Influence of Residue Type and Method of Placement on Dynamics of Decomposition and Nitrogen Release in Maize-Wheat-Mungbean Cropping on Permanent Raised Beds: A Litterbag Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Parameters
2.2. Treatments and Experimental Design
2.2.1. Chemical Analysis of Residue Samples and Calculations
2.2.2. Modelling Decomposition and N Release from Plant Residues
2.3. Statistical Analysis and Model Validation
3. Results
3.1. Model Validation for Residue Decomposition and Nitrogen Release
3.2. Residue Decomposition
3.3. Nitrogen Release
4. Discussion
4.1. Residue Decomposition Kinetics
4.2. Nitrogen Release Dynamics
4.3. Humification Coefficient
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, M.R.; Parihar, C.M.; Jat, S.L.; Singh, A.K.; Kumar, D.; Pooniya, V.; Parihar, M.D.; Saveipune, D.; Parmar, H.; Jat, M.L. Effectoflong-term till ageand diversified crop rotations on nutrient up-take, profitability and energetics of maize (Zea mays) in north-western India. Indian J. Agric. Sci. 2016, 86, 743–749. [Google Scholar]
- Sharma, P.C.; Jat, H.S.; Kumar, V.; Gathala, M.K.; Datta, A.; Yaduvanshi, N.P.S.; Choudhary, M.; Sharma, S.; Singh, L.K.; Saharawat, Y.; et al. Sustainable Intensification Opportunities under Current and Future Cereal Systems of North-West India; TechnicalBulletin: CSSRI/Karnal/2015/4; Central Soil Salinity Research Institute: Karnal, India, 2015; p. 46. [Google Scholar]
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of north west China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reicosky, D.C.; Archer, D.W. Moldboard plow tillage depth and short-term carbon dioxide release. Soil Tillage Res. 2007, 94, 109–121. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Remund, A.J.; Tiry, C.M.; Secott, T.E. Litter decomposition of three lignin-deficient mutants of Sorghum bicolor during spring thaw. Acta Oncol. 2018, 91, 16–21. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of conservation agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Manchanda, J.S.; Garg, A.K.; Kumar, S.; Dercon, G.; Nguyen, M.L. Crop production and nutrient use efficiency of conservation agriculture for soybean–wheat rotation in the Indo-Gangetic plains of Northwestern India. Soil Tillage Res. 2012, 120, 50–60. [Google Scholar] [CrossRef]
- Hassan, I.; Hussain, Z.; Akbar, G. Effect of Permanent Raised Beds on Water Productivity for Irrigated Maize-Wheat Cropping System. Australian Centre for International Agriculture Research Proceeding 121, of a Workshop on “Evaluation and Performance of Permanent Raised Bed Cropping Systems in Asia, Australia and Mexico. 2005. Available online: https://www.aciar.gov.au/sites/default/files/legacy/node/703/pr121_pdf_18149.pdf (accessed on 18 December 2021).
- Das, T.K.; Bhattacharyya, R.; Sudhishri, S.; Sharma, A.R.; Saharawat, Y.S.; Bandyopadhyay, K.K.; Sepat, S.; Bana, R.S.; Aggarwal, P.; Sharma, R.K.; et al. Conservation agriculture in an irrigated cotton–wheat system of the western In-do-Gangetic Plains: Crop and water productivity and economic profitability. Field Crops Res. 2014, 158, 24–33. [Google Scholar] [CrossRef]
- Jat, H.S.; Kumar, P.; Sutaliya, J.M.; Kumar, S.; Choudhary, M.; Yadvinder Singh Jat, M.L. Conservation agriculture based sustainable intensification of basmati rice-wheat system in North-West India. Arch. Agron. Soil Sci. 2019, 65, 1370–1386. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Goudeseune, B.; DeCorte, P.; Lichter, K.; Dendooven, L.; Deckers, J. Conservation agriculture as a sustainable option for the central Mexican highlands. Soil Till. Res. 2009, 103, 222–230. [Google Scholar] [CrossRef]
- Jat, H.S.; Singh, G.; Singh, R.; Choudhary, M.; Jat, M.L.; Gathala, M.K.; Sharma, D.K. Management influence on maize–wheat system performance, water productivity and soil biology. Soil Use Manag. 2015, 31, 534–543. [Google Scholar] [CrossRef]
- Gathala, M.K.; Kumar, V.; Sharma, P.C.; Saharawat, Y.S.; Sharma, P.K.; Jat, H.S.; Singh, M.; Kumar, A.; Jat, M.L.; Humphreys, E.; et al. Optimizing intensive cereal-based cropping systems addressing current and future drivers of agricultural change in the north-western Indo-Gangetic plains of India. Agric. Ecosyst. Environ. 2013, 177, 85–97. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ladha, J.K. Placement effects on rice residue decomposition and nutrient dynamics on two soil types during wheat cropping in rice–wheat system in north-western India. Nutr. Cycl. Agroecosyst. 2010, 88, 471–480. [Google Scholar]
- Hiloidhari, M.; Das, D.; Baruah, D.C. Bioenergy potential from crop residue biomass in India. Renew. Sustain. Energy Rev. 2014, 32, 504–512. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter Decomposition Humus Formation. In Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2003; p. 296. [Google Scholar]
- Singh, Y.; Sidhu, H.S. Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic plains of India. Proc. Indian Nat. Sci. Acad. 2014, 80, 95–114. [Google Scholar] [CrossRef]
- National Academy of Agricultural Sciences. Innovative Viable Solution to Rice Residue Burning in Rice-Wheat Cropping System through Concurrent Use of Super Straw Management System-fitted Combines and Turbo Happy Seeder; Policy Brief No.2; National Academy of Agricultural Sciences: New Delhi, India, 2017; 16p. [Google Scholar]
- Scagnozzi, A.; Saviozzi, A.; Levi-Minzi, R.; Riffaldi, R. Nutrient release from decomposing crop residues in soil: A laboratory experiment. Am. J. Altern. Agric. 1997, 12, 10–13. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Goek, M.; Kara, E.E. Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turn-over. Soil Biol. Biochem. 2004, 36, 255–266. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Crop residue removal impacts on soil productivity and environmental quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Nogueirol, R.C.; Menandro, L.M.S.; Bordonal, R.O.; Borges, C.D.; Cantarella, H.; Franco, H.C.J. Agronomic and environmental implications of sugarcane straw removal: A major review. Glob. Chang. Biol. Bioenergy 2016, 9, 1181–1195. [Google Scholar] [CrossRef]
- Stavi, I.; Bel, G.; Zaady, E. Soil functions and ecosystem services in conventional, conservation, and integrated agricultural systems: A review. Agron. Sustain. Dev. 2016, 36, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.M.F.; Wilhelm, W.W.; Karlen, D.L.; Archer, D.W.; Wienhold, B.; Lightle, D.T. Nutrient removal as a function of corn stover cutting height and cob harvest. BioEnergy Res. 2010, 3, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Yadvinder-Singh; Kukal, S.S.; Jat, M.L.; Sidhu, H.S. Improving water productivity of wheat-based cropping systems in South Asia for sustained productivity. Adv. Agron. 2014, 127, 157–258. [Google Scholar]
- Polyakova, O.; Billor, N. Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For. Ecol. Manag. 2007, 253, 11–18. [Google Scholar] [CrossRef]
- Heal, O.W.; Anderson, J.M.; Swift, M.J. Plant residue quality and decomposition: An historical overview. In Driven by Nature: Plant Residue Quality and Decom-Position; Cadisch, G., Giller, K.E., Eds.; CAB Int: Cambridge, UK, 1997; pp. 3–30. [Google Scholar]
- Singh, Y.; Singh, B.; Timsina, J. Crop residue management for nutrient cycling and improving soil productivity in rice-based cropping systems in the tropics. Adv. Agron. 2005, 85, 269. [Google Scholar]
- Abera, G.; Wolde-Meskel, E.; Bakken, L.R. Carbon and nitrogen mineralization dynamics in different soils of the tropics amended with legume residues and contrasting soil moisture contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Kriauciuniene, Z.; Velička, R.; Raudonius, S. The influence of crop residues type on their decomposition rate in the soil: A litterbag study. Zemdirbyste 2012, 99, 227–236. [Google Scholar]
- Trinsoutrot, I.; Recous, S.; Mary, B.; Nicolardot, B. C and N flux of decomposing 13C and 15N Brassica napus L.: Effect of residue composition and N content. Soil Biol. Biochem. 2000, 32, 1717–1730. [Google Scholar] [CrossRef]
- Redin, M.; Guénon, R.; Recous, S.; Schmatz, R.; de Freitas, L.L.; Aita, C.; Giacomini, S.J. Carbon mineralization in soil of roots from twenty crop species, as affected by their chemical composition and botanical family. Plant Soil 2014, 378, 205–214. [Google Scholar] [CrossRef]
- Wang, X.; Sun, B.; Mao, J.; Sui, Y.; Cao, X. Structural convergence of maize and wheat straw during two-year decomposition under different climate conditions. Environ. Sci. Technol. 2012, 46, 7159–7165. [Google Scholar] [CrossRef]
- Thippayarugs, S.; Toomsan, B.; Vityakon, P.; Limpinuntana, V.; Patanothai, A.; Cadisch, G.G. Interactions in decomposition and N mineralization between tropical legume residue components. Agric. Forest. Syst. 2008, 72, 137–148. [Google Scholar] [CrossRef]
- Salinas-Garcia, J.R.; Báez-González, A.D.; Tiscareño-López, M.; Rosales-Robles, E. Residue removal and tillage interaction effects on soil properties under rain-fed corn production in Central Mexico. Soil Tillage Res. 2001, 59, 67–79. [Google Scholar] [CrossRef]
- Yadvinder-Singh; Bijay-Singh; Ladha, J.K.; Khind, C.S.; Khera, T.S.; Bueno, C.S. Effects of residue decomposition on productivity and soil fertility in rice–wheat rotation. Soil Sci. Soc. Am. J. 2004, 68, 854–864. [Google Scholar] [CrossRef]
- Ambus, P.; Jensen, E.S. Crop residue management strategies to reduce N-losses-Interaction with crop N supply. Commun. Soil Sci. Plant Anal. 2001, 32, 981–996. [Google Scholar] [CrossRef]
- Beare, M.H. Fungal and bacterial pathways of organic matter decomposition and nitrogen mineralization in arable in arable soils. In Soil Ecology in Sustainable Agricultural Systems; Brussard, L., Frrera-Cerrato, R., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1997; pp. 37–70. [Google Scholar]
- Cookson, W.R.; Beare, M.H.; Wilson, P.E. Effects of prior crop residue management onmicrobial properties and crop residue decomposition. Appl. Soil Ecol. 1998, 7, 179–188. [Google Scholar] [CrossRef]
- Wang, H.; Curtin, D.; Jame, Y.W.; McConkey, B.G.; Zhou, H.F. Simulation of soil carbon dioxide flux during plant residue decomposition. Soil Sci. Soc. Am. J. 2001, 66, 1304–1310. [Google Scholar] [CrossRef]
- Datta, A.; Jat, H.S.; Yadav, A.K.; Choudhary, M.; Sharma, P.C.; Rai, M.; Singh, L.K.; Majumder, S.P.; Choudhary, V.; Jat, M.L. Carbon mineralization in soil as influenced by crop residue type and placement in an Alfisols of Northwest India. Carbon Manag. 2019, 10, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Asakawa, N.M. Decomposition of leaf litter from tropical forage grasses and legumes. Soil Biol. Biochem. 1993, 25, 1351–1361. [Google Scholar] [CrossRef]
- Cai, A.; Liang, G.; Zhang, X.; Zhang, W.; Li, L.; Rui, Y.; Xu, M.; Luo, Y. Long-term straw decomposition in agroecosystems described by a unified three-exponentiation equation with thermal time. Sci. Total Environ. 2018, 636, 699–708. [Google Scholar] [CrossRef]
- Williams, L.K.; Shaw, J.D.; Sindel, B.M.; Wilson, S.C.; Kristiansen, P. Longevity, growth and community ecology of invasive Poaannua across environmental gradients in the subantarctic. Basic Appl. Ecol. 2018, 29, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Core Team R Nlme: Linear and nonlinear mixed effects models. R Package Version 2016, 3, 1–137. [Google Scholar]
- Coppens, F.; Garnier, P.; Findeling, A.; Merckx, R.; Recous, S. Decomposition of mulched versus incorporated crop residues: Modelling with PASTIS clarifies interactions between residue quality and location. Soil Biol. Biochem. 2007, 39, 2339–2350. [Google Scholar] [CrossRef]
- Da Silva, W.B.; Périco, E.; Dalzochio, M.S.; Santos, M.; Cajaiba, R.L. Are litterfall and litter decomposition processes indicators of forest regeneration in the neotropics? Insights from a case study in the Brazilian Amazon. For. Ecol. Manag. 2018, 429, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Gregorich, E.G.; Janzen, H.; Ellert, B.H.; Helgason, B.L.; Qian, B.; Zebarth, B.J.; Angers, D.A.; Beyaert, R.P.; Drury, C.F.; Duguid, S.D.; et al. Litter decay con-trolled by temperature, not soil properties, affecting future soil carbon. Glob. Chang. Biol. 2016, 23, 1725–1735. [Google Scholar] [CrossRef]
- Lal, R. Crop residues as soil amendments and feedstock for bioethanol production. Waste Manag. 2008, 28, 747–758. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, N.; Wu, L.; Xu, M.; Bingham, I.J.; Li, Z. Effects of enhancing soil organic carbon sequestration in the topsoil by fertilization on crop productivity and stability: Evidence from long-term experiments with wheat-maize cropping systems in China. Sci. Total Environ. 2016, 562, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Amin, B.A.Z.; Chabbert, B.; Moorhead, D.; Bertrand, I. Impact of fine litter chemistry on lignocellu-lolytic enzyme efficiency during decomposition of maize leaf and root in soil. Biogeochemistry 2013, 117, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Piñeiro, G.; Jackson, R.B.; Jobbágy, E.G.; Kim, J.H.; Porporato, A. Analytical models of soil and litter decomposition: Solutions for mass loss and timedependent decay rates. Soil Biol. Biochem. 2012, 50, 66–76. [Google Scholar] [CrossRef]
- Janssen, B.H. A simple method for calculating decomposition and accumulation of ‘young’ soil organic matter. Plant Soil 1984, 76, 297–304. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agriculture handbook no. 379; Agriculture Research Service USDA: Washington, DC, USA, 1970; p. 20.
- Keeny, D.R.; Nelson, D.W. Inorganic forms of nitrogen. In Methods of Soil Analysis, Part 2; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Luo, Y.; Keenan, T.F.; Smith, M. Predictability of the terrestrial carbon cycle. Glob. Chang. Biol. 2015, 21, 1737–1751. [Google Scholar] [CrossRef]
- Ghimire, B.; Ghimire, R.; VanLeeuwen, D.; Mesbah, A. Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization. Sustainability 2017, 9, 2316. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Han, G.; Jia, Y.; Feng, X.; Tian, X. Insight into the temperature sensitivity of forest litter decomposition and soil enzymes in subtropical forest in China. J. Plant Ecol. 2011, 5, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, S.E.; Eddy, W.C.; Buyarski, C.R.; Adair, E.C.; Ogdahl, M.L.; Weisenhorn, P. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol. Monogr. 2012, 82, 389–405. [Google Scholar] [CrossRef]
- Gusewell, S.; Gessner, M.O. N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Loranger, G.; Ponge, J.F.; Imbert, D.; Lavelle, P. Leaf decomposition in two semi-evergreen tropical forests: Influence of litter quality. Biol. Fertil. Soils 2002, 35, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Schomberg, H.H.; Steiner, J.L.; Unger, P.W. Decomposition and nitrogen dynamics of crop residues: Residue quality and water effects. Soil Sci. Soc. Am. J. 1994, 58, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Ghidey, F.; Alberts, E.E. Residue type and placement effects on decomposition: Field study and model evaluation. Trans. Am. Soc. Agric. Eng. 1993, 36, 1611–1617. [Google Scholar] [CrossRef]
- Jat, R.L.; Pramod, J.; Dotaniya, M.L.; Lakaria, B.L.; Rashmi, I.; Meena, B.P.; Shirale, A.O.; Meena, A.L. Carbon and nitrogen mineralization in vertisol as mediated by type and placement method of residue. Environ. Monit. Assess. 2018, 190, 439. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pe’rez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, E.; Nourbakhsh, F.; Basiri, M. Lignin content of range plant residues controls N mineralization in soil. Eur. J. Soil Biol. 2011, 47, 243–246. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Sollenberger, L.E.; Interrante, S.M.; Vendramini, J.M.B.; Stewart, R.L. Litter decomposition and mineralization in Bahia grass pastures managed at different intensities. Crop Sci. 2006, 46, 1305–1310. [Google Scholar] [CrossRef]
- Hamadi, Z.; Steinberger, Y.; Kutiel, P.; Lavee, H.; Barness, G. Decomposition of Avenasterilis litter under arid conditions. J. Arid Environ. 2000, 46, 281–293. [Google Scholar] [CrossRef]
- Liu, K.; Sollenberger, L.E.; Silveira, M.L.; Vendramini, J.M.B.; Newman, Y.C. Grazing intensity and nitrogen fertilization affect litter responses in ‘Tifton 85% Bermuda grass pastures: II. Decomposition and nitrogen mineralization. Agron. J. 2011, 103, 163–168. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir, M.M.; Sabir, N.; Khurshid, M. Impact of the addition of different plant residues on nitrogen mineralization–immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Goh, K.M. Nitrogen release from crop residues and organic amendments as affected by biochemical composition. Commun. Soil Sci. Plant Anal. 2003, 34, 2441–2460. [Google Scholar] [CrossRef]
- Sandhu, O.S.; Gupta, R.K.; Thind, H.S.; Jat, M.L.; Sidhu, H.S.; Yadvinder-Singh. Drip irrigation and nitrogen management for improving crop yields, nitrogen use efficiency and water productivity of maize-wheat system on permanent beds in north-west India. Agric. Water Manag. 2019, 219, 19–26. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.A.; Zhang, W.; Feng, G.; Wang, X.; Xu, M. Decomposition of organic materials in cropland soils across China: A meta-analysis. Acta Pedol. Sin. 2016, 53, 16–27. [Google Scholar]
- Nicolardot, B.; Recous, S.; Mary, B. Simulation of C and N mineralisation during crop residue decomposition: A simple dynamic model based on the C: N ratio of the residues. Plant Soil 2001, 228, 83–103. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- Smith, P.; Martinio, D.; Cai, Z. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
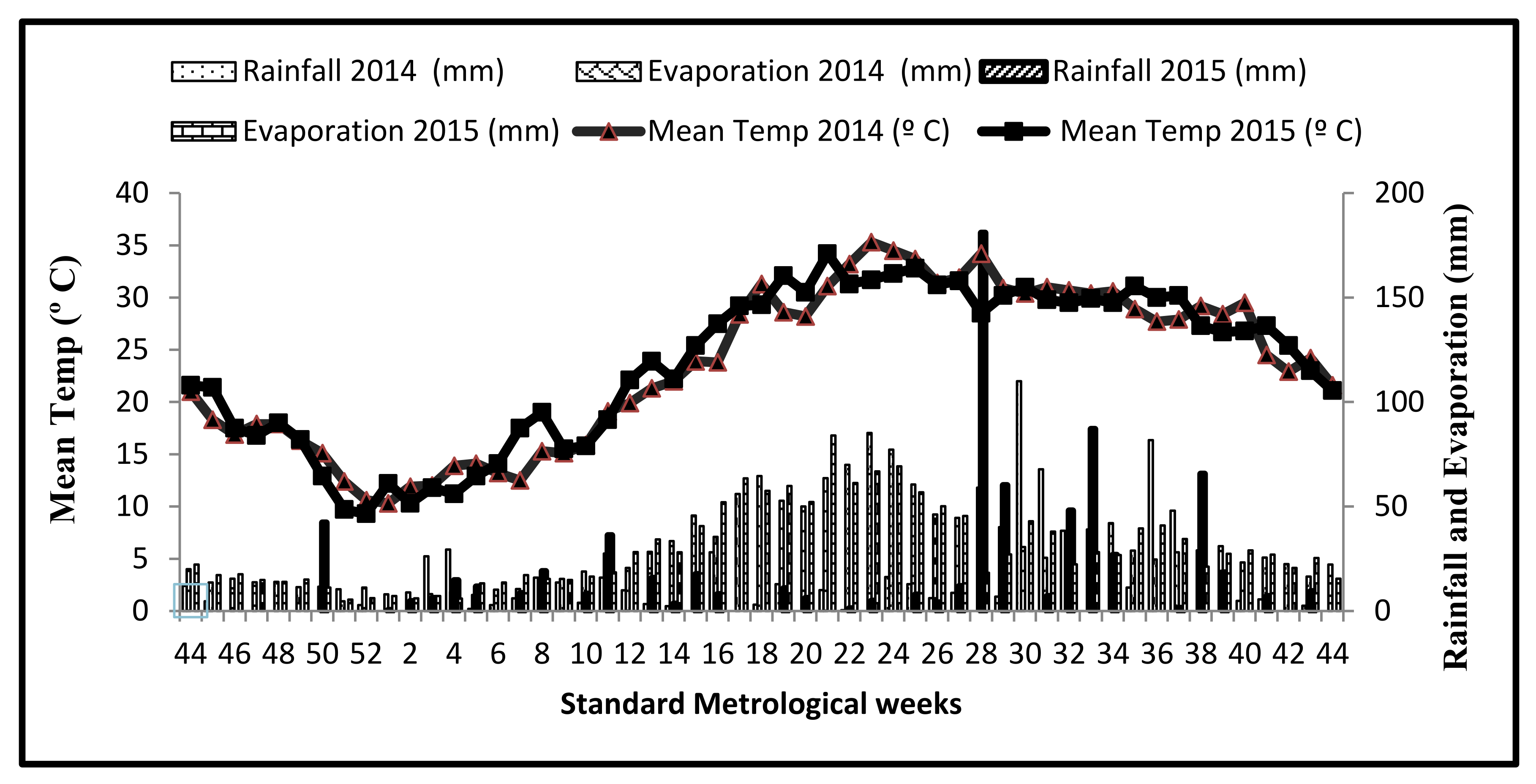
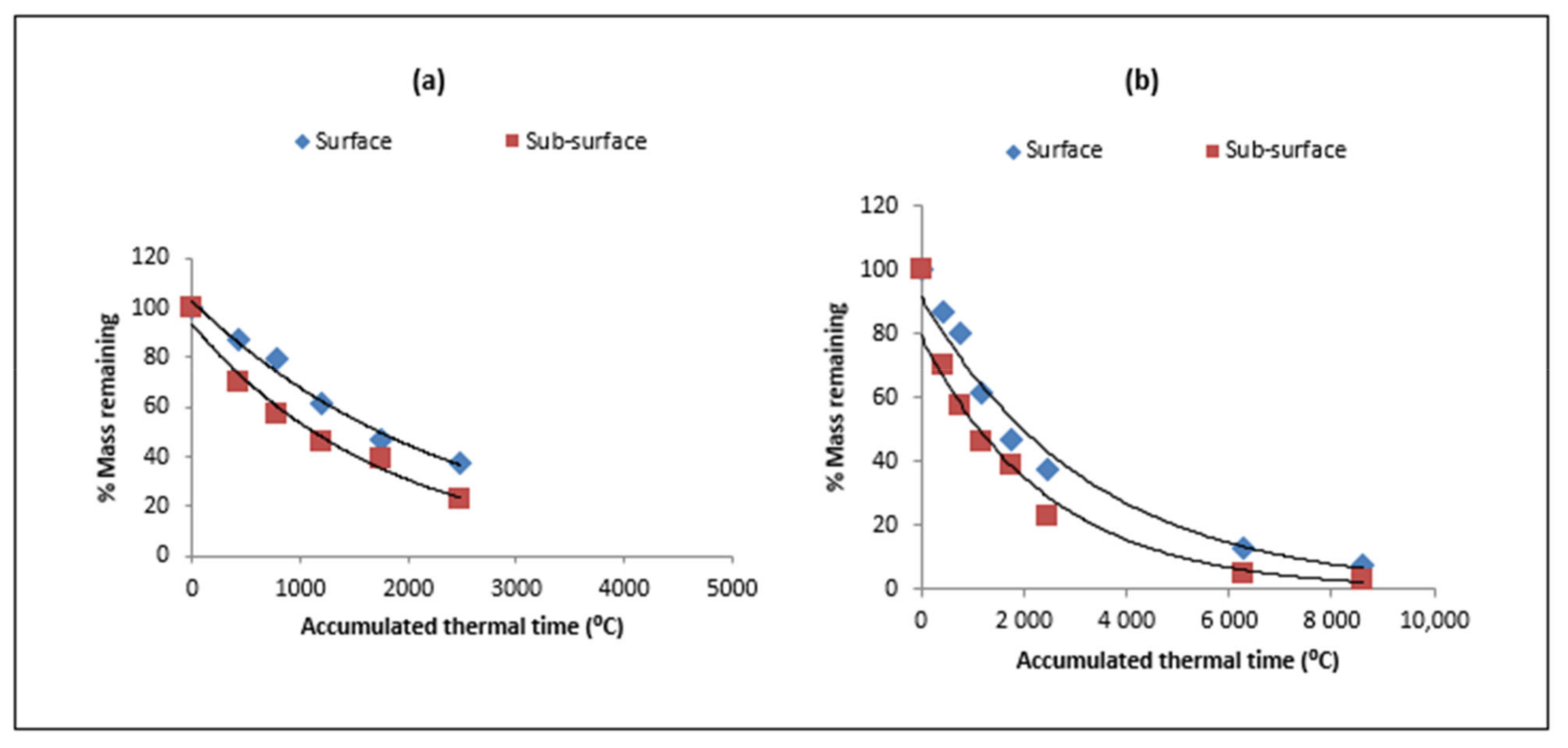
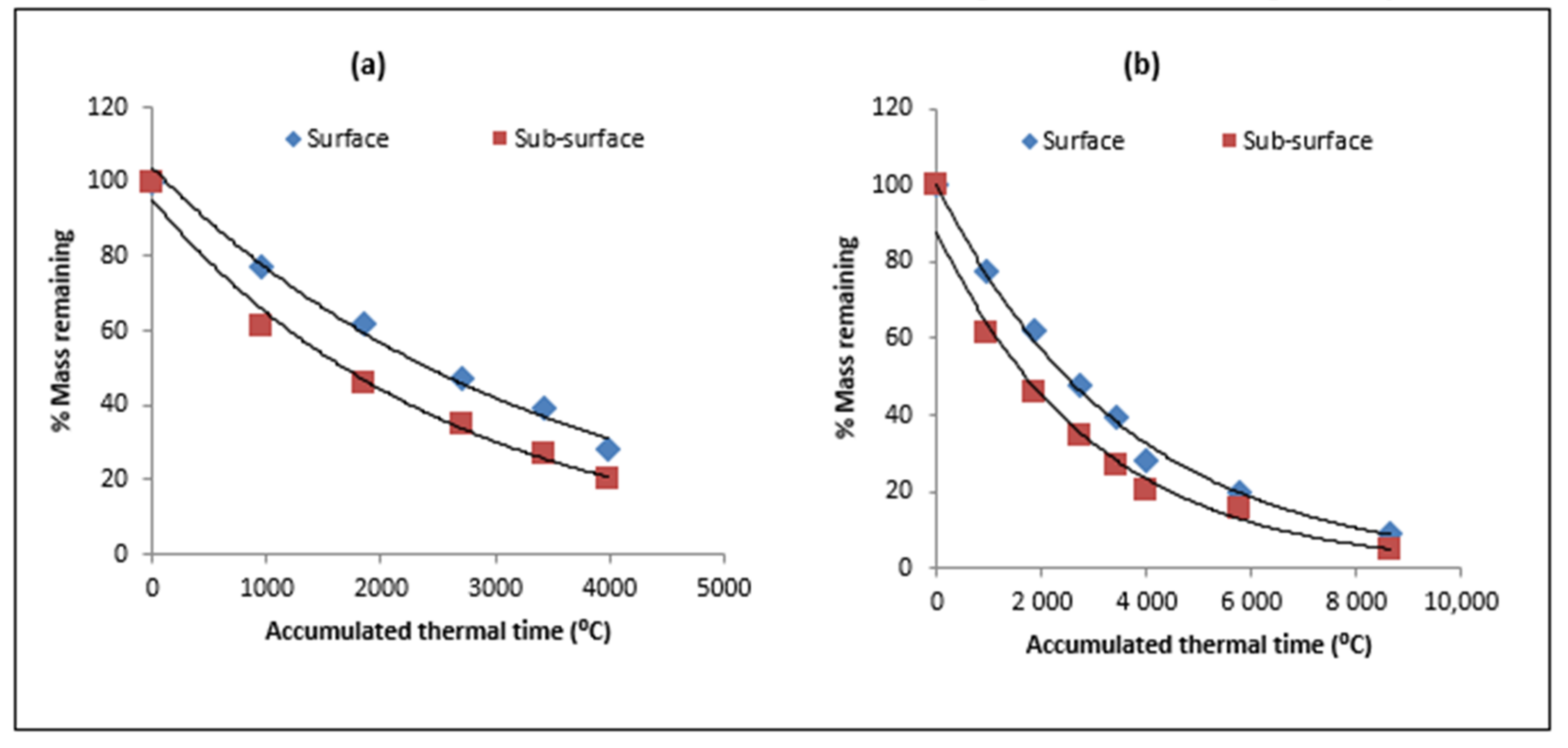
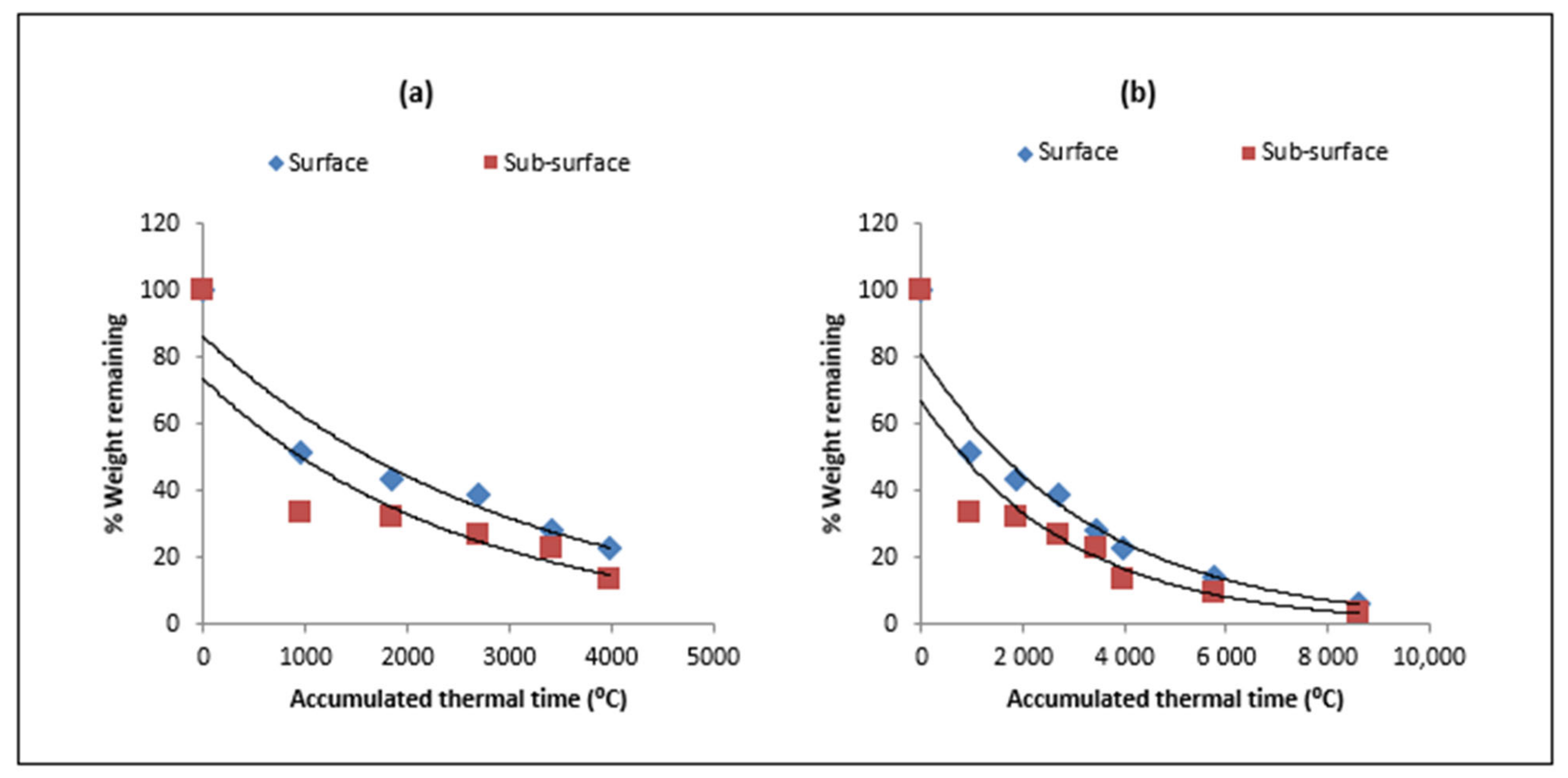
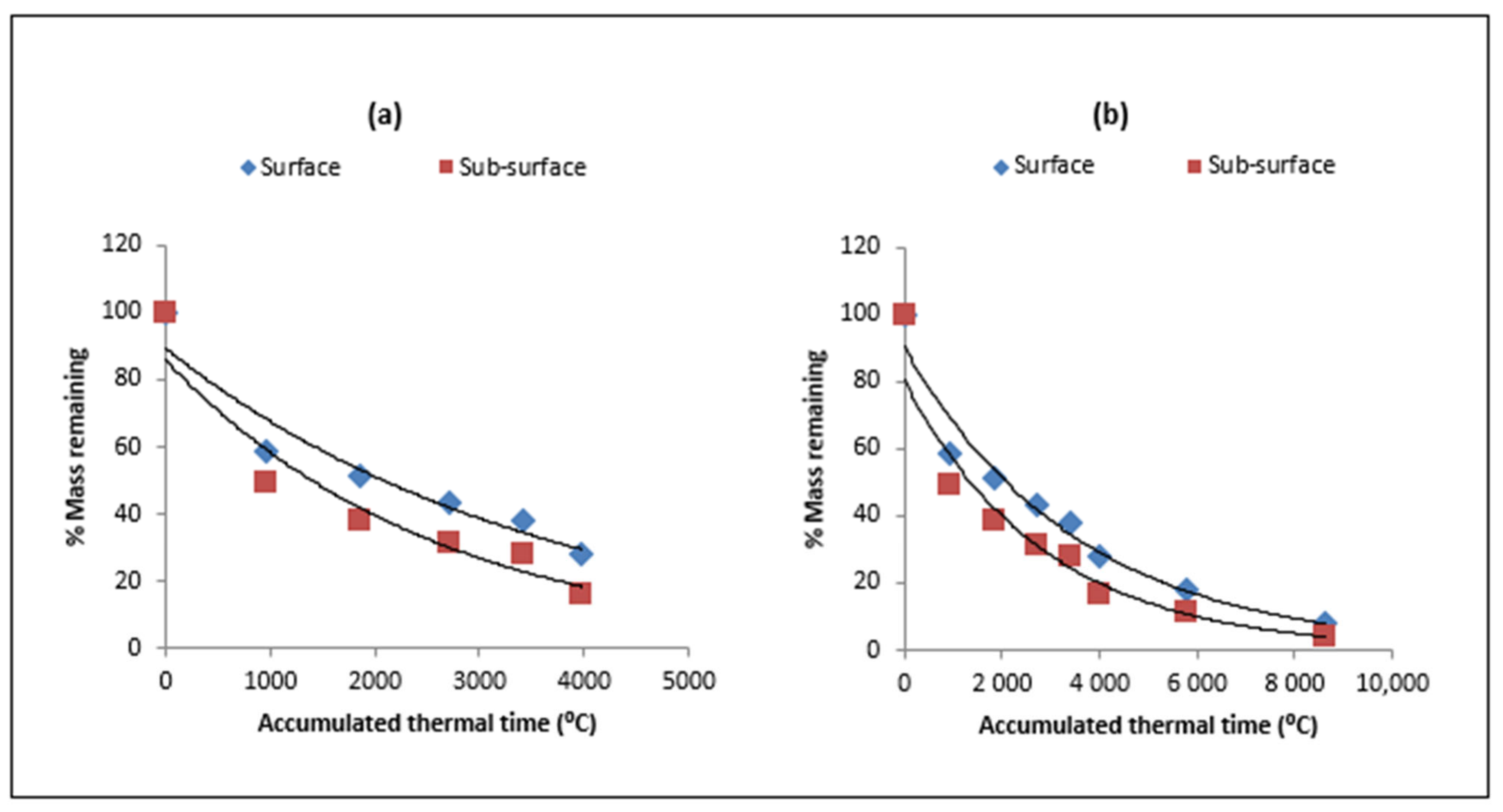
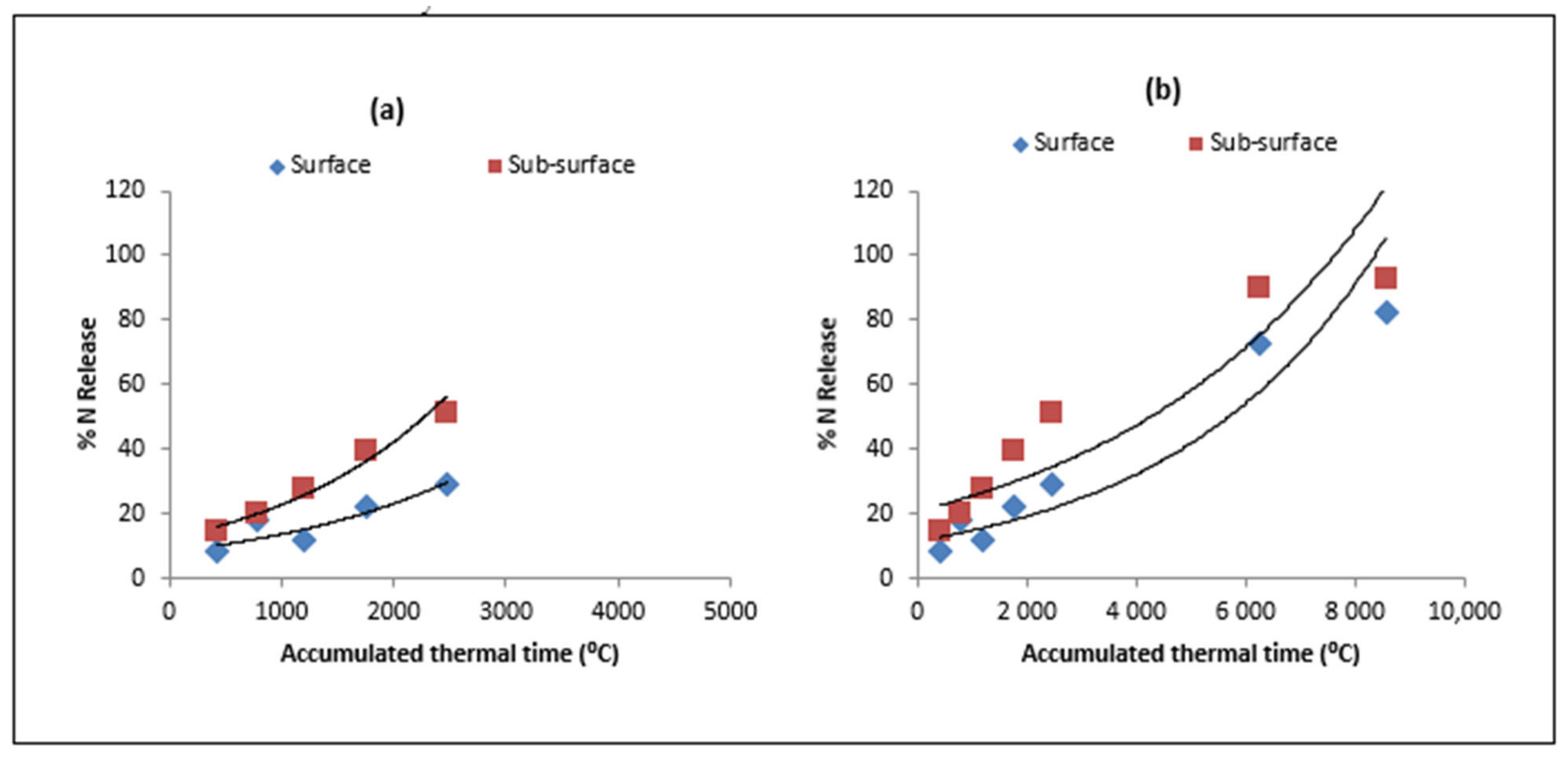

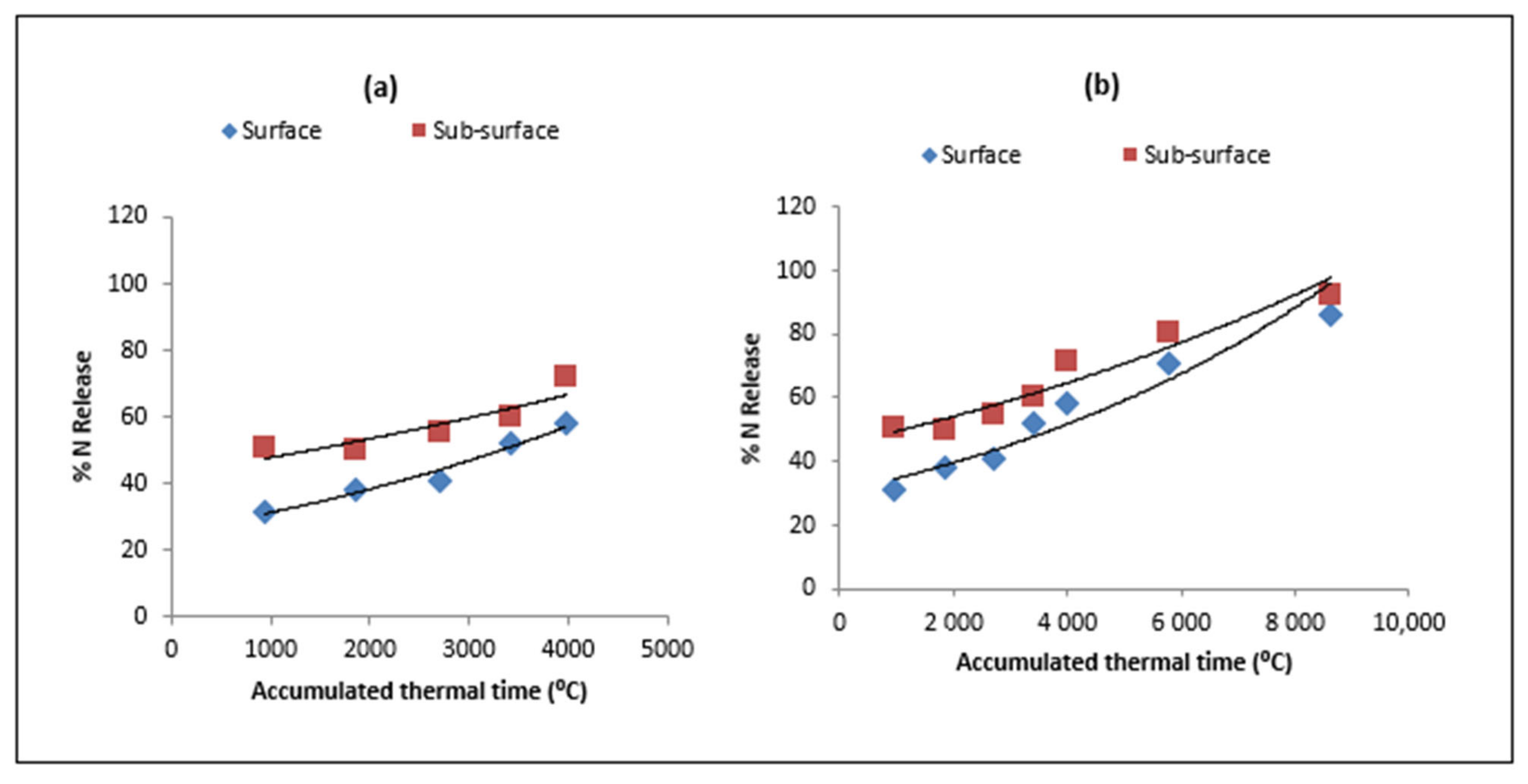
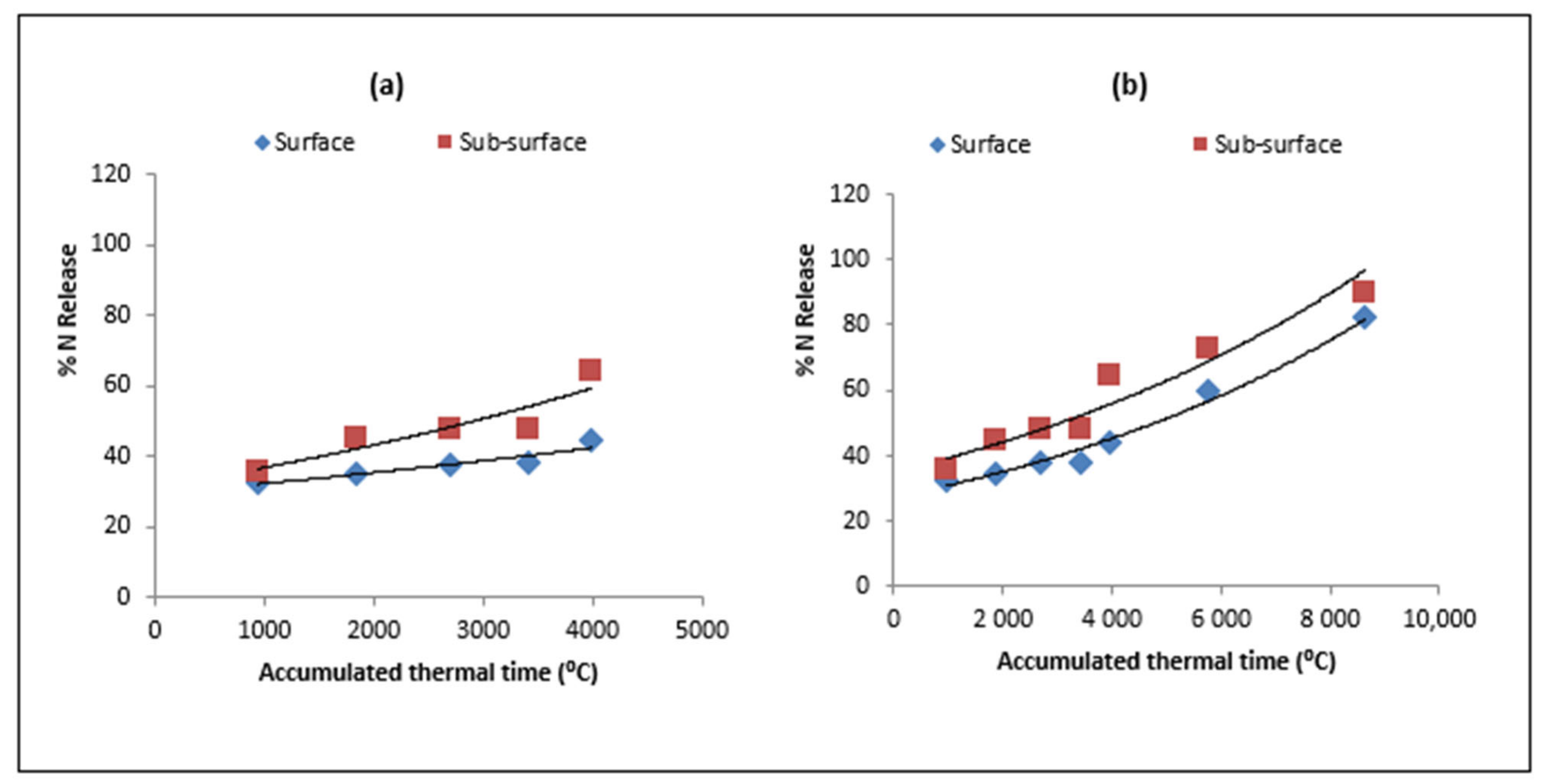
| Type of Residue | Days/ATT after Residue Placement | |||||||
|---|---|---|---|---|---|---|---|---|
| Days | 30 | 60 | 90 | 120 | 150 | 270 | 365 | |
| Maize | ATT (°C) | 420 | 778 | 1195 | 1754 | 2474 | 6265 | 8582 |
| Wheat, mung bean, and wheat + mung bean | ATT (°C) | 952 | 1852 | 2707 | 3425 | 3981 | 5768 | 8634 |
| Type of Residue § | Hemicellulose (%) | Cellulose (%) | Total N (%) | Total C (%) | C:N Ratio |
|---|---|---|---|---|---|
| Maize | 35 | 38 | 0.54 | 42.3 | 84 |
| Wheat | 19 | 45 | 0.32 | 38.3 | 120 |
| Mung bean (MB) | 15 | 44 | 1.08 | 35.4 | 33 |
| Wheat + MB | 23 | 41 | 0.64 | 37.0 | 58 |
| Residue Type | Decomposition Rate (k × 10−4) | R2 | RMSE | NRMSE | EF | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | |
| Maize | 4.1 | 5.6 | 0.985 | 0.982 | 2.85 | 3.79 | 0.041 | 0.068 | 0.98 | 0.98 | <0.001 | <0.001 |
| Wheat | 3.0 | 4.0 | 0.981 | 0.992 | 2.58 | 2.96 | 0.044 | 0.061 | 0.98 | 0.99 | <0.001 | <0.001 |
| Mung bean (MB) | 3.3 | 4.0 | 0.941 | 0.849 | 7.58 | 13.0 | 0.159 | 0.342 | 0.91 | 0.79 | 0.001 | 0.009 |
| Wheat + MB | 2.8 | 3.9 | 0.946 | 0.932 | 6.17 | 7.65 | 0.116 | 0.175 | 0.93 | 0.92 | 0.001 | 0.002 |
| Residue Type | Decomposition Rate (k × 10−4) | R2 | RMSE | NRMSE | EF | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | |
| Maize | 3.1 | 4.1 | 0.988 | 0.981 | 5.56 | 7.71 | 0.117 | 0.221 | 0.97 | 0.94 | <0.001 | <0.001 |
| Wheat | 2.8 | 3.3 | 0.994 | 0.987 | 1.90 | 4.73 | 0.047 | 0.158 | 0.99 | 0.97 | <0.001 | <0.001 |
| Mung bean (MB) | 3.0 | 3.5 | 0.980 | 0.946 | 7.68 | 12.9 | 0.260 | 0.635 | 0.92 | 0.79 | <0.001 | <0.001 |
| Wheat + MB | 2.8 | 3.5 | 0.988 | 0.977 | 5.24 | 7.96 | 0.150 | 0.312 | 0.96 | 0.92 | <0.001 | <0.001 |
| Residue Type | N Release Rate (k × 10−4) | R2 | RMSE | NRMSE | EF | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | |
| Maize | 5.3 | 6.2 | 0.735 | 0.966 | 3.22 | 3.00 | 0.182 | 0.098 | 0.81 | 0.95 | 0.063 | 0.003 |
| Wheat | 1.8 | 2.3 | 0.729 | 0.780 | 3.44 | 4.19 | 0.143 | 0.115 | 0.70 | 0.77 | 0.066 | 0.047 |
| Mung bean (MB) | 2.0 | 1.1 | 0.972 | 0.823 | 1.63 | 3.33 | 0.037 | 0.058 | 0.97 | 0.83 | 0.002 | 0.033 |
| Wheat + MB | 0.91 | 1.6 | 0.896 | 0.848 | 1.35 | 3.86 | 0.036 | 0.080 | 0.88 | 0.83 | 0.015 | 0.026 |
| Residue Type | N Release Rate (k × 10−4) | R2 | RMSE | NRMSE | EF | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | SP | SSP | |
| Maize | 2.6 | 2.1 | 0.873 | 0.807 | 11.3 | 14.8 | 0.326 | 0.307 | 0.83 | 0.75 | <0.01 | <0.01 |
| Wheat | 1.8 | 1.5 | 0.948 | 0.867 | 2.92 | 4.61 | 0.088 | 0.103 | 0.97 | 0.93 | <0.001 | <0.01 |
| Mung bean (MB) | 1.3 | 0.9 | 0.931 | 0.927 | 5.46 | 4.17 | 0.101 | 0.063 | 0.91 | 0.92 | <0.001 | <0.001 |
| Wheat + MB | 1.3 | 1.2 | 0.973 | 0.926 | 2.16 | 4.87 | 0.046 | 0.084 | 0.98 | 0.92 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, O.S.; Jat, M.L.; Gupta, R.K.; Thind, H.S.; Sidhu, H.S.; Singh, Y. Influence of Residue Type and Method of Placement on Dynamics of Decomposition and Nitrogen Release in Maize-Wheat-Mungbean Cropping on Permanent Raised Beds: A Litterbag Study. Sustainability 2022, 14, 864. https://doi.org/10.3390/su14020864
Sandhu OS, Jat ML, Gupta RK, Thind HS, Sidhu HS, Singh Y. Influence of Residue Type and Method of Placement on Dynamics of Decomposition and Nitrogen Release in Maize-Wheat-Mungbean Cropping on Permanent Raised Beds: A Litterbag Study. Sustainability. 2022; 14(2):864. https://doi.org/10.3390/su14020864
Chicago/Turabian StyleSandhu, Opinder Singh, Mangi L. Jat, Rajeev Kumar Gupta, Harmeet Singh Thind, Harminder Singh Sidhu, and Yadvinder Singh. 2022. "Influence of Residue Type and Method of Placement on Dynamics of Decomposition and Nitrogen Release in Maize-Wheat-Mungbean Cropping on Permanent Raised Beds: A Litterbag Study" Sustainability 14, no. 2: 864. https://doi.org/10.3390/su14020864
APA StyleSandhu, O. S., Jat, M. L., Gupta, R. K., Thind, H. S., Sidhu, H. S., & Singh, Y. (2022). Influence of Residue Type and Method of Placement on Dynamics of Decomposition and Nitrogen Release in Maize-Wheat-Mungbean Cropping on Permanent Raised Beds: A Litterbag Study. Sustainability, 14(2), 864. https://doi.org/10.3390/su14020864






