Abstract
In the current study, BaO was doped in Bi2O3-ZnO-B2O3-SLS glass to develop lead-free radiation shielding glasses and to solve the dark brown of bismuth glass. The melt-quenching method was utilized to fabricate (x) BaO (1 − x)[0.3 ZnO 0.2 Bi2O3 0.2 B2O3 0.3 SLS] (where x are 0.01, 0.02, 0.03, 0.04, and 0.05 mol) at 1200 °C. Soda lime silica glass waste (SLS), which is mostly composed of 74.1% SiO2, was used to obtain SiO2. The mass attenuation coefficient () was investigated utilizing X-ray fluorescence (XRF) at 16.61, 17.74, 21.17, and 25.27 keV and narrow beam geometry at 59.54, 662, and 1333 keV. Moreover, the other parameters related to gamma ray shielding properties such as half-value layer (HVL), mean free path (MFP), and effective atomic number () were computed depending on values. The results indicated that HVL and MFP decreased, whereas increased with an increase in BaO concentration. According to these results, it can be concluded that BaO doped in Bi2O3-ZnO-B2O3-SLS glass is a nontoxic, transparent to visible light, and a good shielding material against radiation.
1. Introduction
Heavy metal oxide glasses such as PbO, Bi2O3, and BaO are considered as one of the desirable shielding materials that are used for radiation protection because they possess a lot of interesting properties that make them suitable for radiation protection, such as high density, transparency to visible light, stability in air and water, high interaction cross-section, high infrared transparency, and good absorption of radiation [1,2]. Research interests in the field of radiation shielding materials have changed to using ecofriendly materials instead of PbO due to the toxicity of lead [3,4].
Recently, many research groups have classified Bi2O3 glass as an ideal candidate for radiation protection, and they have confirmed that the ability of Bi2O3 glass to attenuate photons enhances with the increase in the content of Bi2O3 [3,5,6,7].
However, there are few issues with the use of high concentrations of Bi2O3 in glass, such as the color of the glass becoming dark brown or black and the melting temperature increasing [8]. As a result, researchers in the materials sciences and the glasses developers have a technical problem in developing highly high transmission Bi2O3 glass.
Soda lime silica (SLS) glass is one of the most widely used industrial glass products, accounting for up to 90–95% of global glass production and is used for flat glass or container wares and windowpanes. However, SLS glass waste needs a long time to decompose in addition to the limited landfill sites. This contributes to the accumulation of glass waste. To solve environmental problems, researchers are interested in reusing glass waste as an alternative source of SiO2. There are several benefits to using SLS glass waste as a source of SiO2. This reduces production costs, in addition to the good optical and mechanical characteristics of SLS glass waste, such as high thermal stability, high transparency, low melting point, and perfect chemical stability [9,10,11].
The aim of the current study is to prepare glass samples consisting of soda lime silica waste glass that have the ability to attenuate photons’ intensity, which can be used as radiation protection, in addition to improving the optical properties by reducing the dark brown or black color of glass samples. Furthermore, SLS glass waste is utilized as a source of silicon dioxide (SiO2) to reduce the accumulation of SLS glass waste and also reduce production costs. The mass attenuation coefficient was measured at 16.61, 17.74, 21.17, and 25.27 keV utilizing X-ray fluorescence (XRF) and narrow beam geometry at 59.54, 662, and 1333 keV. Moreover, other parameters related to radiation shielding, such as the half-value layer (HVL), mean free path (MFP), the effective atomic number ( ), and the effective electron density () were tested and reported in this study at photon energies of 59.54, 662, and 1333 keV.
2. Materials and Methods
2.1. Glasses Preparation
In the current study, glass samples with the chemical composition of (x) BaO (1 − x)[0.3 ZnO 0.2 Bi2O3 0.2 B2O3 0.3 SLS] (where x are 0.01, 0.02, 0.03, 0.04, and 0.05 mol) were fabricated using the melt quenching technique. SLS glass waste was used to reuse SiO2 contained in it by cleaning and crushing it into a powder utilizing a mortar and pestle. All chemical components such as Bi2O3, ZnO, B2O3, SLS, and BaO were mixed completely for 10 min in an agate mortar and pestle and transferred to an alumina crucible. After that, the crucible was inserted in the electric furnace to melt at a temperature of 1200 °C for 1.5 h. The melted mixture was poured immediately into the preheated cylinder brass plate and then annealed for 2 h at approximately 350 °C in order to prevent internal mechanical stress and strains. Finally, the glass samples were polished after they had cooled to room temperature. These glasses have a thickness of 6 mm and were labeled Ba1, Ba2, Ba3, Ba4, and Ba5, which correspond to doping levels of 0.01, 0.02, 0.03, 0.04, and 0.05 mol BaO (see Figure 1).

Figure 1.
Glass samples of BaO doped in Bi2O3-ZnO-B2O3-SLS glass.
The properties of the crystal structure of the prepared glass samples were tested using a Bruker D8 Advance X-ray diffractometer with Cu Ka radiation, and the X’Pert HighScore software was utilized. The wavelength of the X-ray tube is 1.54180 Å, and it operates at 40 kV, 40 mA. The diffractograms of the glass samples of one phase are in the range of 10° 90°.
2.2. Physical Properties
According to Archimedes’ principle, the density of the glass samples is computed utilizing distilled water as the immersion liquid. The following formula was used to compute density [12].
where indicates glass sample density in (g) cm−3, denotes distilled water density (1 g) cm−3, () denotes the weight g of the glass sample in air, and () denotes the weight of the glass sample.
Molar volume was measured using the following formula [13]:
where indicates the molecular weight of a substance, and the density of the glass sample.
Oxygen packing density OPD was computed utilizing the following equation [14]:
where c denotes the total number of oxygen atoms in the represented composition.
The ion concentration of (N) can be obtained as [15]:
Polaron radius (), inter-nuclear distance (), and field strength (F) were measured depending on ion concentration by using the following equations [16].
where is the Avogadro’s number, and Z is the atomic number.
2.3. Radiation Shielding Features: Theoretical Approach
The Lambert Beer law was used to compute the experimental mass attenuation coefficient depending on the following formula [17,18,19].
whereas the theoretical value of of mixture or compound was measured using the Phy-X/PSD software [20].
where I and indicate the intensity of photons recorded in the detector with and without the glass sample, x indicates glass thickness, and the weight fraction of the component in the compound. The half-value layer (HVL) and mean free path (MFP) are measured using the following equations [21,22]:
The total atomic cross-section for materials and electronic cross-section were used to calculate effective atomic number and electron density through the following formulas [23,24].
where and denote the atomic weight and Avogadro’s number of the ith element in the composition materials, respectively. denotes the number of atoms of element i relative to the total number of atoms of all elements in the composition materials, and is the known atomic number of the ith element in the composition materials.
3. Radiation Shielding Features: Experimental Approach
The experimental of the prepared glass was measured through Equation (8) using X-ray fluorescent equipment (XRF) and narrow beam gamma ray transmission geometry. Figure 2 shows the setup for X-ray fluorescence (XRF). XRF photons were generated through irradiating high-purity metal plates such as Tin (Sn), Palladium (Pd), Molybdenum (Mo), and Niobium (Nb), which are detailed in Table 1 by using 59.54 keV of 100 mCi Am-241. The low-energy germanium (LEGe) detector was used to detect photons that were transmitted through glass samples.
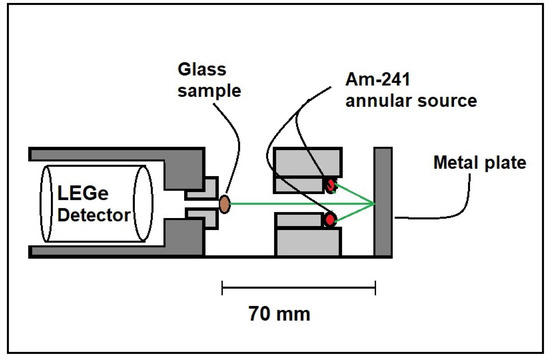
Figure 2.
The setup for X-ray fluorescence (XRF).

Table 1.
Metal plates used in X-ray fluorescence (XRF) configuration.
Figure 3 illustrates the setup of the narrow beam geometry. Am-241 of 45 Ci, Cs-137 of 5 Ci, and Co-60 of 5 Ci were utilized to measure the of the glass samples at energies of 59.54, 662, and 1333 keV, respectively. The Ludlum detector, composed of a flat-face crystal of thallium-activated sodium iodide (NaI [Tl]), was used to detect photons that passed through the glass samples.
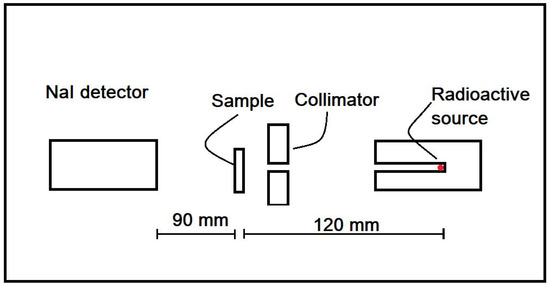
Figure 3.
The setup of narrow beam geometry.
4. Results and Discussion
4.1. Structural Properties
Figure 4 illustrates the XRD pattern of BaBiZnB-SLS glass samples within the range of 10° 90°. It can be seen that all fabricated glasses are amorphous in nature due to the absence of sharp peaks and discrete lines. Furthermore, a broad hump was observed in all fabricated glasses between 25° and 35° [25]. The energy-dispersive X-ray (EDX) technique was used to analyze the chemical content of BaBiZnB-SLS glass samples. It can be observed that these glass samples included elements such as oxygen (O), boron (B), silicon (Si), bismuth (Bi), bismuth (Zn), and barium (Ba), as illustrated in Figure 5. The chemical compositions of the SLS glass waste was analyzed utilizing energy dispersive X-ray fluorescence at Universiti Sains Malaysia’s Centre for Global Archaeological Research. As shown in Figure 6, SLS contains multichemical compounds such as 74.1% SiO2 as well as other minor elements [26].
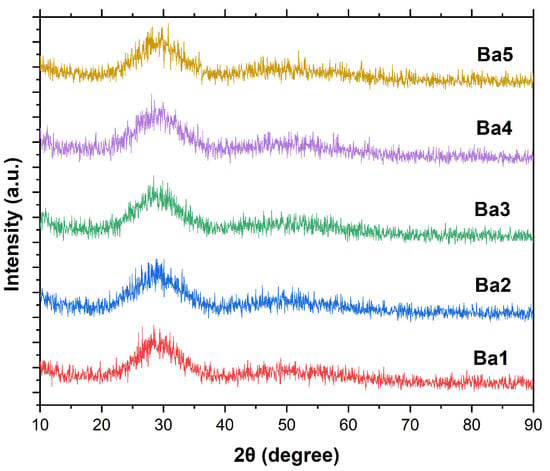
Figure 4.
XRD patterns of BaBiZnB-SLS glass samples.
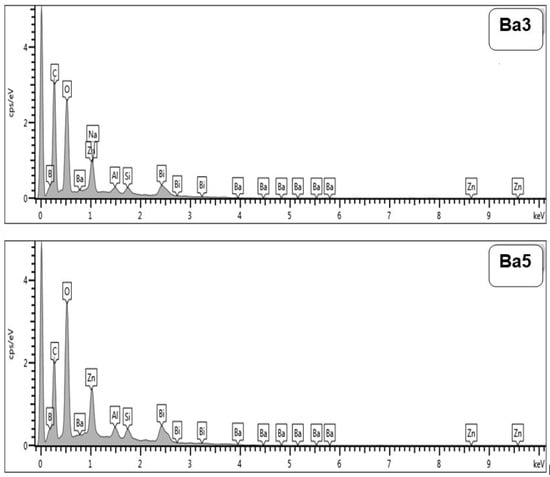
Figure 5.
EDX spectra of Ba3 and Ba5 glass samples.
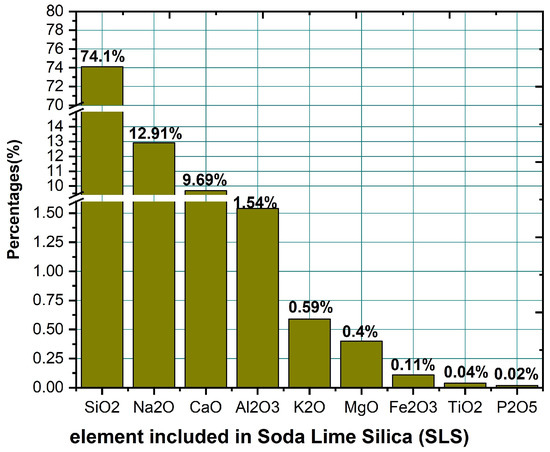
Figure 6.
Element included in soda lime silica (SLS).
4.2. Physical Properties
The physical characteristics values of BaBiZnB-SLS glass samples were measured and are illustrated in Table 2. Figure 7 clarifies the and variation of the prepared glass samples as a function of BaO mol. It can be seen that the slightly increased from 5.157 to 5.256 g cm−3 with increasing BaO content. The increase is due to the replacement of the Bi2O3-ZnO-B2O3-SLS (molecular weight are 149.66 g mol−1) with BaO (molecular weight is 153.33 g mol−1). Furthermore, according to Figure 7, it can be seen that values decreased from 29.1 to 28.49 cm3/mol, indicating resistance to the formation of nonbridging oxygens as well as shrinkage of the glass structure [12,27]. The other physical and structural parameters such as oxygen packing density (OPD), Ba-ion concentration, , , and field strength F of the glass samples were measured to describe BaO’s influence on the BiZnB-SLS glass network. The measured parameters are listed in Table 2. The result indicates that the OPD, , and decreased with increasing BaO concentration. On the other hand, Ba-ion concentration and field strength were increased, hence confirming that the glass network has become more compact [28].

Table 2.
Physical characteristics of BaBiZnB-SLS glass samples.
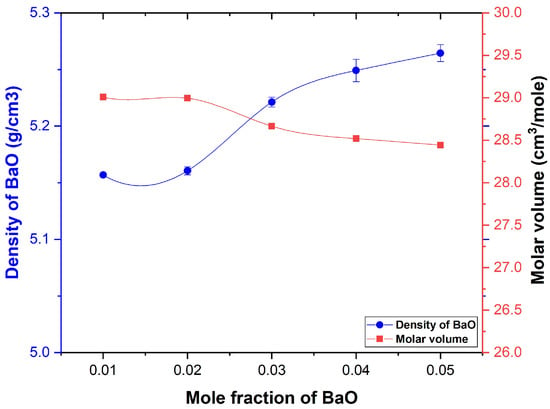
Figure 7.
Density and molar volume of BaBiZnB-SLS glasses as a function of the mole fraction.
4.3. Attenuation of Gamma Rays
The mass attenuation coefficient of BiZnB-SlS glass doped BaO was measured utilizing the X-ray fluorescence technique XRF and narrow beam geometry. The experimental values using XRF for prepared glass were investigated at photon energies of 16.61, 17.74, 21.17, and 25.27 keV and narrow beam geometry at photon energies of 59.54, 662, and 1333 keV. The measured values can be seen in Table 3. It is worth mentioning that the prepared glasses stop almost all the photons with energies of 16.61, 17.74, 21.17, and 25.27 keV, and most of the photons cannot reach the detector. The results indicated that the fabricated glass samples have the ability to prevent photons from penetrating glass and reach the detector when photon energy is less than 25.27 keV. Figure 8 shows the of glass samples as a function of BaO mole fraction. For the energy range from 59.54 to 662 keV, decreased sharply with gamma energy increasing since the photoelectric effect was dominant in this energy range. However, from 662 to 1333 keV, decreased slightly with increasing gamma rays, whereas the Compton effect was dominant in this range [7,29]. According to Figure 8, it can be seen that was increased with increasing BaO concentration at 59.54 keV due to the utilization of BaO whose molecular weight is 153.33 g/mol as compared to the Bi2O3-ZnO-B2O3-SLS glasses whose molecular weight is 149.55 g/mole. However, values remained approximately constant at 662 and 1333 keV with increasing BaO concentration. The mass attenuation coefficient at 59.54 keV is higher than at 662 and 1333 keV because the main interaction process at 59.54 is photoelectric [30,31]. The experimental error was computed depending on the error in transmitted and incident gamma ray intensities, thickness, and density. The calculated error in values was less than 4%.

Table 3.
The experimental mass attenuation coefficient (cm2g−1) for prepared glass a 59.54, 662, and 1333 keV energy photon.
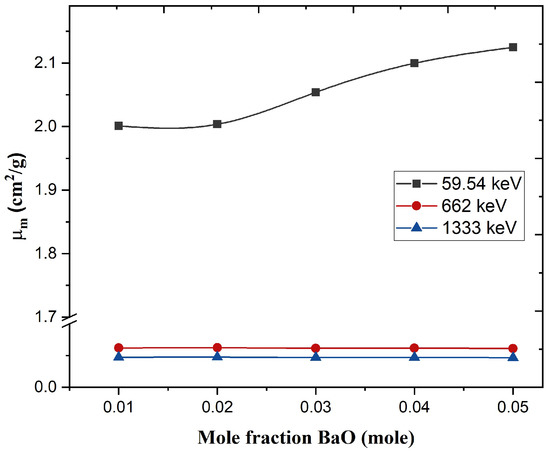
Figure 8.
Mass attenuation coefficient of glass samples as a function of the mole fraction.
Numerous shielding characteristics, such as the , , HVL, and MFP, can be calculated using values as demonstrated in Table 4. According to Table 4 and Figure 9, HVL values increased as gamma energy increased. Furthermore, HVL decreased as BaO concentration increased. In comparison to Barite concrete at 662 and 1333 keV, the current glasses have a lower half-value thickness [27]. Figure 10 illustrates the change in the mean free path of the glasses as a function of the BaO concentration. MFP values were increased as a result of increased photon energy. In addition, MFP decreased as a result of increased BaO content. These values have been compared with Ilmenite concrete at 662 and 1333 keV. It is observed that BaZnB-SLS glasses have lower MFP values than ilmenite concrete. HVL and MFP results support that current glasses have better shielding properties and can be used as radiation shields [7]. and values are mentioned in Table 4. It is obvious that the and values of glass samples have decreased with increasing photon energy. As well, values increased with increased BaO content at 662 and 1333 keV, while they slightly decreased at 59.54 keV. This may be due to the atomic number of barium 56 compared with bismuth 83. The high atomic number indicates that glasses have powerfully absorbed photons [32].

Table 4.
HVL (cm), MFP (cm), , and () (electrons/g) of glass system at 59.54, 662, and 1333 keV.
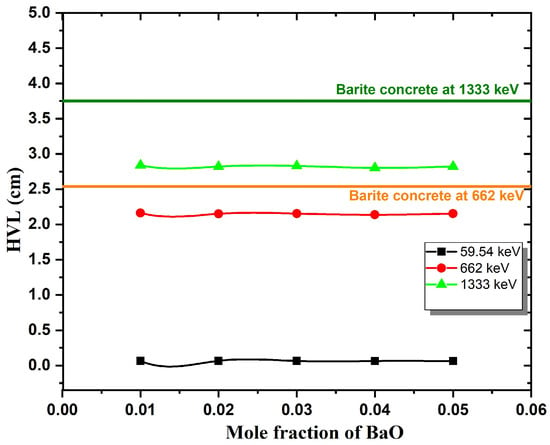
Figure 9.
HVL of BaZnB-SLS glasses as a function of the mole fraction.
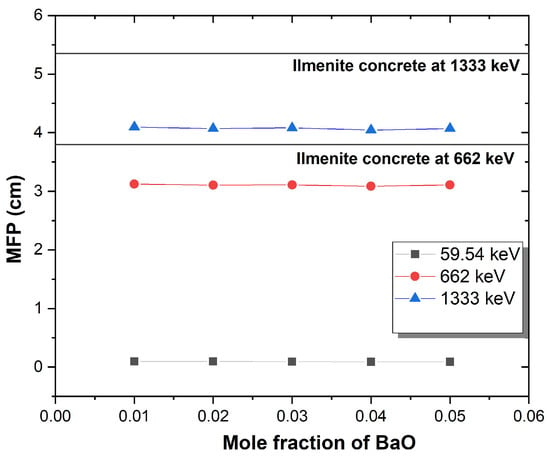
Figure 10.
MFP of BaZnB-SLS glasses as a function of the mole fraction.
5. Conclusions
The glass systems (x) BaO (1 − x)[0.3 ZnO 0.2 Bi2O3 0.2 B2O3 0.3 SLS] (where x are 0.01, 0.02, 0.03, 0.04, and 0.05 mol) were fabricated by using the melt quenching method to be used as a radiation shield and have demonstrated to be a suitable gamma ray shielding material. XRD results confirmed the amorphous nature of the BaBiZnB-SLS glass samples. The XRF analysis revealed that the SLS glass primary composition is 74.1% SiO2. , HVL, MFP, , and were examined. The results showed that glass density and increased and HVL and MFP decreased with an increase in BaO concentration in the glass samples. On the other hand, glass samples are capable of absorbing gamma rays with energies less than 25.27 keV. The study results confirmed that glass samples can be used to develop a lead-free radiation shielding glass that is effective in the specified energy range. In addition, the glass samples are transparent.
Author Contributions
T.H.K.: conception of study, data curation formal analysis, methodology, investigation, and writing—review and editing. I.S.M.: conception of study, investigation, funding acquisition, project administration, investigation, and supervision. M.I.S.: formal analysis, investigation, software, and visualization. A.A.R.: funding acquisition, formal analysis, and visualization. R.H.: software and validation. M.H.M.Z.: formal analysis, data curation, and resources. M.F.I.A.M.: resources and investigation. N.S.E.: methodology and software. H.S.N.: visualization. N.C.K.: data curation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors appreciate the Universiti Sains Malaysia (USM) for the research facilities. Appreciation also goes to the Ministry of Higher Education, Malaysia, for the financial support toward this research under the Fundamental Research Grant Scheme with project code FRGS/1/2019/STG07/ USM/02/19 and project ID 17443 (203/PFIZIK/6711769).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, P.; Singh, D.; Singh, T. Heavy metal oxide glasses as gamma rays shielding material. Nucl. Eng. Des. 2016, 307, 364–376. [Google Scholar] [CrossRef]
- Yasaka, P.; Pattanaboonmee, N.; Kim, H.; Limkitjaroenporn, P.; Kaewkhao, J. Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Dong, M.; Sayyed, M.; Lakshminarayana, G.; Ersundu, M.Ç.; Ersundu, A.; Nayar, P.; Mahdi, M. Investigation of gamma radiation shielding properties of lithium zinc bismuth borate glasses using XCOM program and MCNP5 code. J. Non-Cryst. Solids 2017, 468, 12–16. [Google Scholar] [CrossRef]
- D’Souza, A.N.; Prabhu, N.S.; Sharmila, K.; Sayyed, M.; Somshekarappa, H.; Lakshminarayana, G.; Mandal, S.; Kamath, S.D. Role of Bi2O3 in altering the structural, optical, mechanical, radiation shielding and thermoluminescence properties of heavy metal oxide borosilicate glasses. J. Non-Cryst. Solids 2020, 542, 120136. [Google Scholar] [CrossRef]
- Kurudirek, M.; Chutithanapanon, N.; Laopaiboon, R.; Yenchai, C.; Bootjomchai, C. Effect of Bi2O3 on gamma ray shielding and structural properties of borosilicate glasses recycled from high pressure sodium lamp glass. J. Alloys Compd. 2018, 745, 355–364. [Google Scholar] [CrossRef]
- Bootjomchai, C.; Laopaiboon, J.; Yenchai, C.; Laopaiboon, R. Gamma-ray shielding and structural properties of barium–bismuth–borosilicate glasses. Radiat. Phys. Chem. 2012, 81, 785–790. [Google Scholar] [CrossRef]
- Sanz, O.; Haro-Poniatowski, E.; Gonzalo, J.; Navarro, J.F. Influence of the melting conditions of heavy metal oxide glasses containing bismuth oxide on their optical absorption. J. Non-Cryst. Solids 2006, 352, 761–768. [Google Scholar] [CrossRef]
- Kurtulus, R.; Kavas, T. Investigation on the physical properties, shielding parameters, glass formation ability, and cost analysis for waste soda-lime-silica (SLS) glass containing SrO. Radiat. Phys. Chem. 2020, 176, 109090. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Kumar, A.; Yang, H.; Sayyed, M.; Liu, S.; Bu, E. A novel method of utilization of hot dip galvanizing slag using the heat waste from itself for protection from radiation. J. Hazard. Mater. 2018, 344, 602–614. [Google Scholar] [CrossRef]
- Almasri, K.A.; Matori, K.A.; Zaid, M.H.M. Effect of sintering temperature on physical, structural and optical properties of wollastonite based glass-ceramic derived from waste soda lime silica glasses. Results Phys. 2017, 7, 2242–2247. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, R.; Sayyed, M.; Rashad, M.; Singh, M.; Ali, A.M. Physical, structural, optical and gamma ray shielding behavior of (20+x) PbO–10 BaO–10 Na2O–10 MgO–(50-x) B2O3 glasses. Phys. B Condens. Matter 2019, 552, 110–118. [Google Scholar] [CrossRef]
- Pawar, P.; Munishwar, S.; Gautam, S.; Gedam, R. Physical, thermal, structural and optical properties of Dy3+ doped lithium alumino-borate glasses for bright W-LED. J. Lumin. 2017, 183, 79–88. [Google Scholar] [CrossRef]
- Pawar, P.; Munishwar, S.; Gedam, R. Intense white light luminescent Dy3+ doped lithium borate glasses for W-LED: A correlation between physical, thermal, structural and optical properties. Solid State Sci. 2017, 64, 41–50. [Google Scholar] [CrossRef]
- Ichoja, A.; Hashim, S.; Ghoshal, S.; Hashim, I.; Omar, R. Physical, structural and optical studies on magnesium borate glasses doped with dysprosium ion. J. Rare Earths 2018, 36, 1264–1271. [Google Scholar] [CrossRef]
- Mhareb, M.; Hashim, S.; Ghoshal, S.; Alajerami, Y.; Bqoor, M.; Hamdan, A.; Saleh, M.; Karim, M.A. Effect of Dy2O3 impurities on the physical, optical and thermoluminescence properties of lithium borate glass. J. Lumin. 2016, 177, 366–372. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Liu, S.; Yang, H.; Li, Z.; Sayyed, M.; Agar, O. Using iron concentrate in Liaoning Province, China, to prepare material for X-Ray shielding. J. Clean. Prod. 2019, 210, 653–659. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.A. Effective atomic number and mass attenuation coefficient of PbO–BaO–B2O3 glass system. Radiat. Phys. Chem. 2016, 120, 33–37. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Sayyed, M.; Khandaker, M.U.; Bradley, D. The potential use of boron containing resources for protection against nuclear radiation. Radiat. Phys. Chem. 2021, 188, 109601. [Google Scholar] [CrossRef]
- Sayyed, M.; Elmahroug, Y.; Elbashir, B.; Issa, S.A. Gamma-ray shielding properties of zinc oxide soda lime silica glasses. J. Mater. Sci. Mater. Electron. 2017, 28, 4064–4074. [Google Scholar] [CrossRef]
- Kavaz, E.; Tekin, H.; Yorgun, N.Y.; Özdemir, Ö.; Sayyed, M. Structural and nuclear radiation shielding properties of bauxite ore doped lithium borate glasses: Experimental and Monte Carlo study. Radiat. Phys. Chem. 2019, 162, 187–193. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Barbi, S.; Mugoni, C.; Montorsi, M.; Affatigato, M.; Gatto, C.; Siligardi, C. Structural and optical properties of cerium oxide doped barium bismuth borate glasses. J. Non-Cryst. Solids 2018, 499, 183–188. [Google Scholar] [CrossRef]
- Kurtulus, R.; Kavas, T.; Akkurt, I.; Gunoglu, K. An experimental study and WinXCom calculations on X-ray photon characteristics of Bi2O3-and Sb2O3-added waste soda-lime-silica glass. Ceram. Int. 2020, 46, 21120–21127. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, K.; Anand, V. Correlation of gamma ray shielding and structural properties of PbO–BaO–P2O5 glass system. Nucl. Eng. Des. 2015, 285, 31–38. [Google Scholar] [CrossRef]
- Saeed, A.; Elbashar, Y.; El Khameesy, S. A novel barium borate glasses for optical applications. Silicon 2018, 10, 569–574. [Google Scholar] [CrossRef]
- Waly, E.S.A.; Fusco, M.A.; Bourham, M.A. Gamma-ray mass attenuation coefficient and half value layer factor of some oxide glass shielding materials. Ann. Nucl. Energy 2016, 96, 26–30. [Google Scholar] [CrossRef]
- Kaewkhao, J.; Limsuwan, P. Mass attenuation coefficients and effective atomic numbers in phosphate glass containing Bi2O3, PbO and BaO at 662 keV. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 619, 295–297. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Kedkaew, C.; Chewpraditkul, W.; Pokaipisit, A.; Limsuwan, P. Study on interaction of Bi2O3, PbO and BaO in silicate glass system at 662 keV for development of gamma-rays shielding materials. Prog. Nucl. Sci. Technol. 2011, 1, 106–109. [Google Scholar] [CrossRef]
- Bagheri, R.; Adeli, R. Gamma-ray shielding properties of phosphate glasses containing Bi2O3, PbO, and BaO in different rates. Radiat. Phys. Chem. 2020, 174, 108918. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).