Abstract
The main purpose of the presented research is to characterize the relationship between the amount of bleaching earth used in the bleaching process of rapeseed oil and the efficiency of this process. Changes in the content of chlorophyll and carotenoid pigments were examined using spectrophotometric and colorimetric methods. The process was carried out on a plate filter with different thicknesses of bleaching earth (BE) layers (1–3% in relation to the amount of oil). On the basis of the conducted research, a decrease in the content of chlorophylls (from 59 to 97%) and carotenoids (from 55 to 78%) was found, which affected the overall color of the oil. Based on the research results, it was found that the bleaching earth does not lose all of its adsorption properties after reaching the maximum, but they decrease in the next cycle. For some time, the sorbent can be used successfully, but to a lesser extent (40–45% of the original properties). This information is crucial as it will reduce BE consumption by up to 30% and shorten the oil bleaching cycle, as it will reduce the setup time of the machine.
1. Introduction
Rapeseed oil is one of the most popular edible oils, the annual production of which in 2020 was estimated at approx. 35,406 million tones [1,2]. This product is rich in unsaturated fatty acids, making it an essential component of a balanced diet [3]. It contains approximately 1.5–6.0% palmitic acid, 0.5–3.1% stearic acid, 28.0% oleic acid, 11.0–23.0% linoleic acid, and 5.0–13.0% linolenic acid. Rapeseed oil is therefore characterized by a favorable content of polyunsaturated fatty acids due to the ratio of linoleic (n-6) and linolenic (n-3) acids being 2:1 [4,5,6]. Dyes, phospholipids, free fatty acids, waxes, and heavy metal compounds are undesirable substances in crude rapeseed oil because they have an adverse effect on its physicochemical properties and sensory attributes [7,8,9].
The color of rapeseed oil depends on the content of two types of dyes, i.e., carotenoid and chlorophyll dyes [10,11,12]. Carotenoids accompany chlorophylls in the green parts of plants, in their chloroplasts, and are also found in flowers, fruits, seeds, and roots. They are synthesized exclusively by plants, giving them a yellow, orange, or red color. They are very soluble in nonpolar solvents such as cooking oils. However, they do not dissolve in water. The color of carotenoids results from conjugated double bonds in the all-trans configuration in their molecules. Because they have antioxidant properties, these dyes are considered to be an effective natural quencher of singlet oxygen, transforming it into a triplet form [13]. This means that carotenoids have the opposite effect to chlorophylls, allowing them to tolerate their adverse impact on the product [14]. A diet rich in carotenoid products inhibits the development of cardiovascular diseases, e.g., coronary artery disease [15]. An essential advantage of carotenoid dyes is their durability. Their losses are low during the proper processing and storage of food products rich in carotenoids. The presence of carotenoids in oils is also desirable due to lutein and zeaxanthin, which are believed to support the process of good vision in humans.
Chlorophyll dyes are considered the least durable of all plant dyes. The high content of chlorophylls in the oil negatively affects its stability and sensory attributes, such as appearance, taste, or smell [16]. Reducing the content of chlorophylls is especially important in cold-pressed oils, and thus rich in polyunsaturated fatty acids. Chlorophyll dyes take part in photochemical reactions transforming triplet oxygen into a singlet form (more reactive), thus initiating an avalanche process of oxidation of unsaturated fatty acids [17,18]. The presence of both carotenoids and chlorophylls gives rapeseed oil a dark brown color that consumers do not desire. For this reason, cooking oils must be pre-purified by a refining process that removes substances from the oil that are chemically, not fat [19,20]. The main result of oil refining is an increase in their oxidative stability, thanks to which they can be, among other things, stored longer [21,22]. One of the refining stages is the bleaching process, mainly consisting of the removal of chlorophyll and carotenoid dyes with the use of various types of adsorbents, i.e., bleaching earth [23,24,25], silica gel [26], and active carbon [27]. It is crucial to properly select the bleaching parameters and optimize this process. It has been reported the use of aggressive bleaching conditions, i.e., high temperature or long bleaching time, leads to a significant darkening of the color of the refined oil. Additionally, as a result of the phenomenon called “heat of bleaching”, up to 98% of dyes can be damaged carotenoids, which leads to the fact that refined oil has almost no health-promoting properties [28]. Analysis of the literature made it possible to formulate research questions that require answers based on empirical data:
- RQ1:
- Does bleaching earth retain adsorption properties in subsequent bleaching cycles?
- RQ2:
- How does the efficiency of the oil bleaching process change when the adsorbent mass increases?
The aim of this publication is to investigate the changes in the adsorption properties of bleaching earth in rapeseed oil’s low-temperature bleaching process. The adsorption properties of BE will be characterized based on oil color changes in subsequent bleaching cycles. The color of the oil will be measured using spectroscopic and colorimetric methods. The effectiveness of the bleaching process will be characterized by different weights of the adsorbent used.
2. Materials and Methods
2.1. Reagents
The test oil was obtained from Bastik, an oil producer in Poland. The rapeseed oil was obtained by the two-stage pressing method (cold and hot, after the extrusion process). In the first stage, the oil was pressed at 45 °C without preheating the rapeseed. Then, the pomace was extruded at about 140 °C and the oil was pressed again. The products obtained from the two pressing steps were mixed in the ratio of 84% cold-pressed oil and 16% hot-pressed oil. The physical and chemical properties of rapeseed oil are presented in Table 1.

Table 1.
Physicochemical properties of rapeseed oil.
Hexane was used without further purification as supplied from Aldrich. The composition of the bleaching earth (supplied from Sepigel) is shown in Table 2 [29].

Table 2.
Selected physicochemical properties of bleaching earth used in the process.
2.2. Equipment
The process of low-temperature bleaching of rapeseed oil was carried out on a plate filter with dimensions of 200 × 200 mm manufactured by Farmet (Czech Republic). The scheme of the frame-plate filter structure is shown in Figure 1.
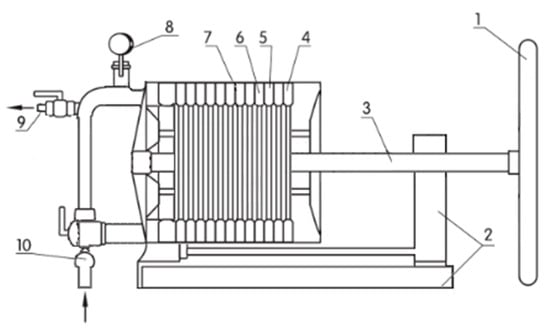
Figure 1.
The scheme of the plate filter structure (1—handwheel; 2—drain tub; 3—reinforcing support; 4—stop plate; 5—filter membrane; 6—slime frame; 7—filter plate; 8—manometer; 9—filtrate discharge channel; 10—cloudy fluid feed channel).
Rapeseed oil was filtered using the AMAFILTER filter, model NVD1200-40/1800, with an area of 40 m2 and a working pressure of 6 bar, which allows obtaining from 0.006 to 0.015 L of clean oil from 1 m2 of filter surface during 1 h. Specord 210 spectrophotometer (Analytic Jena AG, Jena, Germany) was used for the spectrophotometric measurement of the absorbance (A) of the content of chlorophyll and carotenoid dyes and the evaluation of the overall color. The color of the oils was also determined based on the measured values of the parameters L* (photometric brightness), a* (intensity of red), b* (intensity of yellow), C (color saturation), and h° (color tone angle) in the CIE Lab system [30] at using a Minolta Chroma Meter CR-310 reflection colorimeter equipped with a D65 light source and a measuring cell with a diameter of 8 mm. The colorimeter was connected to a computer equipped with Minolta SpectraMagic NX software. The values of h° and C parameters were calculated according to the formulas:
C = (a*2 + b*2) ½
h° = tg−1 (b*/a*)
2.3. Methods
The spectroscopic method described in the PN-A 86934: 1995 standard was used to determine the content of chlorophyll and carotenoid pigments [31]. To determine the content of chlorophyll dyes in the sample, 3.0 m of n-hexane was added to 3.0 mL of oil. After mixing, the solution was transferred to a spectrophotometric cuvette, and the absorbance was measured at a wavelength of 668 nm. To determine the content of carotenoid dyes in the tested oils, samples with 1 mL of oil was diluted by adding 5 mL of n-hexane. The mixture was made up to 11 mL with n-hexane. After mixing, the resulting solution was transferred to a spectrophotometric cuvette, and the absorbance was measured at 442 nm. Based on the PN-A 86934:1995 standard, the sum of the obtained absorbance values for chlorophyll and carotenoid dyes of the tested oils was expressed as a general evaluation of the color.
The methodology of the tests described in this publication was based on five cycles of the bleaching process (I-V). In each of them, 20 dm3 of crude rapeseed oil and a different amount of bleaching earth were used, which in the first cycle amounted to 200 g and increased by 100 g in subsequent cycles, up to the value of 600 g in the 5th cycle. The use of more than 600 g of bleaching earth caused blockage of the plate filter. Bleaching of the obtained rapeseed oil on a plate filter in each cycle was started by mixing the minimum oil necessary to fill the system (4 dm3) with the weight of bleaching earth appropriate for a given cycle in a buffer tank. Mixing was carried out using a mechanical stirrer and continued until the phases were thoroughly mixed and an emulsion was formed. As a result, a dark brown colored homogeneous mixture was obtained. The mixture was poured into the reservoir at the centrifugal pump, which, when actuated, transferred the mixture to the membranes and frame of the plate filter. Then, another 4 dm3 of raw oil was added to the tank, and the bleaching process continued. After receiving the first 4 dm3 of bleached oil, a sample was taken for laboratory tests, and the next portions (4 dm3) of raw oil were added to the tank.
2.4. Statistical Analysis
The obtained results were statistically analyzed using the Statistica® 13.3 software (StatSoft Inc., Poland, 2019). The compliance of the distribution of features with the normal distribution was checked using the Shapiro–Wilk test. The calculations considered the values of arithmetic means and standard deviations (s) for the analyzed features. One-way analysis of variance was performed in the orthogonal system according to the linear model. The statistical significance of differences between the mean values of the analyzed features within the analyzed experimental factors was estimated using Duncan’s multiple range test at the confidence level of p ≤ 0.05. The tables present the average values and their standard deviations. The charts were made using Excel 365 software.
3. Results
3.1. Spectrophotometric Color Determination
Based on the results of the spectrophotometric color determination tests (Table 3), it was found that the average content of chlorophyll dyes in the bleached rapeseed oil decreased with the increase in the amount of added bleaching earth. In each bleaching cycle (I-V), the bleaching earth achieved its maximum adsorption properties in the case of the bleaching of sample B. Spectroscopic analysis of consecutive samples (C-E) of bleached oil showed that they were characterized by a higher content of chlorophylls, which indicates a decrease in the adsorption capacity of the bleaching earth. The oil bleaching efficiency was also calculated using Formula (3) [32]:
where:

Table 3.
Statistical analysis of changes in the content of chlorophyll and carotenoid pigments and total color in rapeseed oil.
- BE—bleaching efficiency,
- AV—absorbance value for starting oil,
- AB—absorbance value for bleached oil.
The decrease in the content of chlorophyll pigments in rapeseed oil lightened its green-brown color. The results in Table 3 suggest the possibility of a continuous bleaching process, i.e., preserving some of the adsorption capacity of the bleaching earth used. In each of the performed bleaching cycles (I-V), sample D had a color similar to that of samples A, taken before reaching the maximum adsorption properties of the bleaching earth. Analyzing the above data, it can be concluded that the adsorption properties of the bleaching earth increase with its mass as its effective adsorption surface increases. During bleaching with the maximum possible dose of BE (600 g), more than 97% of chlorophyll dyes are removed from sample B.
As shown by the research, the average content of carotenoids decreased with the increased amount of added bleaching earth until the maximum adsorption capacity was reached (test B). Exceeding this point by continuing the bleaching process is synonymous with an increase in the average content and a partial loss of adsorption properties of the bleaching earth. The observed decrease in carotenoid adsorption is more dramatic than in the case of chlorophyll adsorption. However, relative stability can be observed at each cycle in the final stages of the bleaching cycle (samples D-E). Figure 2 shows the percentage change in the overall color of rapeseed oil during the low-temperature bleaching process. During the final stages of the bleaching process (trials D-E), relative stabilization of the adsorption value of dyes from rapeseed oil was found. The diagram shows that in the case of the bleaching cycle in which 300 g of bleaching earth was used, a different arrangement of the points was observed. This is due to the lower initial carotenoid content in the oil intended for this bleaching cycle (Table 3). The effectiveness of bleaching depends on the initial content of natural dyes.
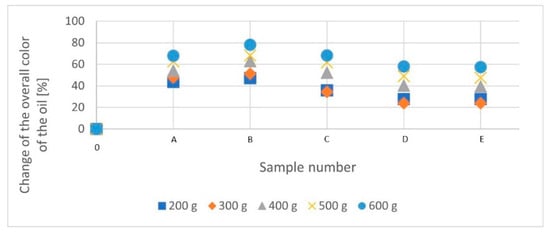
Figure 2.
Percentage change in the overall color of rapeseed oil during low-temperature bleaching. [A–E]—consecutive stages of a given bleaching cycle; (A—20 dm3 of rapeseed oil in the system; B—16 dm3; C—12 dm3; D—8 dm3; E—4 dm3).
Based on the statistical analysis of the results (Table 3), significant differences were found in the content of chlorophyll and carotenoid dyes and the value of the total color of rapeseed oil in individual oil samples (samples 0 and A-E) in each of the bleaching cycles. Sample 0 shows the color of the crude oil before the bleaching process begins. The content of chlorophylls and carotenoids in natural rapeseed oils is significantly higher than in the analyzed samples of bleached oil. In sample A, the start of the bleaching process was observed, as evidenced by significantly lower dye contents in each bleaching cycle. In sample B, the statistically lowest contents of the analyzed dyes were found, which was caused by achieving the maximum adsorption properties of the bleaching earth. In sample C, an increase in the content of chlorophylls and carotenoids (compared to sample B) was observed in all analyzed bleaching cycles. This was due to the decrease in the adsorption properties of the bleaching earth. In samples D and E, they did not show any significant differences in the content of chlorophyll and carotenoid dyes in the analyzed oils. Moreover, these results are similar to the values obtained in the first samples taken. It was due to the preservation of some of the sorption properties of the bleaching earth.
3.2. Colorimetric Evaluation of Oil Color Parameters According to the CIE Lab System
The CIE Lab system is used for color measurements, e.g., food products, including edible oils. Three parameters define the color space in the CIE Lab system: the L* parameter, i.e., the photometric brightness, and its monochromatic components, i.e., a* (intensity of the red color) and b* (intensity of the yellow color). Based on the parameters mentioned above, the following parameters are calculated: color saturation (C) and color tone angle (h°) [33]. The results of the measurements of the parameters determined by the CIE Lab system are presented in Table 4. The statistical analysis of the results showed that all tested B samples were characterized by the significantly highest parameters L* (photometric brightness) and h° (color tone angle), regardless of the weight of the added bleaching earth (from 200 g to 600 g). The samples mentioned above, analyzed with the spectrophotometric method, in terms of the content of chlorophyll and carotenoid dyes, showed the lowest values. Therefore, it can be concluded that the values of the photometric brightness and the color tone angle are higher when the content of dyes in the oil is lower. Moyano et al. [34] proved in their work that there is a relationship between the value of the yellow color intensity of oils and the carotenoid content.

Table 4.
The result of the statistical analysis of the color parameters (L*, a*, b*, C, and h°) of rapeseed oils.
The lowest L* and h° parameters were recorded for the blank tests in all bleaching cycles. This means that these samples are the least clear (transparent) of all the probes. Clarity (transparency) increases until the maximum adsorption properties are achieved. It then drops until it reaches relative stability in the two final samples. The opposite is true for the remaining parameters a*, b*, and C. The highest values were recorded in the blank samples. Their value decreases until the maximum adsorption properties are achieved and then increases until the relative stability is conducted in the last two tests, which corresponds to the transition of the color from green-brown (or brown-green) to yellow-golden. The L* parameter, which is responsible for the photometric brightness and the related transparency of a given substance, from the parameters discussed, best reflects the changes taking place in the oil. Increasing transparency is associated with a decrease in the content of solid impurities and natural dyes, thus lightening the oil’s color. Therefore, it was decided to check the relationship between the percentage change in photometric brightness and the individual samples in each bleaching cycle (Figure 3).
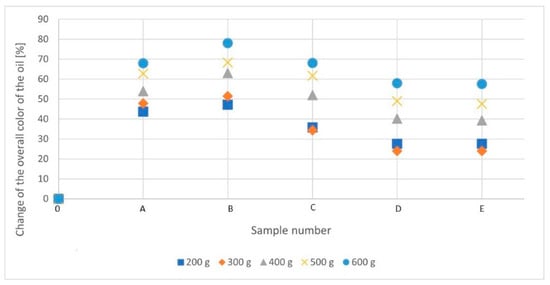
Figure 3.
Rapeseed oil’s percentage of color change was measured by colorimetric methods during different low-temperature bleaching cycles. [A–E]—consecutive stages of a given bleaching cycle; (A—20 dm3 of rapeseed oil in the system; B—16 dm3; C—12 dm3; D—8 dm3; E—4 dm3).
3.3. Colorimteric Oil Color Measurements—Summary
The only parameter that can be considered individually is the parameter L*, which determines the level of photometric brightness and thus the clarity of the oil. Parameters a* and b* were interpreted together, as they are responsible for the balance between different shades of color of the test substance. The obtained measurement results were used to create the L* a* b* spatial vectors, which would allow locating the appropriate point on the color scale, and thus it was possible to compare the color changes of the tested oil. The results are shown in Table 5.

Table 5.
Lab spatial vectors and colors of oils samples.
As it results from the analyzes conducted in the previous chapters, the color of the bleached oil shifts towards a golden-yellow color, and after exceeding the point of maximum adsorption capacity of the bleaching earth (sample B), it slightly darkens, but the bleaching process is still carried out because the color remains lighter than in the blank test (sample 0). It follows that the used bleaching earth retains some of its adsorption capacity, i.e., it can be used for a certain time. This result is consistent with the results of the color change analysis using spectrophotometric methods.
4. Conclusions
This study aimed to characterize the properties of bleaching earth during the bleaching of low-temperature rapeseed oil. Based on the research results, it was found that the bleaching earth does not lose all of its adsorption properties after reaching the maximum, but they decrease. For some time, the sorbent can be used successfully, but to a lesser extent (40–45% of the original properties). This information is crucial as it will reduce BE consumption by up to 30% and shorten the oil bleaching cycle as it will reduce the setup time of the machine. An additional advantage of reusing the adsorbent is the reduction of waste [35]. The used bleaching earth requires particular disposal to not self-ignite or pollute the environment with the emitted greenhouse gases because only a tiny part of the used adsorbent is reused in another industry, and the storage of adsorption residues is problematic. The spectrophotometric measurement of the content of chlorophyll and carotenoid dyes showed that more chlorophylls are removed during the low-temperature bleaching process [36]. It is related to the change of the color of the oil from brown-green (crude oil) to golden-yellow (bleached oil), which is caused by the remains of carotenoid pigments. This was confirmed by colorimetric color measurement using the CIE Lab method by creating, on the basis of the readings from the Minolta CR-310 reflection colorimeter, spatial vectors matched to the color scale. Both measurements of the color change of the oils during bleaching showed that its color initially brightened and turned golden-yellow to become darker when the bleaching earth had its maximum adsorption capacity, but this change did not qualify the bleaching as complete. The oil is still deprived of natural dyes in a significant amount, which means that within the adopted time frame of the process and for the amount of raw material used, the process continues with satisfactory results. Research has shown that at the moment of reaching the maximum of the adsorption properties at the time of oil bleaching, as much as 97% of chlorophyll dyes were removed, which is particularly important as they accelerate the process of rancid oils. However, it is not desirable to remove carotenoid dyes to such an extent (when the maximum adsorption properties are reached, the moment is the removal of about 78% of their content), as in the experiment carried out, because of their pro-health properties. The publication shows that bleaching earth retain their adsorption properties also in subsequent bleaching cycles. In the future, we would like to explore the topic of regeneration of bleaching earth by using non-toxic organic solvents, which will have a positive impact on the environment. It will also allow some of the oil adsorbed on the surface of the bleaching earth to be recovered.
Author Contributions
Conceptualization, D.M. and K.C.; methodology, D.M.; validation, M.B. and K.C.; formal analysis, D.M.; investigation, D.M. and K.C.; resources, M.W. and M.B.; writing—original draft preparation, K.C., P.K. and D.M.; writing—review and editing, D.M., P.K. and M.W.; visualization, K.C.; supervision, D.M.; project administration, D.M.; funding acquisition, D.M. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
Publication was co-financed with the project entitled ‘Excellent science’ program of the Ministry of Education and Science as a part of the contract No. DNK/513265/2021 ‘Role of agriculture in implementing concept of sustainable food system “from field to table”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- US Department of Agriculture Foreign Agricultural Service. Oilseeds: World Markets and Trade 2022. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 8 October 2022).
- Larina, Y.; Galchynska, J.; Kucheruk, P.; Zghurska, O.; Ortina, G.; Al-Nadzhar, F.; Marusei, T.; Kuboń, M.; Dzieniszewski, G. Estimation of the Domestic Agricultural Sector Potential for the Growth of Energy Cultures for Bioenergy Fuel Production. Agric. Eng. 2021, 25, 73–82. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, L.; Zhou, P.; Liu, Z.; Fan, S.; Yang, D.; Li, J.; Liu, Q. Dietary Exposure of General Chinese Population to Fatty Acid Esters of 3-Monochloropropane-1, 2-Diol (3-MCPD) from Edible Oils and Oil-Containing Foods. Food Addit. Contam. Part A 2021, 38, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, R.; Wang, Z.; Wang, B.; Yang, Y.; Ju, X.; He, R. The Effect of Refining Process on the Physicochemical Properties and Micronutrients of Rapeseed Oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, D.; Rukowicz, B.; Golimowski, W.; Czechlowski, M.; Krzaczek, P.; Piekarski, W. Effect of selected depressants on cold filter plugging point for methyl esters obtained from transesterification of waste vegetable and animal fats. Przem. Chem. 2017, 1, 121–124. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an Electronic Nose for Determination of Pre-pressing Treatment of Rapeseed Based on the Analysis of Volatile Compounds Contained in Pressed Oil. Int. J. Food Sci. Technol. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Karabagias, I.K. Advances of Spectrometric Techniques in Food Analysis and Food Authentication Implemented with Chemometrics. Foods 2020, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, A.; Müller, K.; Schmid, M.; Eisner, P. Rapeseed Proteins for Technical Applications: Processing, Isolation, Modification and Functional Properties—A Review. Ind. Crops Prod. 2020, 158, 112986. [Google Scholar] [CrossRef]
- Serrano-Bermúdez, L.M.; Monroy-Peña, C.A.; Moreno, D.; Abril, A.; Imbachi Niño, A.D.; Martínez Riascos, C.A.; Buitrago Hurtado, G.; Narváez Rincón, P.C. Kinetic Models for Degumming and Bleaching of Phospholipids from Crude Palm Oil Using Citric Acid and Super Flo B80® and Tonsil®. Food Bioprod. Process. 2021, 129, 75–83. [Google Scholar] [CrossRef]
- Abedi, E.; Amiri, M.J.; Sahari, M.A. Kinetic, Isotherm and Thermodynamic Investigations on Adsorption of Trace Elements and Pigments from Soybean Oil Using High Voltage Electric Field-Assisted Bleaching: A Comparative Study. Process Biochem. 2020, 91, 208–222. [Google Scholar] [CrossRef]
- Hew, K.S.; Asis, A.J.; Tan, T.B.; Yusoff, M.M.; Lai, O.M.; Nehdi, I.A.; Tan, C.P. Revising Degumming and Bleaching Processes of Palm Oil Refining for the Mitigation of 3-Monochloropropane-1,2-Diol Esters (3-MCPDE) and Glycidyl Esters (GE) Contents in Refined Palm Oil. Food Chem. 2020, 307, 125545. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, R.; Kmiecik, D.; Gawrysiak-Witulska, M.; Malaga-Toboła, U.; Tabor, S.; Findura, P.; Siger, A.; Gancarz, M. Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose. Sensors 2021, 21, 303. [Google Scholar] [CrossRef]
- Kruk, J.; Szymańska, R. Singlet Oxygen Oxidation Products of Carotenoids, Fatty Acids and Phenolic Prenyllipids. J. Photochem. Photobiol. B Biol. 2021, 216, 112148. [Google Scholar] [CrossRef] [PubMed]
- Gurak, P.D.; Mercadante, A.Z.; González-Miret, M.L.; Heredia, F.J.; Meléndez-Martínez, A.J. Changes in Antioxidant Capacity and Colour Associated with the Formation of β-Carotene Epoxides and Oxidative Cleavage Derivatives. Food Chem. 2014, 147, 160–169. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Nascimento, T.C.; Pinheiro, P.N.; Vendruscolo, R.G.; Wagner, R.; de Rosso, V.V.; Jacob-Lopes, E.; Zepka, L.Q. Bioaccessibility of Microalgae-Based Carotenoids and Their Association with the Lipid Matrix. Food Res. Int. 2021, 148, 110596. [Google Scholar] [CrossRef]
- Khaligh, B. Investigation of the Bleaching Potential of Aluminum and Magnesium Oxides in Edible Oil Industry. Food Sci. Technol. 2021, 18, 21–33. [Google Scholar] [CrossRef]
- Wroniak, M.; Kwiatkowska, M.; Krygier, K. Charakterystyka wybranych olejów tłoczonych na zimno. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2006, 47, 46–58. [Google Scholar]
- Aachary, A.A.; Liang, J.; Hydamaka, A.; Eskin, N.A.M.; Thiyam-Holländer, U. A New Ultrasound-Assisted Bleaching Technique for Impacting Chlorophyll Content of Cold-Pressed Hempseed Oil. LWT-Food Sci. Technol. 2016, 72, 439–446. [Google Scholar] [CrossRef]
- Ribeiro, J.A.A.; Almeida, E.S.; Neto, B.A.D.; Abdelnur, P.V.; Monteiro, S. Identification of Carotenoid Isomers in Crude and Bleached Palm Oils by Mass Spectrometry. LWT-Food Sci. Technol. 2018, 89, 631–637. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Xia, F.; Shen, G.; Feng, J. An Expert System Based on 1H NMR Spectroscopy for Quality Evaluation and Adulteration Identification of Edible Oils. J. Food Compos. Anal. 2019, 84, 103316. [Google Scholar] [CrossRef]
- Szczurko, K.; Kolodziej, M.; Konieczny, R.; Golimowski, W. Effect of selected grain parameters on the quality of the extruded oil. Przem. Chem. 2019, 98, 82–85. [Google Scholar]
- McDowell, D.; Defernez, M.; Kemsley, E.K.; Elliott, C.T.; Koidis, A. Low vs. High Field 1h Nmr Spectroscopy for the Detection of Adulteration of Cold Pressed Rapeseed Oil with Refined Oils. LWT-Food Sci. Technol. 2019, 111, 490–499. [Google Scholar] [CrossRef]
- Dijkstra, A.J. What to Do with Spent Bleaching Earth? A Review. J. Am. Oil Chem. Soc. 2020, 97, 565–575. [Google Scholar] [CrossRef]
- Rincón, L.A.; Ramírez, J.C.; Orjuela, A. Assessment of Degumming and Bleaching Processes for Used Cooking Oils Upgrading into Oleochemical Feedstocks. J. Environ. Chem. Eng. 2021, 9, 104610. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Ceriani, R.; Verhé, R.; Stevens, C.; De Greyt, W.; Meirelles, A.J.A. Adsorption of Carotenes and Phosphorus from Palm Oil onto Acid Activated Bleaching Earth: Equilibrium, Kinetics and Thermodynamics. J. Food Eng. 2013, 118, 341–349. [Google Scholar] [CrossRef]
- Yener, N.; Biçer, C.; Pekdemir, A.D.; Sarıkaya, Y.; Önal, M. Preparation and Characterization of Nanoporous Powders from Bentonite by Hydrochloric Acid Leaching and Using as Bleaching Earth. SN Appl. Sci. 2020, 2, 717. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, K.; Yin, K.; Zuo, S.; Yao, C. Clay-Activated Carbon Adsorbent Obtained by Activation of Spent Bleaching Earth and Its Application for Removing Pb(II) Ion. Environ. Sci. Pollut. Res. 2021, 28, 711–723. [Google Scholar] [CrossRef]
- Su, D.; Xiao, T.; Gu, D.; Cao, Y.; Jin, Y.; Zhang, W.; Wu, T. Ultrasonic Bleaching of Rapeseed Oil: Effects of Bleaching Conditions and Underlying Mechanisms. J. Food Eng. 2013, 117, 8–13. [Google Scholar] [CrossRef]
- Łaska-Zieja, B.; Marcinkowski, D.; Golimowski, W.; Niedbała, G.; Wojciechowska, E. Low-Cost Investment with High Quality Performance. Bleaching Earths for Phosphorus Reduction in the Low-Temperature Bleaching Process of Rapeseed Oil. Foods 2020, 9, 603. [Google Scholar] [CrossRef]
- Hernández, B.; Sáenz, C.; Alberdi, C.; Diñeiro, J.M. CIELAB Color Coordinates versus Relative Proportions of Myoglobin Redox Forms in the Description of Fresh Meat Appearance. J. Food Sci. Technol. 2016, 53, 4159–4167. [Google Scholar] [CrossRef]
- PN-A-86934:1995; Oils and fats vegetable and animal—Determination of the general spectrophotometric colour. Polish Committee for Standardization: Warszawa, Poland, 1995; ISBN 83-7001-835-1.
- Asgari, S.; Sahari, M.A.; Barzegar, M. Practical Modeling and Optimization of Ultrasound-Assisted Bleaching of Olive Oil Using Hybrid Artificial Neural Network-Genetic Algorithm Technique. Comput. Electron. Agric. 2017, 140, 422–432. [Google Scholar] [CrossRef]
- Kachel, M.; Matwijczuk, A.; Sujak, A.; Czernel, G.; Niemczynowicz, A.; Nowicka, A. The Influence of Copper and Silver Nanocolloids on the Quality of Pressed Spring Rapeseed Oil. Agronomy 2019, 9, 643. [Google Scholar] [CrossRef]
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A Comprehensive Study on the Colour of Virgin Olive Oils and Its Relationship with Their Chlorophylls and Carotenoids Indexes (I): CIEXYZ Non-Uniform Colour Space. Food Res. Int. 2008, 41, 505–512. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Ceriani, R.; Verhé, R.; Stevens, C.; De Greyt, W.; Meirelles, A.J.A. Effect of Type of Bleaching Earth on the Final Color of Refined Palm Oil. LWT-Food Sci. Technol. 2014, 59, 1258–1264. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Daud, W.M.A.W. Textural Characteristics, Surface Chemistry and Activation of Bleaching Earth: A Review. Chem. Eng. J. 2011, 170, 90–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).