is essential for maintaining the enzymatic reactions in the cytoplasm [39]. Since higher Na+/K+ indicates excess accumulation of Na+ which interfere with the energy utilization pattern that plants exploit to acquire water from the soil, osmotic adjustments might have resulted in reduced plant growth and yield [40,41,42,43]. Among different studied genotypes, Co 0238, Co 05011, Co 0118 and Co 06034 maintained low Na+/K+ in all the studied plant parts, due to lower sodium absorption, retaining higher membrane integrity, higher osmolytes and preferential higher K+ uptake.
4.4. Effect of Salinity on Cane Yield
Generally, salinity stress disturbs the water and nutritional balance, decreased source to sink ratio and reduced photosynthetic source size such as leaf area and shoot length of plant, which ultimately affected the ability of the crops to assimilate and utilize the resources that culminate in the final yield [19,24,44]. Present results also revealed that continued saline irrigation of ECiw ~ 4, 8 and 12 dS m−1, respectively, led to 36.4, 68.5 and 83.5% mean reduction in SCW in comparison to control (Table 4). These results suggested that continued irrigation of ECiw ~ 12 dS m−1 was not appropriate for sugarcane production. Continued saline water irrigation disturbed turgor balance, impaired photosynthetic machinery, reduced source-sink ratio, imbalanced nutrient content due to higher accumulation of toxic ions and disturbed enzyme activities especially sucrose and starch synthase which might be the possible reason for such reductions in cane weight of genotypes [19,22].
5. Conclusions
Sugarcane, a water demanding crop requires approx. 1200 to1800 mm in the subtropical zone and out of this, formative phase (critical stage of sugarcane development) requires 500 mm of water. Present results signify that continued irrigation of ECiw ~ 8 and 12 dS m−1 was not suitable for sugarcane production as these resulted in more than 50% reduction in cane yield and gas exchange attributes. Being salt sensitive plant, such continued irrigations of saline water (ECiw ~ 4, 8 and 12 dS m−1) led to drastic reductions in growth and physiological process resulted in declined cane yield and juice quality. However, we still have characterized them based on the obtained results and regression analysis, three genotypes, Co 0238, Co15027 and Co 0118 were identified as tolerant under salt stress while genotypes Co 05011, Co 06034 and Co 15023 were found as sensitive to salt stress. Hence, it is concluded that continued saline irrigation of more than 4 dS m−1 were not suitable for sugarcane, but present results revealed Co 0238, Co 0118, Co 98014 and Co 06034 might have the potential to survive under continued irrigation of ECiw ~ 4 dS m−1 by maintaining better growth, gas exchange traits and lower Na+/K+, exhibiting lower reduction in cane weight.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su142013246/s1, Table S1: Traits prioritization for salinity stress tolerance in Sugarcane through regression analysis; Table S2: Traits modeling for salinity tolerance in sugarcane genotypes though multiple linear regressions approach; Table S3: Regression coefficient, standard error, and significance of the prioritized traits for salinity stress tolerance; Table S4: Predicted Yield and ranks of the sugarcane genotypes at higher salinity level (ECiw ~ 12 dS m−1) estimated through weighted coefficients (βs).
Author Contributions
P.D.: investigation, data visualization, original draft preparation; R.K.: data visualization and investigation; A.K.: conceptualization, original draft preparation, statistical analysis; K.V.: remove plagiarism and final editing; A.K.R.: Editing; S.V.: remove plagiarism and final editing; M.R.M.: original draft preparation and final editing; N.K.: conceptualization; S.K.P.: conceptualization and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions.
Acknowledgments
We thank ICAR-SBI, Regional Center, Karnal and ICAR-CSSRI, Karnal for logistic support to complete this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ECse | Electrical conductivity of saturated extract |
| ECiw | Electrical conductivity of irrigation water |
| Pn | photosynthetic rate |
| gS | stomatal conductance |
| E | transpiration rate |
| NMC | Number of millable canes |
| CCS | Commercial cane sugar |
| SCW | Single cane weight |
| FW | Fresh weight |
| DW | Dry weight |
References
- FAO. Area, Production and Productivity of Livestock, Food and Agriculture Organization of United Nations. Food and Agriculture Organization of the United Nations, Database on Crops, 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 May 2022).
- Pooja; Nandwal, A.S.; Chand, M.; Kumari, A.; Rani, B.; Goel, V.; Singh, S. Genotypic differences in growth behavior and quality parameters of sugarcane (Saccharum officinarum) varieties under moisture stress conditions. Ind. J. Agr. Sci. 2019, 89, 65–72. [Google Scholar]
- Vision S.B.I. 2030; Vision Document; Sugarcane Breeding Institute: Coimbatore, India, 2011.
- Kumar, P.; Sharma, P.K. Soil Salinity and Food Security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Vision C.S.S.R.I. 2050; Vision Document; ICAR—Central Soil Salinity Research Institute: Karnal, India, 2017.
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance current assessment. J. Irrig. Drain. Div. Amer. Soc. Civil Eng. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Rao, V.P.; Sengar, R.S.; Singh, S.; Sharma, V. Molecular and metabolic perspectives of sugarcane under salinity stress pressure. Prog. Agr. 2015, 15, 77–84. [Google Scholar]
- Plaut, Z.; Meinzer, F.C.; Federman, E. Leaf development, transpiration and ion uptake and distribution in Sugarcane cultivarsgrown under salinity. Plant Soil 2000, 218, 59–69. [Google Scholar] [CrossRef]
- Santana, M.J.; Carvalho, J.A.; Souza, K.J.; Sousa, A.M.G.; Vasconcelos, C.L.; Andrade, L.A.B. Lab effects of irrigationwater salinity on sprouting and initial development of sugarcane (saccharum sp.) and in soils with different textural levels. Rev. Cienc. Agríc. 2007, 31, 1470–1476. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; ArtMed: Guelph, ON, Canada, 2017. [Google Scholar]
- Zuffo, A.M.; Steiner, F.; Aguilera, J.G.; Teodoro, P.E.; Teodoro, L.P.R.; Busch, A. Multi-trait stability index: A tool for simultaneous selection of soya bean in drought and saline stress. J. Agron. Crop Sci. 2020, 206, 815–822. [Google Scholar] [CrossRef]
- Simões, W.L.; Calgaro, M.; Coelho, D.S.; Santos, D.B.D.; Souza, M.A.D. Growth of sugar cane varieties under salinity. Rev. Ceres 2016, 63, 265–271. [Google Scholar] [CrossRef]
- Brindha, C.; Vasantha, S.; Arunkumar, R. The response of sugarcane subjected to salinity stress at differentgrowth phases. J. Plant Stress Physiol. 2019, 5, 28–33. [Google Scholar] [CrossRef][Green Version]
- Simões, W.L.; Coelho, D.S.; Mesquita, A.C.; Calgaro, M.; da Silva, J.S. Physiological and biochemical responses of sugarcane varieties to salt stress. Rev. Caatinga 2019, 32, 1069–1076. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relation of cotton plants. The field measurement of water deficit in leaves. New Phytol. 1950, 49, 81–87. Available online: https://www.jstor.org/stable/2428690 (accessed on 14 June 2022). [CrossRef]
- IRRI. International Rice Research Institute (IRRI): Philippines. 2013. Available online: http://bbi.irri.org/products (accessed on 21 May 2017).
- Kumar, R.; Meena, M.R.; Kulshreshtha, N.; Kumar, A.; Ram, B. Genotypic response of recently evolved sugarcane “Co” clones under different levels of saline irrigation water. J. Sugarcane Res. 2017, 7, 159–168. [Google Scholar]
- Pooja; Nandwal, A.S.; Chand, M.; Singh, K.; Mishra, A.K.; Kumar, A.; Kumari, A.; Rani, B. Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Exp. Biol. 2019, 57, 721–732. [Google Scholar]
- Pooja; Kulshreshtha, N.; Kumar, R.; Raja, A.K.; Pandey, S.K.; Goel, V.; Ram, B. Identification of drought-tolerant genotypes based on physiological traits, yield attributes and drought tolerance indices. Sugar Tech 2021, 23, 741–767. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef]
- Vasantha, S.; Venkataramana, S.; Rao, P.N.G.; Gomathi, R. Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Tech 2010, 12, 5–8. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, N.; Kaur, G.; Kumar, A.; Mann, A. Variability of durum wheat in terms of physio-biochemical traits against salinity stress. Cereal Res. Commun. 2021, 49, 45–54. [Google Scholar] [CrossRef]
- Pooja; Nandwal, A.S.; Chand, M.; Pal, A.; Kumari, A.; Rani, B.; Goel, V.; Kulshreshtha, N. Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (saccharum officinarum) varieties differing in maturity. Ind. J. Agr. Sci. 2020, 90, 507–512. [Google Scholar]
- Garg, N.; Singla, R. Growth, photosynthesis, nodule nitrogen and carbon fixationin the chickpea cultivars under salt stress. Braz. J. Plant Physiol. 2004, 16, 137–146. [Google Scholar] [CrossRef]
- Steduto, P.; Albrizio, R.; Giorio, P.; Sorrentino, G. Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000, 44, 243–255. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.P.; Verma, C.L.; Malviya, M.K.; Guo, D.J.; Rajput, V.D.; Sharma, A.; Wei, K.J.; Chen, G.L.; Solomon, S.; et al. Predication of photosynthetic leaf gas exchange of sugarcane (saccharum spp) leaves in response to leaf positions to foliar spray of potassium salt of active phosphorus under limited water irrigation. ACS Omega 2021, 6, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Stepien, P.; Klobus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biologia Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Hassan, M.; Sun, N.; et al. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Pimentel, C. Photoinhibition in a C4 plant, Zea mays L.: A minireview. Theor. Expt. Plant Physiol. 2014, 26, 157–165. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Pincelli, R.P.; Barbosa, M.d.A. Water stress effects on chlorophyll fluorescence and chlorophyll content in sugarcanecultivars with contrasting tolerance. Biosci. J. 2018, 34, 75–87. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Yeo, A.R. Ion accumulation in the cell walls of rice plantsgrowing under saline conditions: Evidence for the Oertli hypothesis. Plant Cell Environ. 1991, 14, 319–325. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinaur Associates Inc.: Sunderlan, MA, USA, 2006. [Google Scholar]
- Kumar, A.; Mishra, A.K.; Singh, K.; Lata, C.; Kumar, A.; Krishnamurthy, S.L.; Kumar, P. Diurnal changes and effect of elevated CO2 on gas exchange under individual and interactive salt and water stress in wheat (Triticum aestivum). Ind. J. Agr. Sci. 2019, 89, 763. [Google Scholar]
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Internat. J. Phytoremed. 2021, 23, 1041–1051. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Lata, C.; Soni, S.; Kumar, N.; Kumar, A.; Pooja; Mann, A.; Rani, S. Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agr. Sci. 2019, 89, 1050–1052. [Google Scholar]
- Watanabe, K.; Takaragawa, H.; Ueno, M.; Kawamitsu, Y. Changes in agronomic and physiological traits of sugarcane grown with saline irrigation water. Agronomy 2020, 10, 722. [Google Scholar] [CrossRef]
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]
- Ashraf, M.; Rahmatullah; Kanwar, S.; Tahi, M.A.; Sarwar, A.; Ali, L. Differential salt tolerance of sugarcane genotypes. Pak. J. Agri. Sci. 2007, 44, 85–89. [Google Scholar]
- Musavi, F.; Lak, S.; Shokuhfar, A.; Modhaj, A. Evaluation of sugarcane (Saccharum officinarum L.) different to the uptake and transport of ions in salt stress conditions. Biol. Forum—Int. J. 2015, 7, 1719–1724. [Google Scholar]
- Granja, M.M.C.; Medeiros, M.J.L.; Silva, M.M.A.; Camara, T.R.; Willadino, L.; Ulisses, C. Response to in vitro salt stress in sugarcane is conditioned by concentration and condition of exposure to NaCl. Acta Biol Colomb. 2018, 23, 30–38. [Google Scholar] [CrossRef]
- Vasantha, S.; Gomathi, R.; Rakkiyappan, P. Sodium content juice and jaggery quality of Sugarcane under salinity. Electron. J. Biol. Sci. 2009, 1, 33–38. [Google Scholar]
- Zhao, D.; Zhu, K.; Momotaz, A.; Gao, X. Sugarcane plant growth and physiological responses to soil salinity during tillering and stalk elongation. Agriculture 2020, 10, 608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).