“Nature-like” Cryoimmobilization of Phototrophic Microorganisms: New Opportunities for Their Long-Term Storage and Sustainable Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Analytical Methods
3. Results
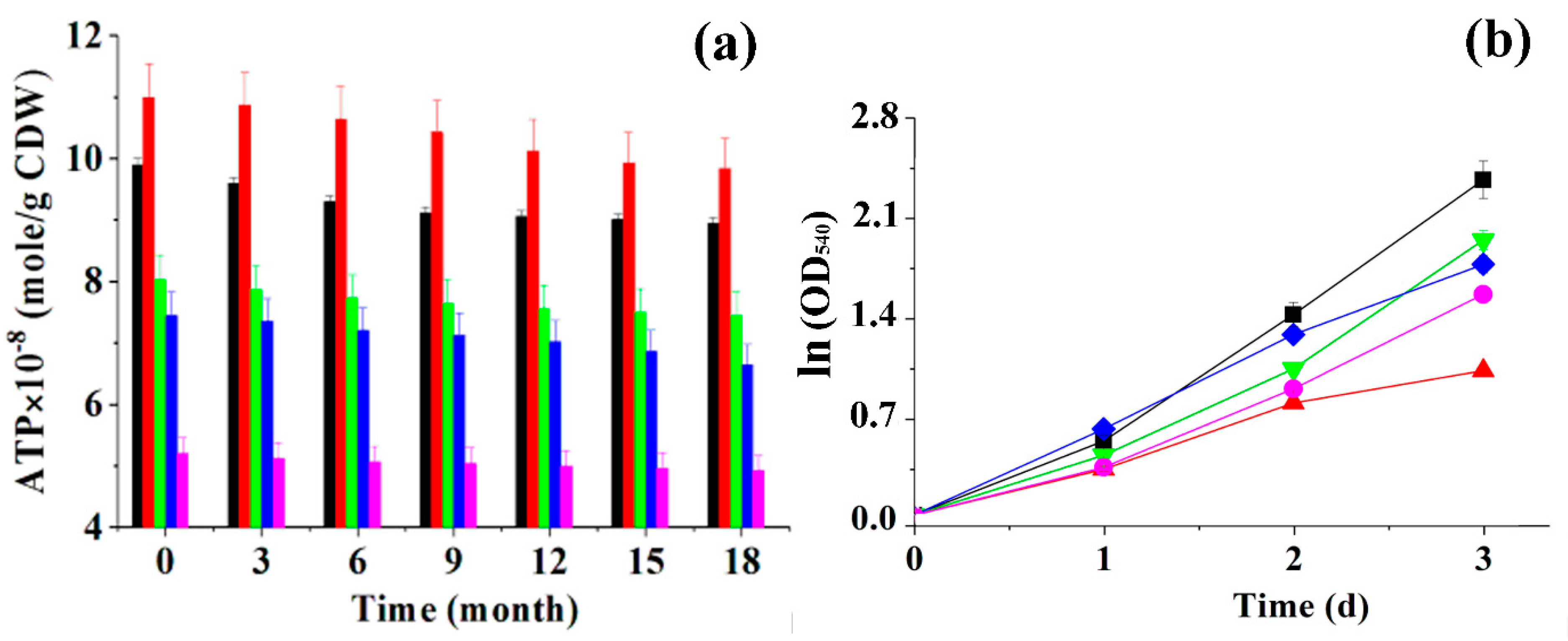
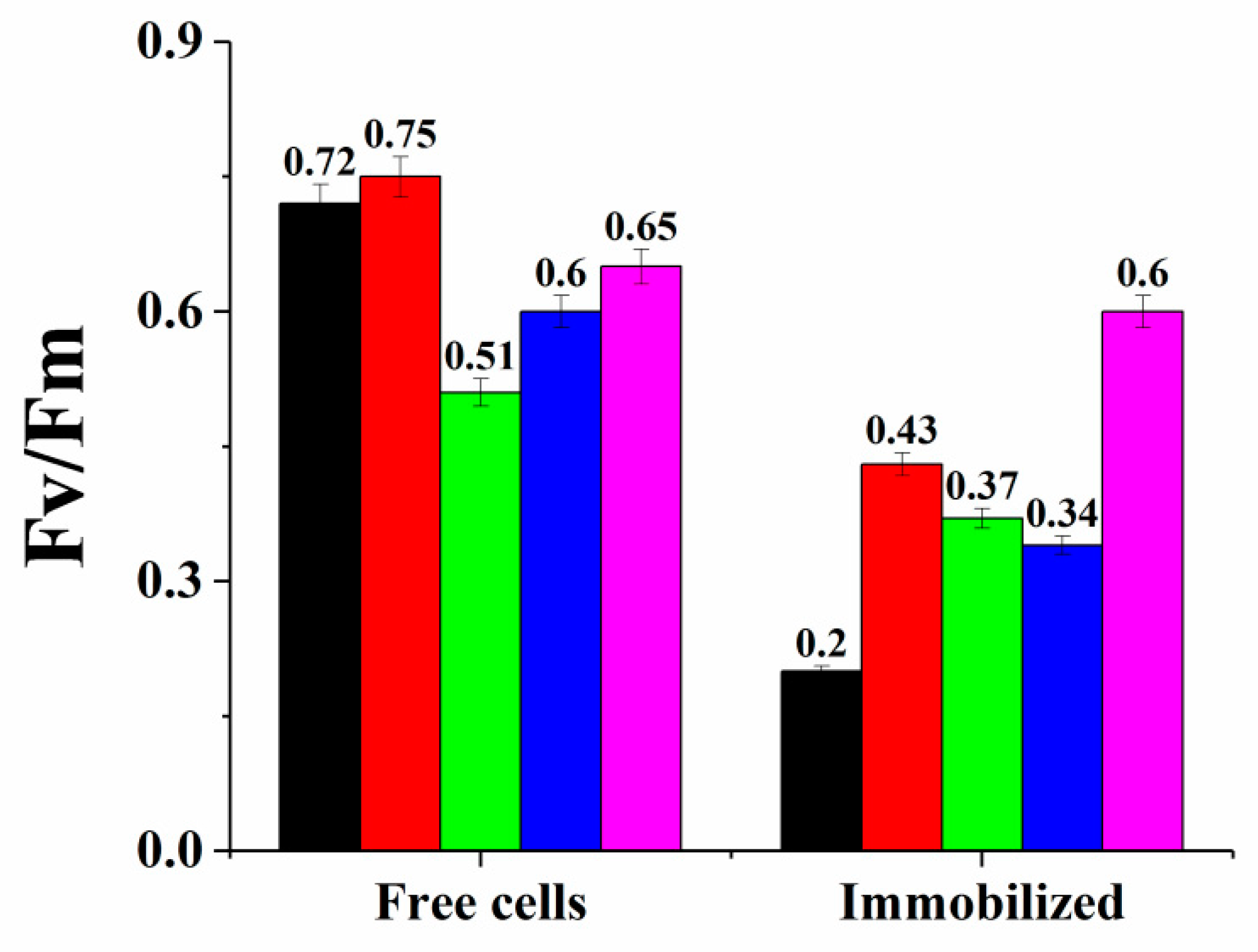
3.1. Cryoimmobilization of Phototrophic Microbial Cells in PVA Cryogel and Their Long-Term Storage
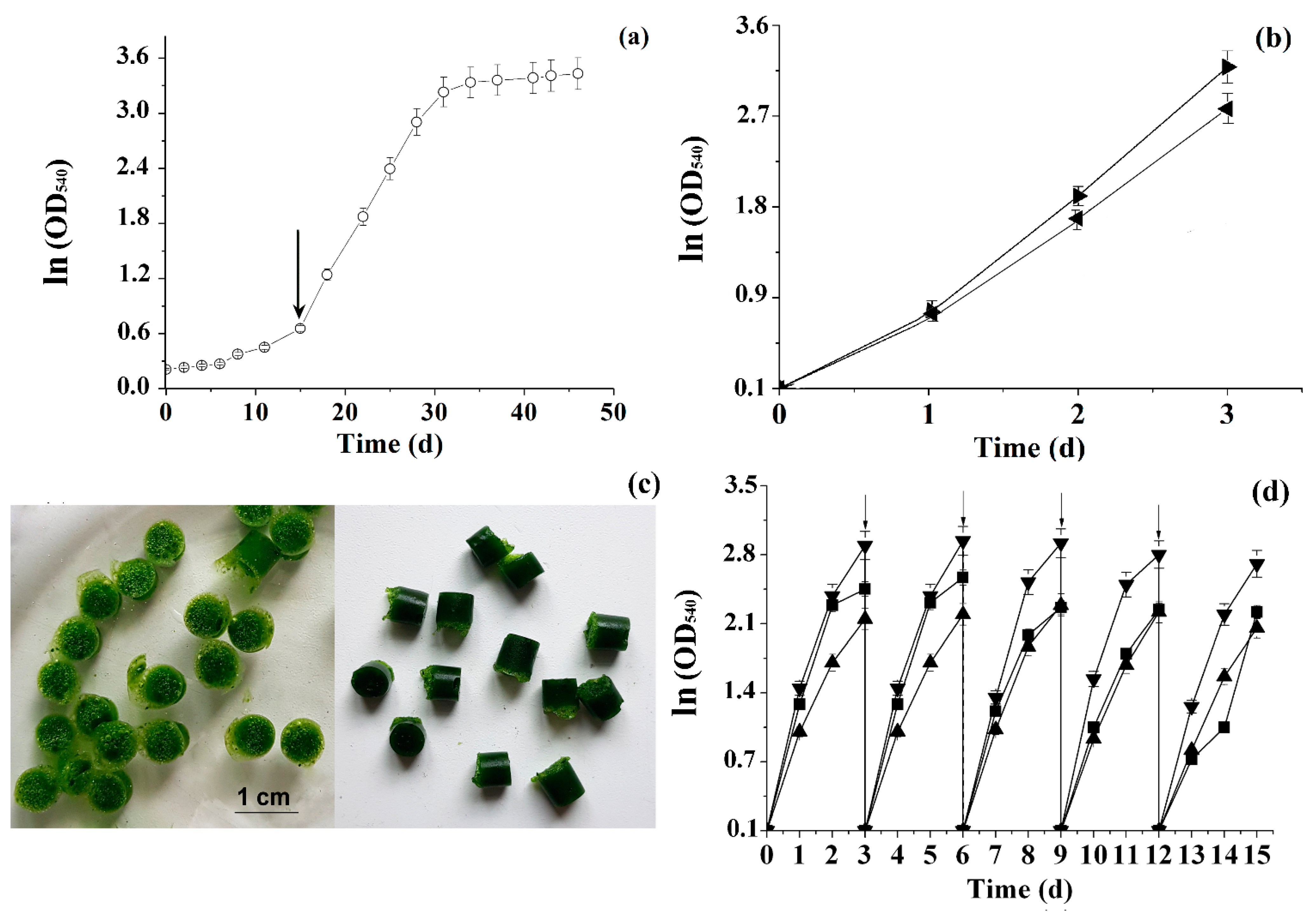
3.2. Accelerating the Accumulation of Biomass of Chlorella Vulgaris via the Use of Immobilized Inoculum
4. Discussion
5. Conclusions
- -
- Their cryopreservation and long-term storage, without the loss of functional, metabolic activity and basic biochemical characteristics of the cells, including in hereditary phototrophic cells.
- -
- The use of immobilized cells as an inoculum for the accumulation of phototrophic cell biomass, including after long-term storage, under mixotrophic conditions, that allows combining wastewater treatment with the growth of microalgae, and accumulation of the phototrophic cell biomass for its conversion to various products in the frame of green chemistry and nature-like processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D.; Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Sen’Ko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Kokkinos, K.; Karayannis, V.; Moustakas, K. Optimizing Microalgal Biomass Feedstock Selection for Nanocatalytic Conversion Into Biofuel Clean Energy, Using Fuzzy Multi-Criteria Decision Making Processes. Front. Energy Res. 2021, 8, 622210. [Google Scholar] [CrossRef]
- O’Neill, E.A.; Neil, J.R. Microalgae as a natural ecological bioindicator for the simple real-time monitoring of aquaculture wastewater quality including provision for assessing impact of extremes in climate variance: A comparative case study from the Republic of Ireland. Sci. Total Environ. 2022, 802, 149800. [Google Scholar] [CrossRef]
- van den Broek, L.A.M.; Wagemakers, M.J.M.; Verschoor, A.M.; Frissen, A.E.; van Haveren, J.; Blaauw, R.; Mooibroek, H. Microalgae as Renewable Raw Material for Bioproducts: Identification and Biochemical Composition of Microalgae from a Raceway Pond in The Netherlands. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value, 1st ed.; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–68. [Google Scholar] [CrossRef]
- Udaiyappan, A.F.M.; Abu Hasan, H.; Takkriff, M.S.; Abdullah, S.R.S. A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J. Water Process Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Lam, M.K.; Yusoff, M.I.; Uemura, Y.; Lim, J.-W.; Khoo, C.G.; Lee, K.T.; Ong, H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renew. Energy 2017, 103, 197–207. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.H.; Buang, A. Immobilized Chlorella vulgaris for efficient palm oil mill effluent treatment and heavy metals removal. Desalinisation Water Treat. 2017, 81, 105–117. [Google Scholar] [CrossRef]
- Makhlis, T.A.; Senko, O.V.; Mamedova, F.T.; Efremenko, E.N. Immobilization of cells as approach to their long-term storage. In Immobilized Cells: Biocatalysts and Processes; Efremenko, E.N., Ed.; RIOR: Moscow, Russia, 2018; pp. 97–122. [Google Scholar]
- Vasilieva, S.G.; Lobakova, E.S.; Lukyanov, A.A.; Solovchenko, A.E. Immobilized microalgae in biotechnology. Mosc. Univ. Biol. Sci. Bull. 2016, 71, 170–176. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Banerjee, S.; Das, D. Microalgal bio-flocculation: Present scenario and prospects for commercialization. Environ. Sci. Pollut. Res. 2021, 28, 26294–26312. [Google Scholar] [CrossRef]
- Osorio, J.H.M.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A Review of Microalgal Biofilm Technologies: Definition, Applications, Settings and Analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Caldwell, G.S.; In-Na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Ng, F.-L.; Phang, S.-M.; Vengadesh, P.; Periasamy, V.; Yunus, K.; Fisher, A.C. Enhancement of Power Output by using Alginate Immobilized Algae in Biophotovoltaic Devices. Sci. Rep. 2017, 7, 16237. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Buzin, M.I.; Golubev, E.K.; Shcherbina, M.A.; Lozinsky, V.I. A Study of Cryostructuring of Polymer Systems. 59. Effect of Cryogenic Treatment of Preliminarily Deformed Poly(vinyl alcohol) Cryogels on Their Physicochemical Properties. Colloid J. 2021, 83, 634–641. [Google Scholar] [CrossRef]
- Senko, O.; Gladchenko, M.; Maslova, O.; Efremenko, E. Long-Term Storage and Use of Artificially Immobilized Anaerobic Sludge as a Powerful Biocatalyst for Conversion of Various Wastes Including Those Containing Xenobiotics to Biogas. Catalysts 2019, 9, 326. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341. [Google Scholar] [CrossRef]
- Razumovsky, S.D.; Efremenko, E.N.; Makhlis, T.A.; Senko, O.V.; Bikhovsky, M.Y.; Podmaster’Ev, V.V.; Varfolomeev, S.D. Effect of immobilization on the main dynamic characteristics of the enzymatic oxidation of methane to methanol by bacteria Methylosinus sporium B-2121. Russ. Chem. Bull. 2008, 57, 1633–1636. [Google Scholar] [CrossRef]
- Ali, P.; Fucich, D.; Shah, A.A.; Hasan, F.; Chen, F. Cryopreservation of Cyanobacteria and Eukaryotic Microalgae Using Exopolysaccharide Extracted from a Glacier Bacterium. Microorganisms 2021, 9, 395. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Huete-Ortega, M.; Day, J.G.; Okurowska, K.; Slocombe, S.P.; Stanley, M.S.; Vaidyanathan, S. Effects of cryopreservation on viability and functional stability of an industrially relevant alga. Sci. Rep. 2019, 9, 2093. [Google Scholar] [CrossRef]
- Nowshari, M.A.; Brem, G. The protective action of polyvinyl alcohol during rapid-freezing of mouse embryos. Theriogenology 2000, 53, 1157–1166. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Ivshina, I.B.; Lozinsky, V.I.; Kuyukina, M.S.; Philp, J.C. Bioremediation of diesel-contaminated soil by microorganisms immobilised in polyvinyl alcohol. Int. Biodeterior. Biodegrad. 2004, 54, 167–174. [Google Scholar] [CrossRef]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Application; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Stepanov, N.; Efremenko, E. Immobilised cells of Pachysolen tannophilus yeast for ethanol production from crude glycerol. New Biotechnol. 2017, 34, 54–58. [Google Scholar] [CrossRef]
- Matsunaga, N.; Uehara, A.; Murase, N.; Kuriyama, A. Cryopreservation of Spirulina (Arthrospira) platensis NIES-46 by snap-freezing considering trichome morphology. Cryobiol. Cryotechnol. 2019, 64, 75–83. [Google Scholar] [CrossRef]
- Shiraishi, H. Cryopreservation of the edible alkalophilic cyanobacterium Arthrospira platensis. Biosci. Biotechnol. Biochem. 2016, 80, 2051–2057. [Google Scholar] [CrossRef]
- Prasad, R.N.; Sanghamitra, K.; Antonia, G.-M.; Juan, G.-V.; Benjamin, R.-G.; Luis, I.-M.J.; Guillermo, V.-V. Isolation, Identification and Germplasm Preservation of Different Native Spirulina Species from Western Mexico. Am. J. Plant Sci. 2013, 04, 65–71. [Google Scholar] [CrossRef]
- Iwamoto, K.; Fukuyo, S.; Okuda, M.; Kobayashi, M.; Shiraiwa, Y. Cryopreservation of the Chlorophyll d-Containing Cyanobacterium Acaryochloris Marina. Procedia Environ. Sci. 2012, 15, 118–125. [Google Scholar] [CrossRef][Green Version]
- Mori, F.; Erata, M.; Watanabe, M.M. Cryopreservation of cyanobacteria and green algae in the NIES-collection. Microbiol. Cult. Coll. 2002, 18, 45–55. [Google Scholar]
- Motham, M.; Peerapornpisal, Y.; Tongsriri, S.; Pumas, C.; Vacharapiyasophon, P. High subzero temperature preservation of Spirulina platensis (Spirulina fusiformis) and its ultrastructure. Chiang Mai J. Sci. 2012, 39, 554–561. Available online: https://www.thaiscience.info/journals/Article/CMJS/10905239.pdf (accessed on 4 January 2022).
- Efremenko, E.N.; Senko, O.V.; Makhls, T.A.; Mamedova, F.T.; Holstov, A.V.; Varfolomeev, S.D. Method of Cryopreservation Phototrophic Microorganisms Cells. RU Patent 2508397, 27 February 2014. [Google Scholar]
- Araujo, G.S.; Matos, L.J.; Fernandes, J.O.; Cartaxo, S.J.; Gonçalves, L.R.; Fernandes, F.A.; Farias, W.R. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- ISO 6060:1989 Water Quality—Determination of the Chemical Oxygen Demand. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6060:ed-2:v1:en (accessed on 4 January 2022).
- ISO 11905-1:1997 Water Quality—Determination of Nitrogen—Part 1: Method Using Oxidative Digestion with Peroxodisulfate. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11905:-1:ed-1:v1:en (accessed on 4 January 2022).
- ISO 11923:1997 Water Quality—Determination of Suspended Solids by Filtration through Glass-Fibre Filters, APHA, AWWA, WEF. Standard Methods for Examination of Water and Wastewater. Available online: https://www.iso.org/obp/ui/fr/#iso:std:iso:11923:ed-1:v1:en (accessed on 4 January 2022).
- ISO 6878:2004 Water Quality—Determination of Phosphorus-Ammonium Molybdate Spectrometric Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6878:ed-2:v1:en (accessed on 4 January 2022).
- Fursova, P.V.; Bobyrev, P.A.; Risnik, D.V.; Voronova, E.N.; Pogosyan, S.I. Bioindicational Potential of Biophysical and Hydrobiological Indicators of Phytoplankton in Experiments with Laboratory Algocenoses. Biol. Bull. Rev. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. [Google Scholar] [CrossRef]
- Breuer, G.; Evers, W.A.C.; De Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of Fatty Acid Content and Composition in Microalgae. J. Vis. Exp. 2013, e50628. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J. Clean. Prod. 2020, 272, 122823. [Google Scholar] [CrossRef]
- Prakash, J.; Kalia, V.C. Application of Quorum Sensing Systems in Production of Green Fuels. In Quorum Sensing and Its Biotechnological Applications; Kalia, V.P., Ed.; Springer: Singapore, 2018; pp. 155–166. [Google Scholar] [CrossRef]
- Rincon, S.M.; Romero, H.M.; Aframehr, W.M.; Beyenal, H. Biomass production in Chlorella vulgaris biofilm cultivated under mixotrophic growth conditions. Algal Res. 2017, 26, 153–160. [Google Scholar] [CrossRef]
- Herrera, N.; Echeverri, F. Evidence of Quorum Sensing in Cyanobacteria by Homoserine Lactones: The Origin of Blooms. Water 2021, 13, 1831. [Google Scholar] [CrossRef]
- Chi, W.; Zheng, L.; He, C.; Han, B.; Zheng, M.; Gao, W.; Sun, C.; Zhou, G.; Gao, X. Quorum sensing of microalgae associated marine Ponticoccus sp. PD-2 and its algicidal function regulation. AMB Express 2017, 7, 59. [Google Scholar] [CrossRef]
- Dow, L. How Do Quorum-Sensing Signals Mediate Algae–Bacteria Interactions? Microorganisms 2021, 9, 1391. [Google Scholar] [CrossRef]
- Tanniou, A.; Turpin, V.; Lebeau, T. Comparison of cryopreservation methods for the long term storage of the marine diatom Haslea ostrearia (simonsen). Cryobiology 2012, 65, 45–50. [Google Scholar] [CrossRef]
- Choudhary, K.K. Post-storage viability and metabolic stability of immobilized cyanobacteria. Nova Hedwig. 2010, 90, 215–226. [Google Scholar] [CrossRef]
- Morschett, H.; Reich, S.; Wiechert, W.; Oldiges, M. Simplified cryopreservation of the microalga Chlorella vulgaris integrating a novel concept for cell viability estimation. Eng. Life Sci. 2016, 16, 36–44. [Google Scholar] [CrossRef]
- Nakanishi, K.; Deuchi, K.; Kuwano, K. Cryopreservation of four valuable strains of microalgae, including viability and characteristics during 15 years of cryostorage. J. Appl. Phycol. 2012, 24, 1381–1385. [Google Scholar] [CrossRef]
- Saadaoui, I.; Al Emadi, M.; Bounnit, T.; Schipper, K.; Al Jabri, H. Cryopreservation of microalgae from desert environments of Qatar. J. Appl. Phycol. 2015, 28, 2233–2240. [Google Scholar] [CrossRef]
- Kumari, N.; Gupta, M.K.; Singh, R.K. Open encapsulation-vitrification for cryopreservation of algae. Cryobiology 2016, 73, 232–239. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Feng, L.-J.; Liu, J.-Z.; Liu, M.; Cai, H.-W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Markou, G. Fed-batch cultivation of Arthrospira and Chlorella in ammonia-rich wastewater: Optimization of nutrient removal and biomass production. Bioresour. Technol. 2015, 193, 35–41. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zheng, C.; Potumarthi, R.; Chen, X.D.; Lu, Y. NaCS–PDMDAAC immobilized autotrophic cultivation of Chlorella sp. for wastewater nitrogen and phosphate removal. Chem. Eng. J. 2012, 187, 185–192. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-H.; Li, C.; Zeng, G.-M.; Ma, D.-H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-H.; Li, C.; Wang, Y.-J.; Jin, W.-H.; Deng, Y.-B. Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour. Technol. 2014, 167, 441–446. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Mu, J.; Liu, M.; Cui, W. Removal of nutrients, organic matter, and metal from domestic secondary effluent through microalgae cultivation in a membrane photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2713–2719. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef]
- Praveen, P.; Loh, K.-C. Nitrogen and phosphorus removal from tertiary wastewater in an osmotic membrane photobioreactor. Bioresour. Technol. 2016, 206, 180–187. [Google Scholar] [CrossRef]
- Boonchai, R.; Seo, G. Microalgae membrane photobioreactor for further removal of nitrogen and phosphorus from secondary sewage effluent. Korean J. Chem. Eng. 2015, 32, 2047–2052. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Akhundov, R.; Ismailov, A.; Efremenko, E. Immobilized Luminescent Bacteria for the Detection of Mycotoxins under Discrete and Flow-Through Conditions. Biosensors 2019, 9, 63. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Maslova, O.V.; Kholstov, A.V.; Senko, O.V.; Ismailov, A.D. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence 2016, 31, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Khesina, Z.B.; Karnaeva, A.E.; Pytskii, I.S.; Buryak, A.K. The mysterious mass death of marine organisms on the Kamchatka Peninsula: A consequence of a technogenic impact on the environment or a natural phenomenon? Mar. Pollut. Bull. 2021, 166, 112175. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic marine microalgae and noxious blooms in the Mediterranean Sea: A contribution to the Global HAB Status Report. Harmful Algae 2021, 102, 101843. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate change and harmful benthic microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef] [PubMed]
| Phototrophic Microorganism Species | Concentration of PVA Solution Used for Cell Immobilization, % | Cell Biomass Concentration (CDW) in the Immobilized Sample, % | PRB *, % |
|---|---|---|---|
| Green microalgae | |||
| C. vulgaris | 7.0 | 4.0 | 95 ± 3 |
| Dunaliella salina | 8.0 | 4.3 | 94 ± 4 |
| Nannochloropsis sp. | 7.5 | 3.8 | 93 ± 4 |
| Chlamydomonas sp. | 6.5 | 4.3 | 90 ± 3 |
| Chlorococcum sp. | 7.0 | 4.2 | 92 ± 3 |
| Cosmarium sp. | 8.0 | 3.7 | 94 ± 4 |
| Red microalgae | |||
| Galdieria partita | 8.0 | 4.1 | 91 ± 3 |
| Haematococcus pluvialis | 7.0 | 4.3 | 92 ± 3 |
| Diatoms | |||
| Thalassiosira weissflogii | 8.7 | 4.0 | 95 ± 4 |
| Cyanobacteria | |||
| Nostoc sp. | 6.5 | 4.0 | 91 ± 3 |
| Arthrospira platensis | 6.0 | 3.8 | 90 ± 3 |
| Gloeotrichia echinulata | 8.5 | 3.7 | 93 ± 4 |
| Phototrophic Microorganism Species | Lipids/ Proteins/ Hydrocarbons, % | Unsaturated Fatty Acids, % from Total Fatty Acids in Lipid Content | RP, mg vit C/g CDW |
|---|---|---|---|
| Green microalgae | |||
| C. vulgaris | 16.5 ± 0.8/7.0 ± 0.3/55.6 ± 2.7 | 53.6 ± 2.6 | 0.01 ± 0.0004 |
| Dunaliella salina | 9.1 ± 0.4/5.4 ± 0.2/69.7 ± 3.4 | 26.3 ± 1.3 | 0.02 ± 0.001 |
| Nannochloropsis sp. | 25.0 ± 1.2/7.0 ± 0.3/54.9 ± 2.7 | 24.4 ± 1.2 | 1.21 ± 0.06 |
| Chlamydomonas sp. | 48.2 ± 2.4/17.0 ± 0.8/21.3 ± 0.9 | 43.6 ± 2.1 | 0.42 ± 0.02 |
| Chlorococcum sp. | 19.3 ± 0.9/52.7 ± 2.6/17.2 ± 0.8 | 47.4 ± 2.3 | 1.09 ± 0.05 |
| Cosmarium sp. | 6.7 ± 0.3/19.9 ± 0.9/58.4 ± 2.9 | 18.4 ± 0.9 | 0.05 ± 0.002 |
| Red microalgae | |||
| Galdieria partita | 7.5 ± 0.3/41.8 ± 2.0/50.0 ± 2.4 | 17.5 ± 0.8 | 1.05 ± 0.05 |
| Haematococcus pluvialis | 15.6 ± 0.7/22.2 ± 1.1/52.7 ± 2.6 | 30.6 ± 1.5 | 3.6 ± 0.1 |
| Diatoms | |||
| Thalassiosira weissflogii | 42.1 ± 2.1/30.6 ± 1.5/22.3 ± 1.2 | 9.4 ± 0.4 | - |
| Cyanobacteria | |||
| Nostoc sp. | 9.2 ± 0.4/20.4 ± 0.9/52.3 ± 2.6 | 15.6 ± 0.7 | 0.83 ± 0.04 |
| Arthrospira platensis | 19.0 ± 0.9/40.9 ± 1.9/40.8 ± 1.9 | 22.9 ± 1.1 | 1.46 ± 0.07 |
| Gloeotrichia echinulata | 15.8 ± 0.7/32.7 ± 1.6/48.5 ± 2.4 | 33.4 ± 1.6 | - |
| Wastewater | Baseline Characteristics (mg/L) | Removal Efficiencies after 72 h (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| COD | TSS | TN | TP | COD | TSS | TN | TP | |
| Horticultural | 780 ± 28 | 70 ± 3 | 47.6 ± 2.1 | 7.3 ± 0.2 | 81 ± 3 | 79 ± 3 | 84 ± 3 | 93 ± 3 |
| Municipal | 230 ± 9 | 50 ± 2 | 38.4 ± 1.6 | 4.6 ± 0.2 | 88 ± 4 | 94 ± 4 | 91 ± 4 | 95 ± 4 |
| Milk plant | 1400 ± 61 | 350 ± 15 | 60.0 ± 2.7 | 8.0 ± 0.3 | 92 ± 4 | 76 ± 3 | 78 ± 4 | 88 ± 3 |
| Strain [Reference] | Cryopreservation of Biomass * | Storage Time | Cell Viability, % |
|---|---|---|---|
| C. vulgaris 211-11b [55] | Freezing and storage at –80 °C using a freezing container or a simple polystyrene box. Cryoprotectant mixture: 10% (v/v) DMSO, 10% (v/v) EG, and 10% (w/v) L-proline. | 24 h | 63 ± 2 |
| C. vulgaris C-27 [56] | Freezing at –40 °C for 4 h and then storage in liquid nitrogen Cryoprotectant mixture: 5% (v/v) DMSO, 5% (v/v) EG, and 5% (w/v) L-proline. | 15 years | 54 ± 1 |
| Chlorella sp. [57] | Cryoprotectant: 10% (v/v) DMSO Cooling-freezing-cryopreservation: gradual decrease of temperature from +25 °C to −30 °C. with rate of 1 °C min−1. | 12 months | 77.5 |
| Cryoprotectant: 5% (v/v) methanol. Direct freezing in liquid nitrogen (−196 °C). | 6 months | 80 | |
| H. ostrearia NCC-Jμ1 [53] | Immobilization of cells in Ca-alginate gel and dehydration in the presence of 0.7 M sucrose solution with further freezing in liquid nitrogen (−196 °C). | 48 h | 57.4 ± 3.9 |
| Immobilization of cells in Ca-alginate gel and dehydration in the presence of 0.7 M sucrose solution. Two-step freezing: freezing for 1 h at −80 °C and then quick transfer into liquid nitrogen (−196 °C). | 76.9 ± 3.3 | ||
| Oocystis sp. [58] | Encapsulation in Ca-alginate microbeads with addition of cryoprotectants supplemented with 100 (μM) glutathione at room temperature. The algal-encapsulated microbeads were exposed to equilibration solution (15% (w/v) glycerol, 7.5% (w/v) ethylene glycol, 7.5% (w/v) dimethyl sulfoxide) followed by vitrification solution (30% (w/v) glycerol, 15% (w/v) ethylene glycol, 15% (w/v) DMSO) at 27 °C for 0.5 h and 0.25 h respectively. Freezing in liquid nitrogen (−196 °C). | 14 days | 79 ± 1.6 |
| Rivularia aquatica and Gloeotrichia echinulata [54] | Cells were entrapped in Ca-alginate gel and stored at −20 °C. | 1 year | 47 and 49, respectively |
| C. vulgaris [Beier.] rsemsu Chv-20/11 [This work] | Mixing with PVA solution and freezing at –70 °C. | 18 month | 90 ± 5 |
| Strain | Cell Form | Wastewater Source [Reference] | Biomass Productivity, mg CDW/L/d |
|---|---|---|---|
| C. saccharophila | Suspended | Water from carpet industry with municipal sewage [59] | 23.0 |
| C. sorokiniana UTEX1230 | Municipal and domestic with water supplemented CO2 [60] | 82.5 | |
| C. vulgaris | Aquaculturing [61] | 42.6 | |
| C. vulgaris SAG 211-11b | Poultry litter [62] | 127.0 | |
| Chlorella sp. | Manure [63] | 90.0 | |
| Artificial [64] | 130.0 | ||
| * Immobilized in polymeric carrier NaCS–DMDAAC | 55.0 | ||
| C. vulgaris | Immobilized on poly(vinylidene fluoride) hollow-fiber membrane | Municipal secondary effluent [65] | 72.0 |
| Municipal water [66] | 39.9 | ||
| Domestic secondary effluent [67] | 50.7 | ||
| Aquaculturing [61] | 42.6 | ||
| C. vulgaris AG30007 + activated sludge | Immobilized in Ca-alginate gel | Municipal wastes [68] | 50.0 |
| C. vulgaris ATCC 13482 | Immobilized on commercial thin film composite membrane | Synthetic wastewater [69] | 31.0 |
| Chlorella sp. ADE4 | Immobilized on high-density polyethylene hollow fiber microfiltration membrane | Treated sewage effluent [70] | 55.0 |
| C. vulgaris [Beier.] rsemsu Chv-20/11 | Immobilized in PVA cryogel | Horticultural wastes | 335.3 ± 16.7 |
| Municipal wastes | 311.3 ± 15.5 | ||
| Milk plant wastes [this work] | 407.3 ± 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. “Nature-like” Cryoimmobilization of Phototrophic Microorganisms: New Opportunities for Their Long-Term Storage and Sustainable Use. Sustainability 2022, 14, 661. https://doi.org/10.3390/su14020661
Senko O, Stepanov N, Maslova O, Efremenko E. “Nature-like” Cryoimmobilization of Phototrophic Microorganisms: New Opportunities for Their Long-Term Storage and Sustainable Use. Sustainability. 2022; 14(2):661. https://doi.org/10.3390/su14020661
Chicago/Turabian StyleSenko, Olga, Nikolay Stepanov, Olga Maslova, and Elena Efremenko. 2022. "“Nature-like” Cryoimmobilization of Phototrophic Microorganisms: New Opportunities for Their Long-Term Storage and Sustainable Use" Sustainability 14, no. 2: 661. https://doi.org/10.3390/su14020661
APA StyleSenko, O., Stepanov, N., Maslova, O., & Efremenko, E. (2022). “Nature-like” Cryoimmobilization of Phototrophic Microorganisms: New Opportunities for Their Long-Term Storage and Sustainable Use. Sustainability, 14(2), 661. https://doi.org/10.3390/su14020661