Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils
Abstract
:1. Introduction
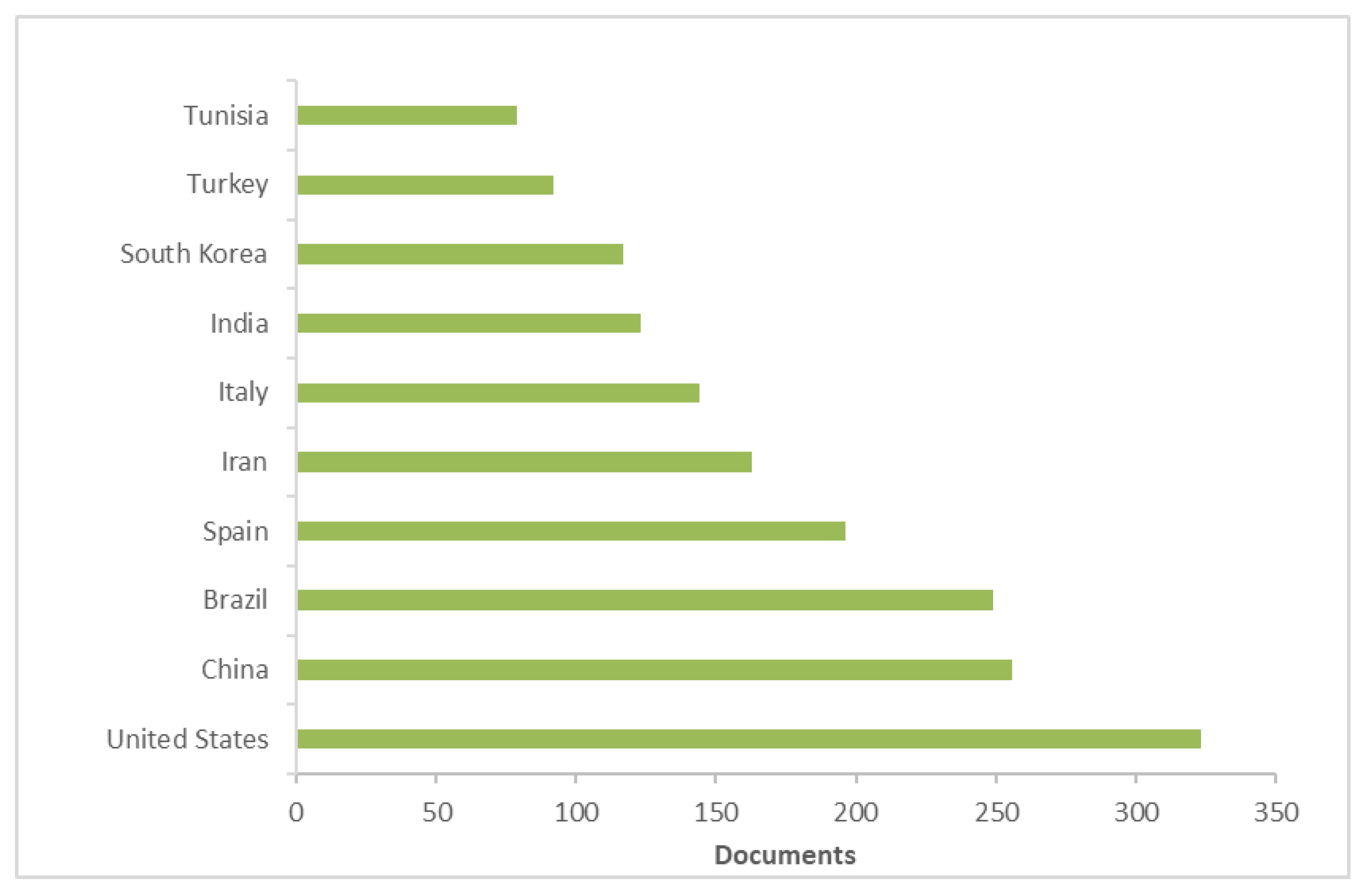
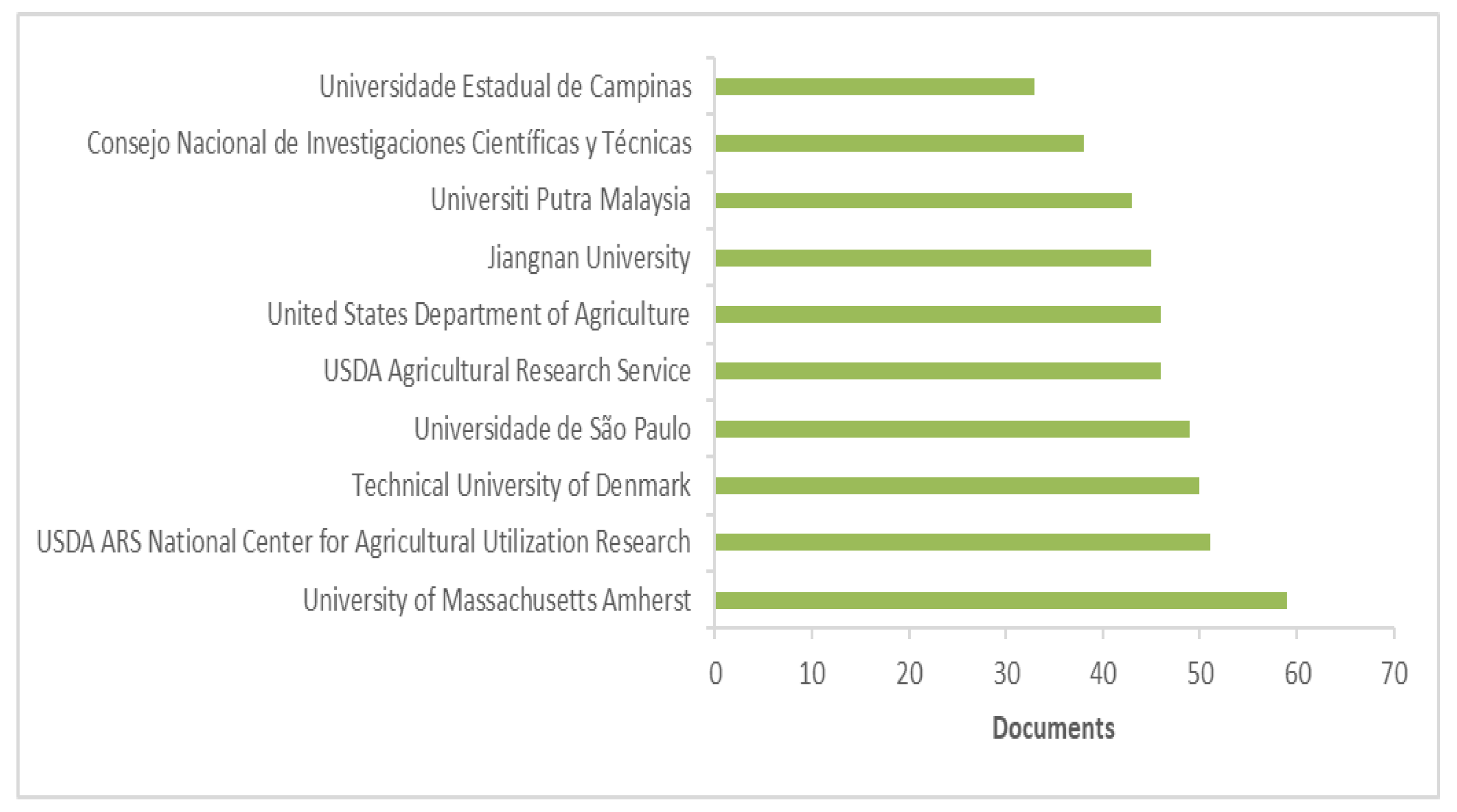
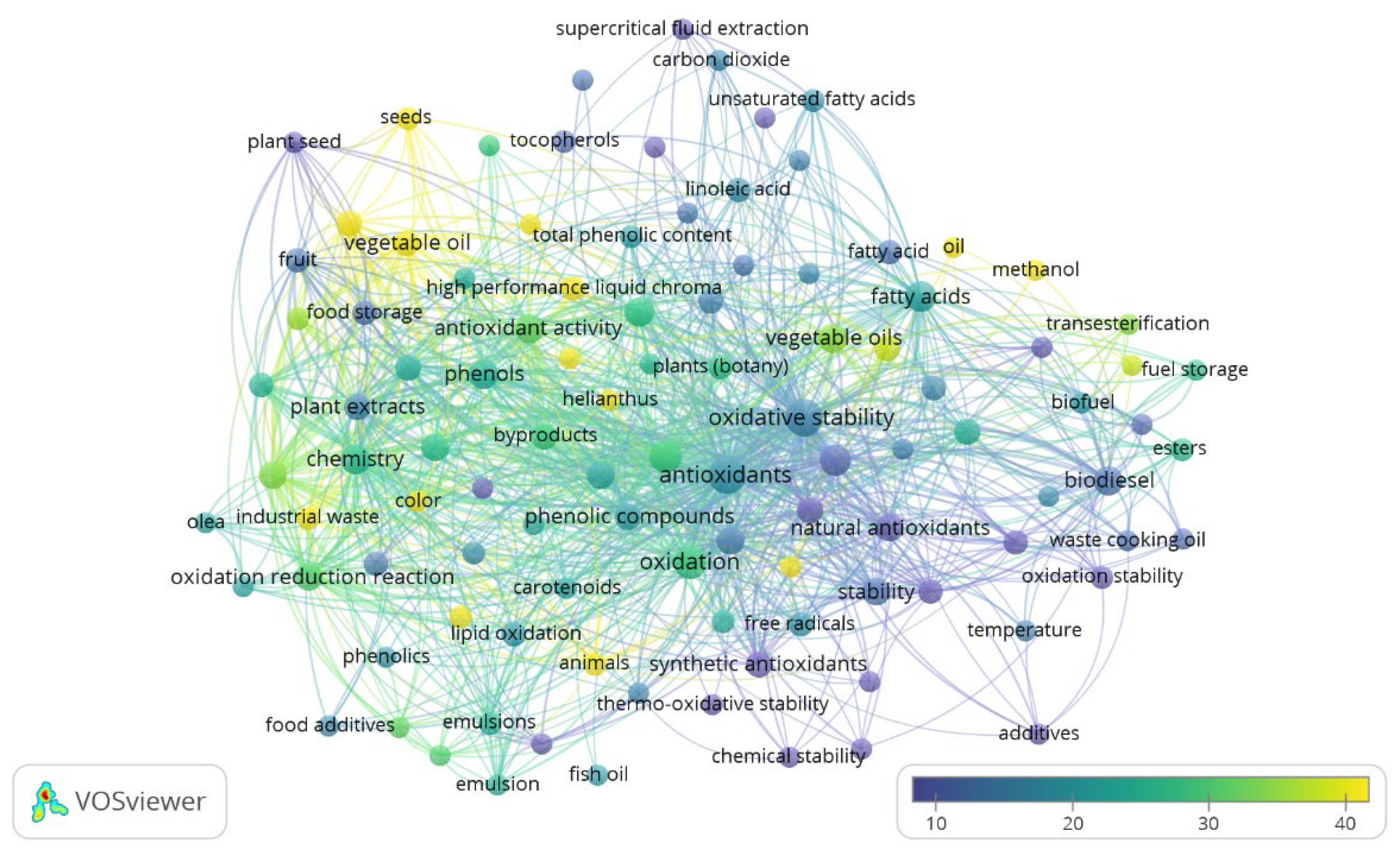
2. Antioxidant, Oil Oxidative Stability, Waste, Biodiversity, and Sustainability: Literature Research Review
3. Oils and Oxidative Stability
3.1. Oils: Classification, Properties, and Trends
3.2. Oil’s Oxidative Stability: Main Features, Mechanisms, and Analytycal Methods for Its Determination
4. Focus on Synthetic and Natural Antioxidants for the Stabilization of Edible Oils
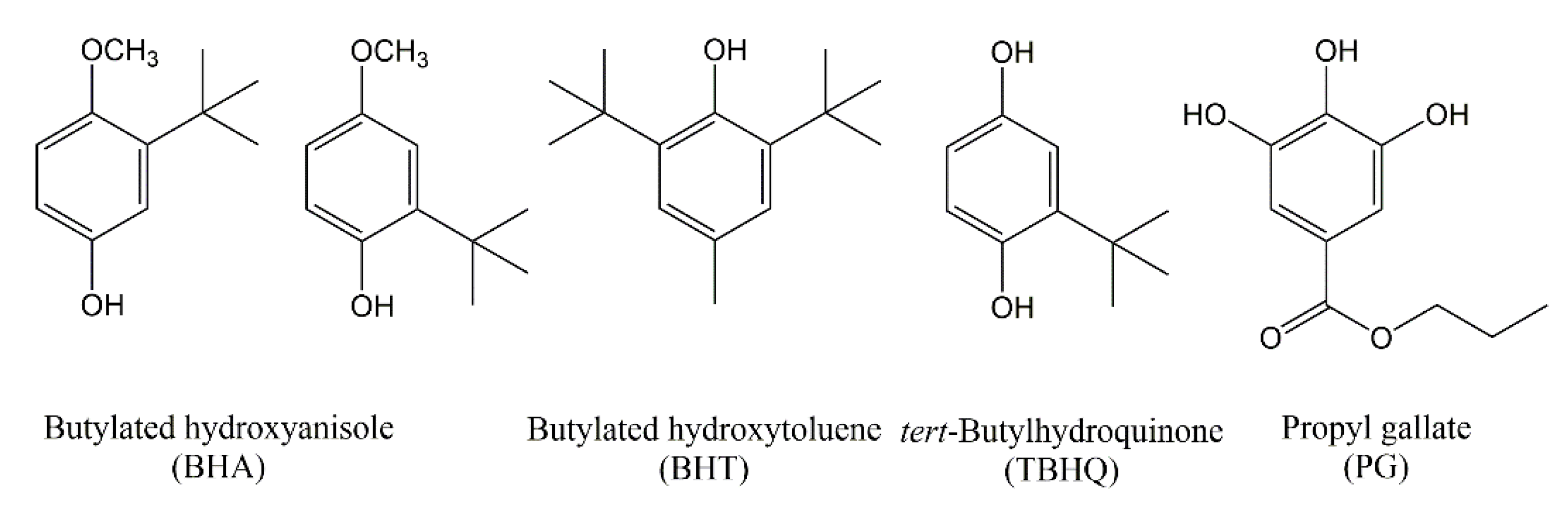
4.1. Synthetic and Natural Antioxidants
4.2. New Sources of Antioxidants: Agri-Food Waste and By-Products in the Perspective of Biorefinery and a Circular Economy
4.3. Antioxidants’ Extraction Methods
5. Enrichment of Edible Oils with Antioxidant Rich Extracts
5.1. Application and Beneficial Outcomes: Case Studies
5.2. Conventional and Emerging Strategies for Edible Oil Enrichment
5.3. Safety Concerns Associated with the Use of Natural Antioxidants
6. Oil Blending as a Tool to Improve an Oil’s Oxidative Stability
| Oil Blend (First + Second Component) | Concentration of the Second Blend Component | Analytical Approach | Main Results | References |
|---|---|---|---|---|
| Sunflower oil + Sclerocarya oil | 10, 20, 30, and 40% | Oxidative stability (Rancimat 120C) and stability at 70C using peroxide value. | The oxidative stability in the Rancimat test was improved from 47 to 147% in oil blends compared with sunflower oil alone. Storage of the blends at 70 °C showed that peroxide value of the blends was remarkably lower than sunflower alone. | [158] |
| Sunflower oil (SFO) + Moringa oleifera oil (MOO)Soybean oil (SBO) + MOO | 20, 40, 60, and 80% | Storage test: oils stored at room temperature for 180 days. Peroxide value (PV), iodine value (IV), and induction period (IP, Rancimat test) were evaluated monthly. Thermal stability test: heating at 180 °C for 42 h (6-h heating cycles each day). | The storage test showed an improvement of the oxidative stability of blends with increasing MOO concentration. Each 20% addition of MOO resulted in decreases of PV and an increases of IP. The heating performance test revealed the SBO:MOO (20:80 w/w) blend was the most stable. | [155] |
| Coconut oil + palm oil Coconut oil + sunflower oil Coconut oil + soybean oil | 20, 50, and 80% | Storage test: 6 weeks at 37 °C. Peroxide value (PV), fatty acid composition, and antioxidant activity. | Blending coconut oil with other vegetable oils increased the medium chain fatty acid concentration and the oxidative stability to the blends. | [159] |
| Rapeseed oil + rice bran oil Rapeseed oil + black cumin oil | 5, 10, and 20% | Oxidative stability index (Rancimat test), phytosterols, fatty acids, and tochochromanols. | The blends with black cumin seed oil had higher level of α- and γ-tocopherols and all isomers of tocotrienols. Blends with rice bran oil had high tocotrienol, β-sitosterol, and squalene levels. Blending resulted in lowering ratio of PUFA:SFA and improved the stability of these oils. | [160] |
| Sunflower oil (SFO) + cold-pressed black cumin oil (BCO) | 5, 10, and 20% | Thermally accelerated oxidation conditions with Rancimat method (110C) and Schaal oven test (60C). Peroxide value (PV), conjugated dienes (CD), and conjugated trienes (CT). Volatile oxidation compounds. | BCO increased the stability of SFO at high temperatures according to induction period times. SFO:BCO (80:20) blends had lower PV at the end of storage at 60 °C (Schaal oven test). Stability of blends was better than SO, most likely due to changes in the levels of thymoquinone and tocopherols found in BCO. | [161] |
| Butter oil + crude mango kernel oil (MKO) | 2.5, 5, 7.5, and 10% | Storage at 25 °C and 55 °C for 6 months. Induction period (rancimat methd) peroxide values, anisidine values, and iodine values were measured at 0, 45, and 90 days. | Supplementation of MKO oil in butter oil inhibited oil oxidation at ambient and accelerated oxidation, as showed by the low PV and anisidine values of blends after 90 days of storage. Induction period of all the treatments increased as a function of the addition of MKO. | [162] |
| Chia seed (Salvia hispanica L.) oil (CO) + walnut (WO), almond (AO), virgin and roasted sesame oils (VSO, RSO) | 60, 70, and 80% | Accelerated storage test (Schaal oven test (SOT) 40 ± 1 °C, 12 days). Peroxide value (PV), conjugated dienes (CD), conjugated trienes (CT), p-anisidine value (PAV), free fatty acid content, antioxidant compounds, and induction time (Rancimat method). | The blends presented a higher induction period than chia oil alone. Sesame oil blends (VSO and RSO) were the most stable, folowed by CO:AO blends. | [156] |
| Perilla seed oil (PO) + extra virgin olive oil (EVOO) | 0, 55, 60, 65, 70, 75, and 100% | Free acidity, peroxide value, conjugated dienes (CD), conjugated trienes (CT), fatty acid composition, sterols, tocopherols, biophenols content, and oxidation stability. | Blends were rich in ω-3 and ω-6fatty acids, biophenols, tocopherols, and sterols. Blends showed higher oxidation stability than PO alone. | [163] |
| Linseed oil + corn, canola, sesame, and bitter almond oils | 33, 66% | Oxidative stability index (OSI) as determined by the Rancimat method at 353, 373, and 393 K. | Blending linseed oil with 66% corn, bitter almond, sesame, and canola oil increased the OSI value of linseed oil by 67.05%, 61.68%, 60.12%, and 59.13%. | [154] |
| Chia oil + sesame oil | 84.5, 89.6, 92.3, and 93.9% | Oxidative stability index (OSI), with the Rancimat method, and shelf life (oxidative stability index at 25 °C, OSI25). Acidity, peroxide value (PV), p-anisidine value (p-AV), and the total oxidation value (TotOx). | The supplementation of chia oil with sesame oil increased the OSI value and the shelf life (oxidative stability index at 25 °C, OSI25). | [164] |
| Soybean oil (SBO) + sesame seed oil (SSO) | 40, 50, and 60% | 3 frying cycles. At the end of each cycle, the free fatty acid (FFA), peroxide value, and acid value were determined. Characterization of fresh and fried oil blends by Fourier transform infrared spectroscopy (FTIR). | The blend with the ratio of 60% had the lowest peroxide value. The blending of SBO with SSO improved the thermal stability, as sesame seed oil contains high levels of antioxidants such as sesamin, sesamol, and tocopherol. | [165] |
| Virgin olive oil (VOO) + sesame (SSO), flaxseed (VFO), hazelnut (VHO), and pistachio (VPO) oils | SSO 5, 15% VFO 3, 10% VPO or VHO 50, 75% | Oxidative stability by Rancimat method. Shelf life testing at room temperature for 24 days (25 °C) and under accelerated shelf-life conditions (55 °C) (ASLT). | The oxidative stability test showed that the oil blends maintained similar stabilities of VOO under normal and accelerated storage conditions. | [166] |
| Sunflower oil (SFO) + pomegranate seed oil (PSO) | 10, 15, 20% (w/w) | Accelerated storage test was conducted using the best blending ratio (85:15) at 60 ± 2 °C for 20 days following the evolution of the peroxide value, ρ-anisidine value, and total oxidation value. | Blending of SO with PSO improved total phenols, total carotenoids, and tocopherol content. Blended oils exhibited better oxidative stability than SO, as shown by the low peroxide and ρ-anisidine values. Blending SO with PSO decreased the rate of hydroperoxides formation during the accelerated storage period. | [157] |
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both Sunflower Oil and Its Headspace throughout the Oxidation Process. Occurrence in the Headspace of Toxic Oxygenated Aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Barbosa de Lima, N.G.; Wobeto, C.; Jorge, N.; Lannes, S.C.D.S. Synthetic and Natural Antioxidants Used in the Oxidative Stability of Edible Oils: An Overview. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Metzner Ungureanu, C.-R.; Poiana, M.-A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to Improve the Thermo-Oxidative Stability of Sunflower Oil by Exploiting the Antioxidant Potential of Blueberries Processing Byproducts. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef] [PubMed]
- Odeh, D.; Kraljić, K.; Benussi Skukan, A.; Škevin, D. Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants 2021, 10, 785. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. LWT 2017, 75, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Carelli, A.A.; Franco, I.C.; Crapiste, G.H. Effectiveness of added natural antioxidants in sunflower oil. Grasas Aceites 2005, 56, 303–310. [Google Scholar] [CrossRef]
- Drinić, Z.; Mudrić, J.; Zdunić, G.; Bigović, D.; Menković, N.; Šavikin, K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020, 333, 127501. [Google Scholar] [CrossRef] [PubMed]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Bienkiewicz, G.; Łopusiewicz, Ł. The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders. Antioxidants 2021, 10, 211. [Google Scholar] [CrossRef]
- Ghorbani Gorji, S.; Smyth, H.E.; Sharma, M.; Fitzgerald, M. Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends Food Sci. Technol. 2016, 56, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to increase the oxidative stability of cold pressed oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Iqbal, S.; Haleem, S.; Akhtar, M.; Zia-ul-Haq, M.; Akbar, J. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Res. Int. 2008, 41, 194–200. [Google Scholar] [CrossRef]
- Next Generation EU. Available online: https://europa.eu/next-generation-eu/index_en (accessed on 16 December 2021).
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Faraji, H.; McClements, D.J.; Decker, E.A. Role of Continuous Phase Protein on the Oxidative Stability of Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2004, 52, 4558–4564. [Google Scholar] [CrossRef]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant Activity of Cysteine, Tryptophan, and Methionine Residues in Continuous Phase β-Lactoglobulin in Oil-in-Water Emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef]
- Inchingolo, R.; Bayram, I.; Uluata, S.; Kiralan, S.S.; Rodriguez-Estrada, M.T.; McClements, D.J.; Decker, E.A. Ability of Sodium Dodecyl Sulfate (SDS) Micelles to Increase the Antioxidant Activity of α-Tocopherol. J. Agric. Food Chem. 2021, 69, 5702–5708. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waltman, L.; van Eck, N.J.; Noyons, E.C.M. A unified approach to mapping and clustering of bibliometric networks. J. Informetr. 2010, 4, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. Text Mining and Visualization Using VOS Viewer. Available online: http://arxiv.org/ftp/arxiv/papers/1109/1109.2058.pdf (accessed on 24 October 2021).
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical Scavenging Activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in Large Quantities of Highly Purified Hydroxytyrosol from Liquid−Solid Waste of Two-Phase Olive Oil Processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods—A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Angelovič, M.; Jablonický, J.; Tkáč, Z.; Angelovič, M. Oxidative stability of fatty acid alkyl esters: A review. Potravin. Slovak J. Food Sci. 2015, 9, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary Characterisation of Wastes Generated from the Rapeseed and Sunflower Protein Isolation Process and Their Valorisation in Delaying Oil Oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Clemente, M.; Grande, E.; Urciuoli, S.; Romani, A.; Torre, L.; Puglia, D.; Bernini, R.; Santi, L. Hydroxytyrosol and Oleuropein-Enriched Extracts Obtained from Olive Oil Wastes and By-Products as Active Antioxidant Ingredients for Poly (Vinyl Alcohol)-Based Films. Molecules 2021, 26, 2104. [Google Scholar] [CrossRef] [PubMed]
- Jolayemi, O.S.; Stranges, N.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Influence of Free and Encapsulated Olive Leaf Phenolic Extract on the Storage Stability of Single and Double Emulsion Salad Dressings. Food Bioprocess Technol. 2021, 14, 93–105. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative Status and Presence of Bioactive Compounds in Meat from Chickens Fed Polyphenols Extracted from Olive Oil Industry Waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef] [Green Version]
- Oils and Fats in the Market Place. Available online: https://lipidlibrary.aocs.org/resource-material/market-trends/oils-and-fats-in-the-market-place (accessed on 2 November 2021).
- Woodgate, S.L.; van der Veen, J.T. Fats and Oils—Animal Based. In Food Processing; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 481–499. [Google Scholar]
- The Four Major Vegetable Oils. Available online: https://lipidlibrary.aocs.org/resource-material/market-trends/individual-commodity-oils-and-fats (accessed on 2 November 2021).
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.-H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Akoh, C.C. (Ed.) Food Lipids. Chemistry, Nutrition, and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Li, R.; Xia, Q.; Tang, M.; Zhao, S.; Chen, W.; Lei, X.; Bai, X. Chemical composition of Chinese palm fruit and chemical properties of the oil extracts. Afr. J. Biotechnol. 2012, 11, 9377–9382. [Google Scholar] [CrossRef]
- Rasor, A.S.; Duncan, S.E. Fats and Oils—Plant Based. In Food Processing; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 457–480. [Google Scholar]
- Boskou, D. (Ed.) Olive Oil. Minor Constituents and Health, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Fung, T.T.; Isanaka, S.; Hu, F.B.; Willett, W.C. International food group–based diet quality and risk of coronary heart disease in men and women. Am. J. Clin. Nutr. 2018, 107, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; Purriños, L.; Tomasevic, I.; Domínguez, R.; Brnčić, M.; Barba, F.J.; Lorenzo, J.M. Consumer Acceptance and Quality Parameters of the Commercial Olive Oils Manufactured with Cultivars Grown in Galicia (NW Spain). Foods 2020, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- Matthäus, B.; Guillaume, D.; Gharby, S.; Haddad, A.; Harhar, H.; Charrouf, Z. Effect of processing on the quality of edible argan oil. Food Chem. 2010, 120, 426–432. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Quality parameters for the evaluation of cold-pressed edible argan oil. J. Verbrauch. Lebensm. 2015, 10, 143–154. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Ruiz, A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol. 2005, 107, 36–47. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Matthäus, B.; Charrouf, Z. Oxidative stability of edible argan oil: A two-year study. LWT-Food Sci. Technol. 2011, 44, 1–8. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Roller, M.; Birtić, S. Mass Transport Phenomena in Lipid Oxidation and Antioxidation. Annu. Rev. Food Sci. Technol. 2017, 8, 391–411. [Google Scholar] [CrossRef]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasaikina, V.D.K.O.T. Lipid Oxidation in Homogeneous and Micro-Heterogeneous Media in Presence of Prooxidants, Antioxidants and Surfactants. In Lipid Peroxidation; Catala, A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Haman, N.; Bodner, M.; Ferrentino, G.; Scampicchio, M. Lipid autoxidation of fish, lard, corn and linseed oils by isothermal calorimetry. Ital. J. Food Sci. 2018, 31, 323. [Google Scholar] [CrossRef]
- Na, L.; Lu, H.; Xin, G.; Zhiping, T.; Jun, L. DFT Study of Oxidation Reaction Paths for Ethanol Gasoline. J. Energy Nat. Resour. 2020, 9, 39–43. [Google Scholar] [CrossRef]
- Gharby, S.; Guillaume, D.; Elibrahimi, M.; Charrouf, Z. Physico-Chemical Properties and Sensory Analysis of Deodorized Argan Oil. ACS Food Sci. Technol. 2021, 1, 275–281. [Google Scholar] [CrossRef]
- Gharby, S.; Guillaume, D.; Nounah, I.; Harhar, H.; Hajib, A.; Matthäus, B.; Charrouf, Z. Shelf-life of Moroccan prickly pear (Opuntia ficus-indica) and argan (Argania spinosa) oils: A comparative study. Grasas Aceites 2021, 72, e397. [Google Scholar] [CrossRef]
- Gharby, S.; Hajib, A.; Ibourki, M.; Sakar, E.H.; Nounah, I.; Moudden, H.E.L.; Elibrahimi, M.; Harhar, H. Induced changes in olive oil subjected to various chemical refining steps: A comparative study of quality indices, fatty acids, bioactive minor components, and oxidation stability kinetic parameters. Chem. Data Collect. 2021, 33, 100702. [Google Scholar] [CrossRef]
- van der Merwe, G.H.; du Plessis, L.M.; Taylor, J.R. Changes in chemical quality indices during long-term storage of palm-olein oil under heated storage and transport-type conditions. J. Sci. Food Agric. 2004, 84, 52–58. [Google Scholar] [CrossRef]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, H.R.; Naderi, M.; Jafari, S.M.; Naeli, M.H. Postmarketing surveillance of the oxidative stability for cooking oils, frying oils, and vanaspati supplied in the retail market. Food Sci. Nutr. 2019, 7, 1455–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Ait Addi, E.H.; Charrouf, Z. Chemical Characterization and Kinetic parameter determination under Rancimat test conditions of four monovarietal virgin olive oils grown in Morocco. OCL 2016, 23, A401. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, I.d.M.; Rios, M.A.d.S.; Cavalcante, C.L.; Luna, F.M.T. Effects of Amine and Phenolic Based Antioxidants on the Stability of Babassu Biodiesel Using Rancimat and Differential Scanning Calorimetry Techniques. Ind. Eng. Chem. Res. 2020, 59, 18–24. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Priol, L. Study of the co-encapsulation of edible oxidable oils and phenolic antioxidants. In Étude de la co-Encapsulation d’huiles Alimentaires Oxydables et d’antioxydants Phénoliques; Université de Technologie de Compiègne: Compiègne, France, 2019. [Google Scholar]
- Houmy, N.; Mansouri, F.; Benmoumen, A.; Elmouden, S.; Boujnah, M.; Sindic, M.; Fauconnier, M.-L.; Serghini-Caid, H.; Elamrani, A. Characterization of almond kernel oils of five almonds varieties cultivated in Eastern Morocco. Cah. Options Méditerranéennes 2016, 119, 317–321. [Google Scholar]
- Cui, L.; Lahti, P.M.; Decker, E.A. Evaluating Electron Paramagnetic Resonance (EPR) to Measure Lipid Oxidation Lag Phase for Shelf-Life Determination of Oils. J. Am. Oil Chem. Soc. 2017, 94, 89–97. [Google Scholar] [CrossRef]
- Merkx, D.W.H.; Plankensteiner, L.; Yu, Y.; Wierenga, P.A.; Hennebelle, M.; Van Duynhoven, J.P.M. Evaluation of PBN spin-trapped radicals as early markers of lipid oxidation in mayonnaise. Food Chem. 2021, 334, 127578. [Google Scholar] [CrossRef] [PubMed]
- Ricca, M.; Fodera, V.; Vetri, V.; Buscarino, G.; Montalbano, M.; Leone, M. Oxidation Processes in Sicilian Olive Oils Investigated by a Combination of Optical and EPR Spectroscopy. J. Food Sci. 2012, 77, C1084–C1089. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Spallaci, M.; Cangiotti, M.; Bacchiocca, M.; Ninfali, P. Electron paramagnetic resonance investigations of free radicals in extra virgin olive oils. J. Agric. Food Chem. 2001, 49, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, V.; Maridakis, G.A.; Sotiroudis, T.G.; Xenakis, A. Antioxidant activity of polar extracts from olive oil and olive mill wastewaters: An EPR and photometric study. Eur. J. Lipid Sci. Technol. 2005, 107, 513–520. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, Y.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the oxidative stability of edible oil by electron spin resonance spectroscopy. Food Chem. 2020, 309, 125714. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Andersen, M.L.; Skibsted, L.H. ESR spin trapping for in situ detection of radicals involved in the early stages of lipid oxidation of dried microencapsulated oils. Food Chem. 2021, 341, 128227. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Molinu, M.G.; Deiana, P.; Sanna, D. Electron Paramagnetic Resonance Spin Trapping of Sunflower and Olive Oils Subjected to Thermal Treatment: Optimization of Experimental and Fitting Parameters. ACS Food Sci. Technol. 2021, 1, 1294–1303. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Jiménez, P.; García, P.; Bustamante, A.; Barriga, A.; Robert, P. Thermal stability of oils added with avocado (Persea americana cv. Hass) or olive (Olea europaea cv. Arbequina) leaf extracts during the French potatoes frying. Food Chem. 2017, 221, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- Dairi, S.; Galeano-Díaz, T.; Acedo-Valenzuela, M.I.; Godoy-Caballero, M.P.; Dahmoune, F.; Remini, H.; Madani, K. Monitoring oxidative stability and phenolic compounds composition of myrtle-enriched extra virgin olive during heating treatment by flame, oven and microwave using reversed phase dispersive liquid–liquid microextraction (RP-DLLME)-HPLC-DAD-FLD method. Ind. Crops Prod. 2015, 65, 303–314. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. (Eds.) Iron, Ruthenium and Osmium. In Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; Chapter 25; pp. 1070–1112. [Google Scholar]
- Perron, N.R.; Wang, H.C.; DeGuire, S.N.; Jenkins, M.; Lawson, M.; Brumaghim, J.L. Kinetics of iron oxidation upon polyphenol binding. Dalton Trans. 2010, 39, 9982–9987. [Google Scholar] [CrossRef] [PubMed]
- Blekas, G.; Tsimidou, M.; Boskou, D. Contribution of α-tocopherol to olive oil stability. Food Chem. 1995, 52, 289–294. [Google Scholar] [CrossRef]
- Oswell, N.J.; Gunstone, F.D.; Pegg, R.B. Vegetable Oils. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2020; pp. 1–30. [Google Scholar]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Olive Oil. In Vegetable Oils in Food Technology; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 243–271. [Google Scholar]
- Grompone, M.A. Sunflower Oil. In Vegetable Oils in Food Technology; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 137–167. [Google Scholar]
- Siger, A.; Nogala-kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; George Truscott, T.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, Z.; Liu, C.; Wang, Y.; Li, J.; Liu, Y. Identification and quantification of synergetic antioxidants and their application in sunflower oil. LWT 2020, 118, 108726. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Maciej, B.; Dimitri, P.M.; Maria-Corina, S.; Amirhossein, S. Editorial: Natural Products as the Integral Part of the Therapy? Curr. Pharm. Des. 2017, 23, 2411–2413. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: A global perspective. Biofuels Bioprod. Biorefining 2014, 8, 686–715. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Leong, H.Y.; Chang, C.-K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef] [PubMed]
- Viganó, J.; Machado, A.P.d.F.; Martínez, J. Sub- and supercritical fluid technology applied to food waste processing. J. Supercrit. Fluids 2015, 96, 272–286. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Villacís-Chiriboga, J.; Vera, E.; Van Camp, J.; Ruales, J.; Elst, K. Valorization of byproducts from tropical fruits: A review, Part 2: Applications, economic, and environmental aspects of biorefinery via supercritical fluid extraction. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2305–2331. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Kiefer, J.; Mahesar, S.A. State-of-the-Art Infrared Applications in Drugs, Dietary Supplements, and Nutraceuticals. J. Spectrosc. 2020, 2020, 1397275. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M. A current shot and re-thinking of antioxidant research strategy. Braz. J. Anal. Chem 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit Wastes as a Valuable Source of Value-Added Compounds: A Collaborative Perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Romani, A.; Sagratini, G.; Bevilacqua, N.; Ieri, F.; Vignolini, P.; Campo, M.; Cecchini, F. Biorefinery for Innovative Production of Bioactive Compounds from Vegetable Biomass. In Biorefinery Production Technologies for Chemicals and Energy; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 89–128. [Google Scholar]
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Romani, A.; Sagratini, G.; Bevilacqua, N.; Ieri, F.; Vignolini, P.; Campo, M.; Cecchini, F. Case-Studies Towards Sustainable Production of Value-Added Compounds in Agro-Industrial Wastes. In Biorefinery Production Technologies for Chemicals and Energy; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 197–219. [Google Scholar]
- Rubió, L.; Motilva, M.-J.; Macià, A.; Ramo, T.; Romero, M.-P. Development of a Phenol-Enriched Olive Oil with Both Its Own Phenolic Compounds and Complementary Phenols from Thyme. J. Agric. Food Chem. 2012, 60, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Romero, M.-P.; Motilva, M.-J. Development of a Phenol-Enriched Olive Oil with Phenolic Compounds from Olive Cake. J. Agric. Food Chem. 2010, 58, 10396–10403. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Ramo, T.; Motilva, M.-J. Stability of a phenol-enriched olive oil during storage. Eur. J. Lipid Sci. Technol. 2011, 113, 894–903. [Google Scholar] [CrossRef]
- Delgado-Adamez, J.; Franco Baltasar, M.N.; Ayuso Yuste, M.C.; Martin-Vertedor, D. Oxidative Stability, Phenolic Compounds and Antioxidant Potential of a Virgin Olive Oil Enriched with Natural Bioactive Compounds. J. Oleo Sci. 2014, 63, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, M.; Valls, R.M.; Romero, M.-P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.-J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [Green Version]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Cao, L.-L.; Pang, M.; Hou, Z.-G.; Jiang, S.-T. Preparation of camellia oil-based W/O emulsions stabilized by tea polyphenol palmitate: Structuring camellia oil as a potential solid fat replacer. Food Chem. 2019, 276, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M.; Gülmez, Ö.; Güçlü, K.; Özyürek, M. Enrichment of Hazelnut Oil with Several Polyphenols: An Alternative Approach to A New Functional Food. J. Oleo Sci. 2021, 70, 11–19. [Google Scholar] [CrossRef]
- Bakkalbaşı, E. Oxidative stability of enriched walnut oil with phenolic extracts from walnut press-cake under accelerated oxidation conditions and the effect of ultrasound treatment. J. Food Meas. Charact. 2019, 13, 43–50. [Google Scholar] [CrossRef]
- Abenoza, M.; Sánchez-Gimeno, A.C. Increasing the stability of Empeltre olive oils by aromatization with rosemary (Rosmarinus officinalis) and garlic (Allium sativum). Int. J. Gastron. Food Sci. 2021, 24, 100333. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Prior, M.Á.; Fernández-Bolaños, J.; Aguilera-Herrera, M.d.l.P.; Rodríguez-Gutiérrez, G. Extra virgin olive oil jam enriched with cocoa bean husk extract rich in theobromine and phenols. LWT 2019, 111, 278–283. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- More, S.B.; Gogate, P.R.; Waghmare, J.S. Bioactives from pomegranate peel and moringa leaves as natural antioxidants for stability of edible oil blends. Braz. J. Chem. Eng. 2021, 1–12. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Smetanska, I.; Ramadan, M.F.; Sarhan, M.A.; Mahmoud, A. Antioxidant potential of sesame (Sesamum indicum) cake extract in stabilization of sunflower and soybean oils. Ind. Crops Prod. 2011, 34, 952–959. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Sobiechowska, D.A.; Tańska, M. Edible flowers as a new source of natural antioxidants for oxidative protection of cold-pressed oils rich in omega-3 fatty acids. Food Res. Int. 2020, 134, 109216. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Poiana, M.-A. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. Int. J. Mol. Sci. 2012, 13, 9240–9259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinello, F.; Lante, A. Accelerated storage conditions effect on ginger- and turmeric-enriched soybean oils with comparing a synthetic antioxidant BHT. LWT 2020, 131, 109797. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Wu, G.; Zhang, M. Impact of thermal processing and storage temperature on the phenolic profile and antioxidant activity of different varieties of lychee juice. LWT 2019, 116, 108578. [Google Scholar] [CrossRef]
- Nwuha, V.; Nakajima, M.; Tong, J.; Ichikawa, S. Solubility study of green tea extracts in pure solvents and edible oils. J. Food Eng. 1999, 40, 161–165. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Li, J.; Fan, L.; Huang, S. Study on the antioxidative mechanism of tocopherol loaded ethyl cellulose particles in thermal-oxidized soybean oil. Carbohydr. Polym. 2021, 276, 118734. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ. Technol. Innov. 2018, 10, 62–70. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Turan, S.; Köroğlu, D.G. Oxidative stability of soybean oil enriched with ethyl acetate extract of olive by-products. Turk. J. Agric. Food Sci. Technol. 2020, 8, 1373–1379. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial activity of phenolic-rich extracts from avocado fruit waste: Influence on the colloidal and oxidative stability of emulsions and nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Asi, M.R.; Chatha, S.A.S. Antioxidant potential of extracts from different agro wastes: Stabilization of corn oil. Grasas Aceites 2008, 59, 205–217. [Google Scholar]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Savoire, R.; Harscoat-Schiavo, C.; Cansell, M.; Subra-Paternault, P. Improvement of the oxidative stability of camelina oil by enrichment with phospholipid-quercetin formulations. Food Chem. 2021, 341, 128234. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Fan, L.; Yan, W. Ethyl cellulose particles loaded with α-tocopherol for inhibiting thermal oxidation of soybean oil. Carbohydr. Polym. 2021, 252, 117169. [Google Scholar] [CrossRef]
- Sayyed-Alangi, S.Z.; Nematzadeh, M. Formulation, development and evaluation of bifunctionalized nanoliposomes containing Trifolium resupinatum sprout methanolic extract: As effective natural antioxidants on the oxidative stability of soybean oil. BMC Chem. 2019, 13, 77. [Google Scholar] [CrossRef]
- Hermund, D.; Jacobsen, C.; Chronakis, I.S.; Pelayo, A.; Yu, S.; Busolo, M.; Lagaron, J.M.; Jónsdóttir, R.; Kristinsson, H.G.; Akoh, C.C.; et al. Stabilization of Fish Oil-Loaded Electrosprayed Capsules with Seaweed and Commercial Natural Antioxidants: Effect on the Oxidative Stability of Capsule-Enriched Mayonnaise. Eur. J. Lipid Sci. Technol. 2019, 121, 1800396. [Google Scholar] [CrossRef] [Green Version]
- Silva Faria, W.C.; Oliveira, M.G.d.; Cardoso da Conceição, E.; Silva, V.B.; Veggi, N.; Converti, A.; Miguel de Barros, W.; Fernandes da Silva, M.; Bragagnolo, N. Antioxidant efficacy and in silico toxicity prediction of free and spray-dried extracts of green Arabica and Robusta coffee fruits and their application in edible oil. Food Hydrocoll. 2020, 108, 106004. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M.; Mahoonak, A.S.; Nikoo, A.M.; Rahmanian, N.; Hajitabar, J.; Meshginfar, N. The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT-Food Sci. Technol. 2014, 56, 124–130. [Google Scholar] [CrossRef]
- Farag, R.S.; El-baroty, G.S.; Basuny, A.M. Safety evaluation of olive phenolic compounds as natural antioxidants. Int. J. Food Sci. Nutr. 2003, 54, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. NeuroMolecular Med. 2008, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Islam, B.u.; Suhail, M.; Khan, M.K.; Zughaibi, T.A.; Alserihi, R.F.; Zaidi, S.K.; Tabrez, S. Polyphenols as anticancer agents: Toxicological concern to healthy cells. Phytother. Res. 2021, 35, 6063–6079. [Google Scholar] [CrossRef]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 day oral toxicity study of blueberry polyphenols in ovariectomized sprague-dawley rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Romanić, R.S.; Lužaić, T.Z.; Radić, B.Đ. Enriched sunflower oil with omega 3 fatty acids from flaxseed oil: Prediction of the nutritive characteristics. LWT 2021, 150, 112064. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Soltani, A.; Hosseini, S.M.H.; Keramat, M. Improving the oxidation kinetics of linseed oil using the blending approach. J. Food Process. Preserv. 2020, 44, e14964. [Google Scholar] [CrossRef]
- Anwar, F.; Hussain, A.I.; Iqbal, S.; Bhanger, M.I. Enhancement of the oxidative stability of some vegetable oils by blending with Moringa oleifera oil. Food Chem. 2007, 103, 1181–1191. [Google Scholar] [CrossRef]
- Bordón, M.G.; Meriles, S.P.; Ribotta, P.D.; Martinez, M.L. Enhancement of Composition and Oxidative Stability of Chia (Salvia hispanica L.) Seed Oil by Blending with Specialty Oils. J. Food Sci. 2019, 84, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Blending of Sunflower Oil with Pomegranate Seed Oil from Blanched Seeds: Impact on Functionality, Oxidative Stability, and Antioxidant Properties. Processes 2021, 9, 635. [Google Scholar] [CrossRef]
- Mariod, A.; Matthäus, B.; Eichner, K.; Hussein, I.H. Improving the oxidative stability of sunflower oil by blending with Sclerocarya birrea and Aspongopus viduatus oils. J. Food Lipids 2005, 12, 150–158. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Prasanth Kumar, P.K.; Hemavathy, J.; Gopala Krishna, A.G. Fatty Acid Composition, Oxidative Stability, and Radical Scavenging Activity of Vegetable Oil Blends with Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 991–999. [Google Scholar] [CrossRef]
- Rudzińska, M.; Hassanein, M.M.; Abdel–Razek, A.G.; Ratusz, K.; Siger, A. Blends of rapeseed oil with black cumin and rice bran oils for increasing the oxidative stability. J. Food Sci. Technol. 2016, 53, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiralan, M.; Ulaş, M.; Özaydin, A.; Özdemır, N.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Blends of Cold Pressed Black Cumin Oil and Sunflower Oil with Improved Stability: A Study Based on Changes in the Levels of Volatiles, Tocopherols and Thymoquinone during Accelerated Oxidation Conditions. J. Food Biochem. 2017, 41, e12272. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Iqbal, Z.; Abbas, N.; Mahmud, A. Enhancement of the Oxidative Stability of Butter Oil by Blending with Mango (Mangifera indica L.) Kernel Oil in Ambient and Accelerated Oxidation. J. Food Process. Preserv. 2017, 41, e12957. [Google Scholar] [CrossRef]
- Torri, L.; Bondioli, P.; Folegatti, L.; Rovellini, P.; Piochi, M.; Morini, G. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem. 2019, 286, 584–591. [Google Scholar] [CrossRef]
- Rodríguez, G.; Villanueva, E.; Cortez, D.; Sanchez, E.; Aguirre, E.; Hidalgo, A. Oxidative Stability of Chia (Salvia hispanica L.) and Sesame (Sesamum indicum L.) Oil Blends. J. Am. Oil Chem. Soc. 2020, 97, 729–735. [Google Scholar] [CrossRef]
- Garg, M.; Wason, S.; Meena, P.L.; Chopra, R.; Sadhu, S.D.; Dhyani, A. Effect of frying on physicochemical properties of sesame and soybean oil blend. J. Appl. Nat. Sci. 2021, 13, 820–829. [Google Scholar] [CrossRef]
- González-Gamallo, S.; Salvador, M.D.; Fregapane, G. Design and Characteristics of Novel Sensory and Nutritionally Oriented Olive, Seed, and Nut Virgin Oils’ Blendings. Eur. J. Lipid Sci. Technol. 2021, 123, 2100008. [Google Scholar] [CrossRef]

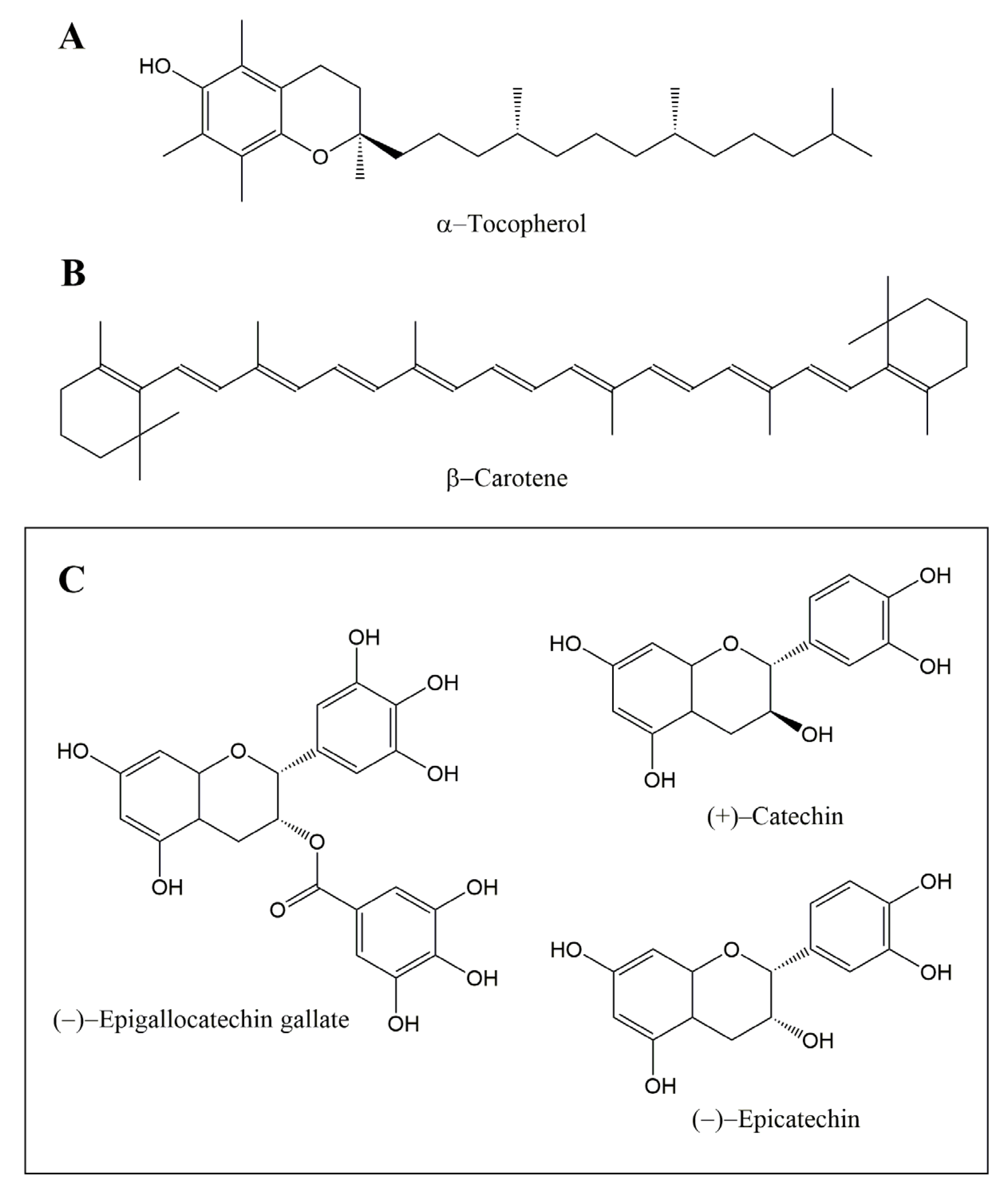

| Type of Waste or By-Product | Type of Extract | Extract Concentration in the Oil | Oil-Enriched and Oxidative Treatment Conditions | Analytical Approach | Main Results | References |
|---|---|---|---|---|---|---|
| Olive mill wastewaters | Water extract (WE) | 50 mg hydroxytirosol L−1 of oil | Sunflower oil (SFO) stored for 90 days at 10 or 25 °C | Refractive index, peroxide value, oxitest oxidation test reactor, DPPH, ABTS, and ORAC | WE increased the oxidative stability of SFO and decreased the peroxide value | [120] |
| Tomato peel and seed by-product | Oleoresin extracted with hexane | 250, 500, 1000, and 2000 µg g−1 of oil | Refined olive oil (ROO) and SFO Storage for 19 weeks at 50 °C | Free acidity, peroxide value, k232, k270 | 250 and 2000 µg g−1 increased the stability of ROO and SFO, respectively | [125] |
| Olive mill wastewaters | Water extract | 500, 1000, 2000, and 3000 mg L−1 of oil | Extra-virgin and refined kernel olive oil Heating at 100 °C for 30 min Heating at 160 °C for 120 min | DPPH, peroxide value, k270, Totox index | Olive polyphenols were efficient against oxidation of both oil types at 1000–3000 mg L−1. Oils treated with olive polyphenols and heated had similar levels of PV than fresh oils. | [133] |
| Mango peel by-product of mango industrial processing | supercritical CO2 extract | 200, 500, and 1000 mg extract kg−1 oil | Sunflower oil (SFO) | Rancimat method | Mango peel extracts protected sunflower oil against lipid oxidation when evaluated between 200 and 1000 mg extract kg−1 oil | [134] |
| Sesame cake by-product of oil extraction | Methanolic sesame cake extract (SCE) | 5, 10, 50, 100, and 200 mg kg−1 of oil | SFO and soybean oil (SBO) Stored for 72 h at 70 °C | Peroxide value, p-anisidine, k232, k270, Schaal oven test | SCE at 200 mg kg−1 inhibited SBO oxidation in SFO SCE controlled peroxide and p-anisidine formation | [122] |
| Olive mill wastewaters (OMWW) and olive pomace (PO) | Ethyl acetate extract | 1 mg g−1 of oil | SBO subjected to accelerated oxidation at 60 °C for 21 days | Schaal oven test, k232, and k270 | Under accelerated oxidation conditions, PO and OMWW extracts were more effective than BHT. They decreased K232 but had no effect on K270. | [135] |
| Blueberry juice by-product (BBE) | Hydro-alcoholic extract, | 200, 500, and 800 mg kg−1 of oil | SFO Simulated frying conditions at 180 °C for 12 h | Peroxide value, p-anisidine, Totox, thiobarbituric acid test | BBE 800 mg kg−1 inhibited the formation of primary oxidation products, BBE significantly inhibited lipid thermo-oxydation, and BBE at 500 mg kg−1 was as effective as BHT 200 ppm. | [3] |
| Pumpkin peel | Water extracts obtained with subcritical water extraction (SWE) Ethanol or water extracts obtained with supercritical CO2 extraction (SFE) | 400 mg kg−1 of oil | Canola oil Storage at 30 °C for 60 days | Peroxide value, carbonyl value, acid value | Both SWE and SFE inhibited the formation of peroxides and carbonyl compounds during storage. The mix of SFE and SWE was more effective than the extracts alone. Oils with SFE had lower acidity than SWE-enriched oils. | [136] |
| Peel (AP) and seeds (AS) from avocado waste | Freeze dried ethanolic extract | 0.5% (w/w) | Avocado oil and water emulsions and nanoemulsions stored for 50 days at room temperature, oxidation was induced by the addition of Fe2+ solution | Peroxide value and thiobarbituric acid test | AP and AS had no effect on the inhibition of peroxide production but accelerated their degradation. Ap and As limited the formation of secondary oxidation products. | [137] |
| Rice bran, rice hull, wheat bran, wheat husk, peels of citrus, banana, apple, pomegranate | Methanolic extracts | 600 mg kg−1 of oil | Corn oil heating at 60 °C (8 h of heating per day) for 30 days | Peroxide value (PV), p-anisidine, conjugated dienes (k232), conjugated trienes (k268) | Methanolic extracts decreased the rise in peroxide value, conjugated dienes, conjugated trienes, and p-anisidine compared with the control. Pomegranate peel extract was the most effective, while rice hull extract was the least effective. | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. https://doi.org/10.3390/su14020849
Fadda A, Sanna D, Sakar EH, Gharby S, Mulas M, Medda S, Yesilcubuk NS, Karaca AC, Gozukirmizi CK, Lucarini M, et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability. 2022; 14(2):849. https://doi.org/10.3390/su14020849
Chicago/Turabian StyleFadda, Angela, Daniele Sanna, El Hassan Sakar, Said Gharby, Maurizio Mulas, Silvia Medda, Nese Sahin Yesilcubuk, Asli Can Karaca, Celale Kirkin Gozukirmizi, Massimo Lucarini, and et al. 2022. "Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils" Sustainability 14, no. 2: 849. https://doi.org/10.3390/su14020849
APA StyleFadda, A., Sanna, D., Sakar, E. H., Gharby, S., Mulas, M., Medda, S., Yesilcubuk, N. S., Karaca, A. C., Gozukirmizi, C. K., Lucarini, M., Lombardi-Boccia, G., Diaconeasa, Z., & Durazzo, A. (2022). Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability, 14(2), 849. https://doi.org/10.3390/su14020849














