Hedgerows on Crop Field Edges Increase Soil Carbon to a Depth of 1 meter
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Site Selection
2.3. Soil Sampling and Profile Descriptions
2.4. Bulk Density
2.5. Soil Chemical Properties
2.6. Statistical Analyses
2.7. Scenario Estimates
3. Results and Discussion
3.1. Soil Organic Carbon Stocks (0–100 cm)
3.2. SOC Concentrations and Stocks by Depth
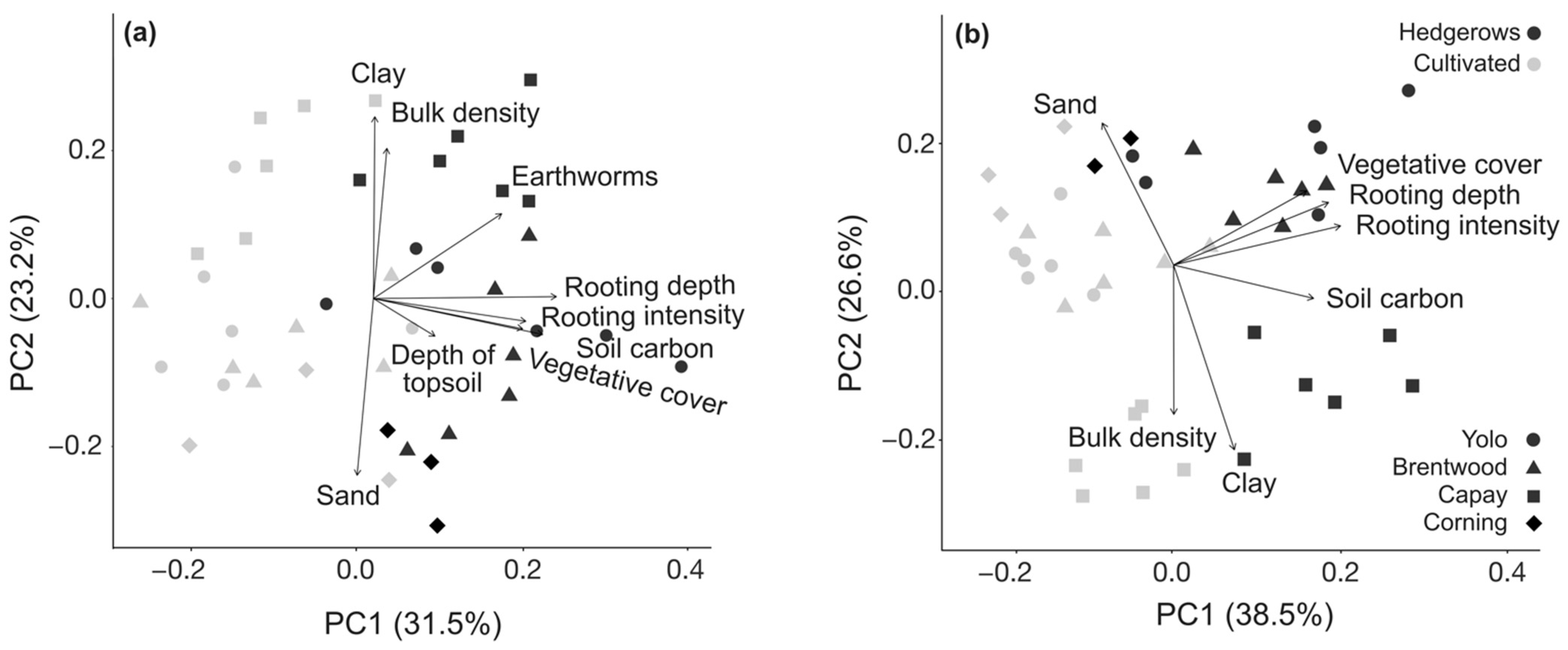
3.3. Multivariate Analysis of Management Effects
3.4. Scaling up—Potential Carbon Sinks State-wide
3.5. Future Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amundson, R. The Carbon Budget in Soils. Annu. Rev. Earth Planet. Sci. 2001, 29, 535–562. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil Carbon Debt of 12,000 Years of Human Land Use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Sato, M.; Kharecha, P.; Von Schuckmann, K.; Beerling, D.J.; Cao, J.; Marcott, S.; Masson-Delmotte, V.; Prather, M.J.; Rohling, E.J.; et al. Young People’s Burden: Requirement of Negative CO2 Emissions. Earth Syst. Dyn. 2017, 8, 577–616. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Change Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Amelung, W.; Bossio, D.; de Vries, W.; Kögel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a Global-Scale Soil Climate Mitigation Strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-Nutrient Stoichiometry to Increase Soil Carbon Sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; van Kessel, C.; Hungate, B.A.; Oenema, O.; Powlson, D.S.; van Groenigen, K.J. Sequestering Soil Organic Carbon: A Nitrogen Dilemma. Environ. Sci. Technol. 2017, 51, 4738–4739. [Google Scholar] [CrossRef]
- Janzen, H.H.; van Groenigen, K.J.; Powlson, D.S.; Schwinghamer, T.; van Groenigen, J.W. Photosynthetic Limits on Carbon Sequestration in Croplands. Geoderma 2022, 416, 115810. [Google Scholar] [CrossRef]
- Poulton, P.; Johnston, J.; Macdonald, A.; White, R.; Powlson, D. Major Limitations to Achieving “4 per 1000” Increases in Soil Organic Carbon Stock in Temperate Regions: Evidence from Long-Term Experiments at Rothamsted Research, United Kingdom. Glob. Change Biol. 2018, 24, 2563–2584. [Google Scholar] [CrossRef]
- Amundson, R.; Biardeau, L. Soil Carbon Sequestration Is an Elusive Climate Mitigation Tool. Proc. Natl. Acad. Sci. USA 2018, 115, 11652–11656. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-Smart Soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The Role of Soil Carbon in Natural Climate Solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Paustian, K.; Collier, S.; Baldock, J.; Burgess, R.; Creque, J.; DeLonge, M.; Dungait, J.; Ellert, B.; Frank, S.; Goddard, T.; et al. Quantifying Carbon for Agricultural Soil Management: From the Current Status toward a Global Soil Information System. Carbon Manag. 2019, 10, 567–587. [Google Scholar] [CrossRef]
- Tautges, N.E.; Chiartas, J.L.; Gaudin, A.C.M.; O’Geen, A.T.; Herrera, I.; Scow, K.M. Deep Soil Inventories Reveal That Impacts of Cover Crops and Compost on Soil Carbon Sequestration Differ in Surface and Subsurface Soils. Glob. Change Biol. 2019, 25, 3753–3766. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing Soil Carbon for Climate Change Mitigation and Adaptation in Mediterranean Cropping Systems: A Meta-Analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Schoeneberger, M.M. Agroforestry: Working Trees for Sequestering Carbon on Agricultural Lands. Agrofor. Syst. 2009, 75, 27–37. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.; Zou, B.; Guo, Z.; Li, Z.; Zhu, W. Biomass Accumulation and Carbon Sequestration in Four Different Aged Casuarina Equisetifolia Coastal Shelterbelt Plantations in South China. PLoS ONE 2013, 8, e77449. [Google Scholar] [CrossRef]
- Thiel, B.; Smukler, S.M.; Krzic, M.; Gergel, S.; Terpsma, C. Using Hedgerow Biodiversity to Enhance the Carbon Storage of Farmland in the Fraser River Delta of British Columbia. J. Soil Water Conserv. 2015, 70, 247–256. [Google Scholar] [CrossRef]
- Brodt, S.B.; Fontana, N.M.; Archer, L.F. Feasibility and Sustainability of Agroforestry in Temperate Industrialized Agriculture: Preliminary Insights from California. Renew. Agric. Food Syst. 2020, 35, 513–521. [Google Scholar] [CrossRef]
- Ghazavi, G.; Thomas, Z.; Hamon, Y.; Marie, J.C.; Corson, M.; Merot, P. Hedgerow Impacts on Soil-Water Transfer Due to Rainfall Interception and Root-Water Uptake. Hydrol. Process. 2008, 22, 4723–4735. [Google Scholar] [CrossRef]
- Long, R.; Anderson, J. Establishing Hedgerows on Farms in California; University of California Agriculture and Natural Resources: Richmond, CA, USA, 2010; ISBN 978-1-60107-662-5. [Google Scholar]
- Morandin, L.; Long, R.; Pease, C.; Kremen, C. Hedgerows Enhance Beneficial Insects on Farms in California’s Central Valley. Calif. Agric. 2011, 65, 197–201. [Google Scholar] [CrossRef]
- Morandin, L.A.; Long, R.F.; Kremen, C. Pest Control and Pollination Cost–Benefit Analysis of Hedgerow Restoration in A Simplified Agricultural Landscape. J. Econ. Entomol. 2016, 109, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Heath, S.K.; Long, R.F. Multiscale Habitat Mediates Pest Reduction by Birds in an Intensive Agricultural Region. Ecosphere 2019, 10, e02884. [Google Scholar] [CrossRef]
- Smukler, S.M.; Sánchez-Moreno, S.; Fonte, S.J.; Ferris, H.; Klonsky, K.; O’Geen, A.T.; Scow, K.M.; Steenwerth, K.L.; Jackson, L.E. Biodiversity and Multiple Ecosystem Functions in an Organic Farmscape. Agric. Ecosyst. Environ. 2010, 139, 80–97. [Google Scholar] [CrossRef]
- Long, R.; Garbach, K.; Morandin, L. Hedgerow Benefits Align with Food Production and Sustainability Goals. Calif. Agric. 2017, 71, 117–119. [Google Scholar] [CrossRef]
- Falloon, P.; Powlson, D.; Smith, P. Managing Field Margins for Biodiversity and Carbon Sequestration: A Great Britain Case Study. Soil Use Manag. 2006, 20, 240–247. [Google Scholar] [CrossRef]
- Drexler, S.; Gensior, A.; Don, A. Carbon Sequestration in Hedgerow Biomass and Soil in the Temperate Climate Zone. Reg. Environ. Change 2021, 21, 74. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, H.; Qin, Q.; Wang, Y. Effect of Plant Hedgerows on Agricultural Non-Point Source Pollution: A Meta-Analysis. Environ. Sci. Pollut. Res. 2020, 27, 24831–24847. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees Increase Soil Organic Carbon and Nutrient Availability in Temperate Agroforestry Systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Cardinael, R.; Guenet, B.; Chevallier, T.; Dupraz, C.; Cozzi, T.; Chenu, C. High Organic Inputs Explain Shallow and Deep SOC Storage in a Long-Term Agroforestry System—Combining Experimental and Modeling Approaches. Biogeosciences 2018, 15, 297–317. [Google Scholar] [CrossRef]
- Walter, C.; Merot, P.; Layer, B.; Dutin, G. The Effect of Hedgerows on Soil Organic Carbon Storage in Hillslopes. Soil Use Manag. 2003, 19, 201–207. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil Organic Carbon Sequestration in Agroforestry Systems. A Review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Cardinael, R.; Mao, Z.; Prieto, I.; Stokes, A.; Dupraz, C.; Kim, J.H.; Jourdan, C. Competition with Winter Crops Induces Deeper Rooting of Walnut Trees in a Mediterranean Alley Cropping Agroforestry System. Plant Soil 2015, 391, 219–235. [Google Scholar] [CrossRef]
- Biffi, S.; Chapman, P.J.; Grayson, R.P.; Ziv, G. Soil Carbon Sequestration Potential of Planting Hedgerows in Agricultural Landscapes. J. Environ. Manag. 2022, 307, 114484. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.-D. Maximum Rooting Depth of Vegetation Types at the Global Scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Upson, M.A.; Burgess, P.J. Soil Organic Carbon and Root Distribution in a Temperate Arable Agroforestry System. Plant Soil 2013, 373, 43–58. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Halberg, N.; Nicolaisen, M.; Olesen, J.E.; Crews, T.E.; Hinsinger, P.; Kirkegaard, J.; Pierret, A.; Dresbøll, D.B. Digging Deeper for Agricultural Resources, the Value of Deep Rooting. Trends Plant Sci. 2020, 25, 406–417. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Rasmussen, C.; Throckmorton, H.; Liles, G.; Heckman, K.; Meding, S.; Horwath, W.R. Controls on Soil Organic Carbon Partitioning and Stabilization in the California Sierra Nevada. Soil Syst. 2018, 2, 41. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W. Soil Organic Matter in Major Pedogenic Soil Groups. Geoderma 2021, 384, 114785. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon Accumulation in Agricultural Soils after Afforestation: A Meta-Analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Doetterl, S.; Stevens, A.; Six, J.; Merckx, R.; Van Oost, K.; Casanova Pinto, M.; Casanova-Katny, A.; Muñoz, C.; Boudin, M.; Zagal Venegas, E.; et al. Soil Carbon Storage Controlled by Interactions between Geochemistry and Climate. Nat. Geosci. 2015, 8, 780–783. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Luetzow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Liess, M.; Garcia-Franco, N.; et al. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Robins, P.; Holmes, R.B.; Laddish, K. Bring Farm Edges back to Life! Yolo County RCD: Woodland, CA, USA, 2001. [Google Scholar]
- Brodt, S.; Klonsky, K.; Jackson, L.; Brush, S.B.; Smukler, S. Factors Affecting Adoption of Hedgerows and Other Biodiversity-Enhancing Features on Farms in California, USA. Agrofor. Syst. 2009, 76, 195–206. [Google Scholar] [CrossRef]
- Suddick, E.C.; Scow, K.M.; Horwath, W.R.; Jackson, L.E.; Smart, D.R.; Mitchell, J.; Six, J. The Potential for California Agricultural Crop Soils to Reduce Greenhouse Gas Emissions: A Holistic Evaluation. Adv. Agron. 2010, 107, 123–162. [Google Scholar]
- Hayhoe, K.; Cayan, D.; Field, C.B.; Frumhoff, P.C.; Maurer, E.P.; Miller, N.L.; Moser, S.C.; Schneider, S.H.; Cahill, K.N.; Cleland, E.E.; et al. Emissions Pathways, Climate Change, and Impacts on California. Proc. Natl. Acad. Sci. USA 2004, 101, 12422–12427. [Google Scholar] [CrossRef]
- Romanyà, J.; Rovira, P. An Appraisal of Soil Organic C Content in Mediterranean Agricultural Soils. Soil Use Manag. 2011, 27, 321–332. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Organic Carbon Stocks in Mediterranean Soil Types under Different Land Uses (Southern Spain). Solid Earth 2012, 3, 375–386. [Google Scholar] [CrossRef]
- Williams, J.N.; Hollander, A.D.; O’Geen, A.T.; Thrupp, L.A.; Hanifin, R.; Steenwerth, K.; McGourty, G.; Jackson, L.E. Assessment of Carbon in Woody Plants and Soil across a Vineyard-Woodland Landscape. Carbon Balance Manag. 2011, 6, 11. [Google Scholar] [CrossRef]
- Andrews, W.F. Soil Survey of Yolo County, California; US Department of Agriculture, Soil Conservation Service: Washington, DC, USA, 1972.
- Culman, S.W.; Young-Mathews, A.; Hollander, A.D.; Ferris, H.; Sánchez-Moreno, S.; O’Geen, A.T.; Jackson, L.E. Biodiversity Is Associated with Indicators of Soil Ecosystem Functions over a Landscape Gradient of Agricultural Intensification. Landsc. Ecol. 2010, 25, 1333–1348. [Google Scholar] [CrossRef]
- Earnshaw, S. Hedgerows for California Agriculture; Community Alliance for Family Farmers: Davis, CA, USA, 2004. [Google Scholar]
- Garbach, K.; Long, R.F. Determinants of Field Edge Habitat Restoration on Farms in California’s Sacramento Valley. J. Environ. Manag. 2017, 189, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yolo County. Yolo County Climate Action Plan: A Strategy for Smart Growth Implementation, Greenhouse Gas Reduction, and Adaptation to Global Climate Change; Yolo County Board of Supervisors: Woodland, CA, USA, 2011. [Google Scholar]
- Series Extent Explorer, Soil Web. California Soil Resource Lab. 2017. Available online: https://casoilresource.lawr.ucdavis.edu/see/ (accessed on 20 September 2021).
- O’Geen, A.; Walkinshaw, M.; Beaudette, D. SoilWeb: A Multifaceted Interface to Soil Survey Information. Soil Sci. Soc. Am. J. 2017, 81, 853–862. [Google Scholar] [CrossRef]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Field Book for Describing and Sampling Soils; Government Printing Office: Washington, DC, USA, 2012; ISBN 978-0-16-091542-0.
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press: London, UK, 1985. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Particle Density. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1986; pp. 377–382. ISBN 978-0-89118-864-3. [Google Scholar]
- Miller, R.O.; Kissel, D.E. Comparison of Soil PH Methods on Soils of North America. Soil Sci. Soc. Am. J. 2010, 74, 310–316. [Google Scholar] [CrossRef]
- Gavlak, R.; Horneck, D.; Miller, R.O. Soil, Plant and Water Reference Methods for the Western Region. Western Region Extension Publication (WREP-125). WERA-103 Technical Committee; University of Alaska: Fairbanks, AK, USA, 2005. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Hedges, J.; Stern, J. Carbon and Nitrogen Determinations of Carbonate-Containing Solids. Limnol. Oceanogr. 1984, 29, 657–663. [Google Scholar] [CrossRef]
- Batjes, N.H. Total Carbon and Nitrogen in the Soils of the World. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1; R Core Team: Vienna, Austria, 2008; pp. 1–89. [Google Scholar]
- Wickham, H. Manipulating Data. Ggplot2; Springer: New York, NY, USA, 2002; pp. 157–175. [Google Scholar]
- Oksanen, J. Constrained Ordination: Tutorial with R and Vegan. R-Packace Vegan 2012, 1, 1–10. [Google Scholar]
- Jolliffe, I.T. Principal Components in Regression Analysis. In Principal Component Analysis; Springer: New York, NY, USA, 2002; pp. 167–198. [Google Scholar]
- NASS National Agricultural Statistics Service. California Production Data. 2021. Available online: https://www.nass.usda.gov/ (accessed on 1 August 2021).
- Laura, V.V.; Bert, R.; Steven, B.; Pieter, D.F.; Victoria, N.; Paul, P.; Kris, V. Ecosystem Service Delivery of Agri-Environment Measures: A Synthesis for Hedgerows and Grass Strips on Arable Land. Agric. Ecosyst. Environ. 2017, 244, 32–51. [Google Scholar] [CrossRef]
- Lim, S.-S.; Baah-Acheamfour, M.; Choi, W.-J.; Arshad, M.A.; Fatemi, F.; Banerjee, S.; Carlyle, C.N.; Bork, E.W.; Park, H.-J.; Chang, S.X. Soil Organic Carbon Stocks in Three Canadian Agroforestry Systems: From Surface Organic to Deeper Mineral Soils. For. Ecol. Manag. 2018, 417, 103–109. [Google Scholar] [CrossRef]
- Viaud, V.; Kunnemann, T. Additional Soil Organic Carbon Stocks in Hedgerows in Crop-Livestock Areas of Western France. Agric. Ecosyst. Environ. 2021, 305, 107174. [Google Scholar] [CrossRef]
- Young-Mathews, A.; Culman, S.W.; Sánchez-Moreno, S.; Toby O’Geen, A.; Ferris, H.; Hollander, A.D.; Jackson, L.E. Plant-Soil Biodiversity Relationships and Nutrient Retention in Agricultural Riparian Zones of the Sacramento Valley, California. Agro for. Syst. 2010, 80, 41–60. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil Carbon Saturation: Concept, Evidence and Evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- West, T.O.; Six, J. Considering the Influence of Sequestration Duration and Carbon Saturation on Estimates of Soil Carbon Capacity. Clim. Change 2007, 80, 25–41. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Nandal, D.P.; Kapoor, K.K. Soil Organic Matter, Microbial Biomass and Enzyme Activities in a Tropical Agroforestry System. Biol. Fertil. Soils 1998, 27, 168–172. [Google Scholar] [CrossRef]
- Ramos, H.M.N.; Vasconcelos, S.S.; Kato, O.R.; Castellani, D.C. Above- and Belowground Carbon Stocks of Two Organic, Agroforestry-Based Oil Palm Production Systems in Eastern Amazonia. Agrofor. Syst. 2018, 92, 221–237. [Google Scholar] [CrossRef]
- Lehmann, J.; Zech, W. Fine Root Turnover of Irrigated Hedgerow Intercropping in Northern Kenya. Plant Soil 1998, 198, 19–31. [Google Scholar] [CrossRef]
- Nair, P.R.; Nair, V.D.; Kumar, B.M.; Showalter, J.M. Carbon sequestration in agroforestry systems. Adv. Agron. 2010, 108, 237–307. [Google Scholar] [CrossRef]
- Sánchez, I.A.; Lassaletta, L.; McCollin, D.; Bunce, R.G.H. The Effect of Hedgerow Loss on Microclimate in the Mediterranean Region: An Investigation in Central Spain. Agrofor. Syst. 2010, 78, 13–25. [Google Scholar] [CrossRef]
- Kanzler, M.; Böhm, C.; Mirck, J.; Schmitt, D.; Veste, M. Microclimate Effects on Evaporation and Winter Wheat (Triticum Aestivum L.) Yield within a Temperate Agroforestry System. Agrofor. Syst. 2019, 93, 1821–1841. [Google Scholar] [CrossRef]
- Veste, M.; Littmann, T.; Kunneke, A.; du Toit, B.; Seifert, T. Windbreaks as Part of Climate-Smart Landscapes Reduce Evapotranspiration in Vineyards, Western Cape Province, South Africa. Plant Soil Environ. 2020, 66, 119–127. [Google Scholar] [CrossRef]
- Clinch, R.L.; Thevathasan, N.V.; Gordon, A.M.; Volk, T.A.; Sidders, D. Biophysical Interactions in a Short Rotation Willow Intercropping System in Southern Ontario, Canada. Agric. Ecosyst. Environ. 2009, 131, 61–69. [Google Scholar] [CrossRef]
- Dubbert, M.; Mosena, A.; Piayda, A.; Cuntz, M.; Correia, A.C.; Pereira, J.S.; Werner, C. Influence of Tree Cover on Herbaceous Layer Development and Carbon and Water Fluxes in a Portuguese Cork-Oak Woodland. Acta Oecologica 2014, 59, 35–45. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-Carbon Response to Warming Dependent on Microbial Physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The Temperature Response of Soil Microbial Efficiency and Its Feedback to Climate. Nat. Clim. Change 2013, 3, 395–398. [Google Scholar] [CrossRef]
- Mérot, P. The Influence of Hedgerow Systems on the Hydrology of Agricultural Catchments in a Temperate Climate. Agronomie 1999, 19, 655–669. [Google Scholar] [CrossRef]
- Ilstedt, U.; Bargués Tobella, A.; Bazié, H.R.; Bayala, J.; Verbeeten, E.; Nyberg, G.; Sanou, J.; Benegas, L.; Murdiyarso, D.; Laudon, H.; et al. Intermediate Tree Cover Can Maximize Groundwater Recharge in the Seasonally Dry Tropics. Sci. Rep. 2016, 6, 21930. [Google Scholar] [CrossRef]
- Germon, A.; Cardinael, R.; Prieto, I.; Mao, Z.; Kim, J.; Stokes, A.; Dupraz, C.; Laclau, J.-P.; Jourdan, C. Unexpected Phenology and Lifespan of Shallow and Deep Fine Roots of Walnut Trees Grown in a Silvoarable Mediterranean Agroforestry System. Plant Soil 2016, 401, 409–426. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling Downwards—Dissolved Organic Matter in Soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Toosi, E.R.; Doane, T.A.; Horwath, W.R. Abiotic Solubilization of Soil Organic Matter, a Less-Seen Aspect of Dissolved Organic Matter Production. Soil Biol. Biochem. 2012, 50, 12–21. [Google Scholar] [CrossRef]
- Wilkinson, M.T.; Richards, P.J.; Humphreys, G.S. Breaking Ground: Pedological, Geological, and Ecological Implications of Soil Bioturbation. Earth-Sci. Rev. 2009, 97, 257–272. [Google Scholar] [CrossRef]
- Rovira, A.D.; Smettem, K.R.J.; Lee, K.E. Effect of Rotation and Conservation Tillage of Earthworms in a Red-Brown Earth under Wheat. Aust. J. Agric. Res 1987, 38, 829–834. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Schmidt, O. Conventional Tillage Decreases the Abundance and Biomass of Earthworms and Alters Their Community Structure in a Global Meta-Analysis. Glob. Change Biol. 2017, 23, 4396–4419. [Google Scholar] [CrossRef] [PubMed]
- Errouissi, F.; Ben Moussa-Machraoui, S.; Ben-Hammouda, M.; Nouira, S. Soil Invertebrates in Durum Wheat (Triticum Durum L.) Cropping System under Mediterranean Semi Arid Conditions: A Comparison between Conventional and No-Tillage Management. Soil Tillage Res. 2011, 112, 122–132. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Albrecht, S.L.; Smiley, R.W. Soil C and N Changes under Tillage and Cropping Systems in Semi-Arid Pacific Northwest Agriculture. Soil Tillage Res. 1998, 47, 197–205. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Ghimire, R.; Machado, S.; Rhinhart, K. Long-Term Crop Residue and Nitrogen Management Effects on Soil Profile Carbon and Nitrogen in Wheat–Fallow Systems. Agron. J. 2015, 107, 2230–2240. [Google Scholar] [CrossRef]
- Akinremi, O.O.; McGinn, S.M.; McLean, H.D.J. Effects of Soil Temperature and Moisture on Soil Respiration in Barley and Fallow Plots. Can. J. Soil. Sci 1999, 79, 5–13. [Google Scholar] [CrossRef]
- Fernandez, R.; Quiroga, A.; Noellemeyer, E.; Funaro, D.; Montoya, J.; Hitzmann, B.; Peinemann, N. A Study of the Effect of the Interaction between Site-Specific Conditions, Residue Cover and Weed Control on Water Storage during Fallow. Agric. Water Manag. 2008, 95, 1028–1040. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shrestha, A.; Irmak, S. Trade-Offs between Winter Cover Crop Production and Soil Water Depletion in the San Joaquin Valley, California. J. Soil Water Conserv. 2015, 70, 430–440. [Google Scholar] [CrossRef]
- Mathieu, J.A.; Hatté, C.; Balesdent, J.; Parent, É. Deep Soil Carbon Dynamics are Driven More by Soil Type than by Climate: A Worldwide Meta-Analysis of Radiocarbon Profiles. Glob. Change Biol. 2015, 21, 4278–4292. [Google Scholar] [CrossRef]
- Torres-Sallan, G.; Creamer, R.E.; Lanigan, G.J.; Reidy, B.; Byrne, K.A. Effects of Soil Type and Depth on Carbon Distribution within Soil Macroaggregates from Temperate Grassland Systems. Geoderma 2018, 313, 52–56. [Google Scholar] [CrossRef]
- Follain, S.; Walter, C.; Legout, A.; Lemercier, B.; Dutin, G. Induced Effects of Hedgerow Networks on Soil Organic Carbon Storage within an Agricultural Landscape. Geoderma 2007, 142, 80–95. [Google Scholar] [CrossRef]
- California Air Resources Board. AB 32 Climate Change Scoping Plan. 2017. Available online: https://ww2.arb.ca.gov/sites/default/files/classic/cc/scopingplan/scoping_plan_2017.pdf (accessed on 5 September 2021).
- US Census Bureau (MCD): Cheryl Cornish, S.C. Characteristics of New Housing. 2011. Available online: https://www.census.gov/construction/chars/highlights.html (accessed on 12 August 2021).
- Goldstein, B.; Gounaridis, D.; Newell, J.P. The Carbon Footprint of Household Energy Use in the United States. Proc. Natl. Acad. Sci. USA 2020, 117, 19122–19130. [Google Scholar] [CrossRef] [PubMed]
- Asmus, P. Technologies and Policies to Consider for Reducing GHG Emissions in California; Economic and Technology Advancement Advisory Committee: Sacramento, CA, USA, 2008.
- Aertsens, J.; De Nocker, L.; Gobin, A. Valuing the Carbon Sequestration Potential for European Agriculture. Land Use Policy 2013, 31, 584–594. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Robertson, G.P. Whole-Profile Soil Carbon Stocks: The Danger of Assuming Too Much from Analyses of Too Little. Soil Sci. Soc. Am. J. 2011, 75, 235–240. [Google Scholar] [CrossRef]
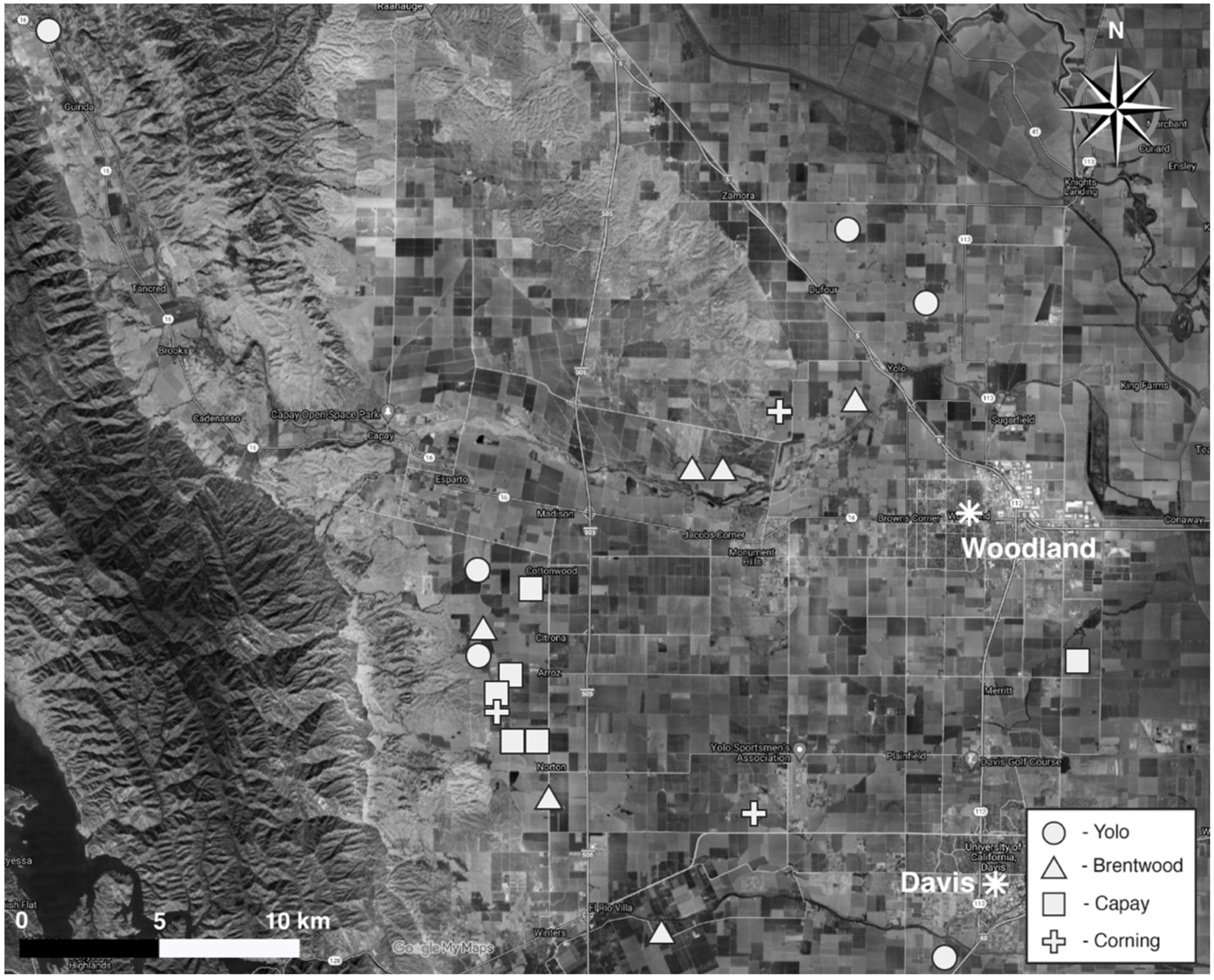
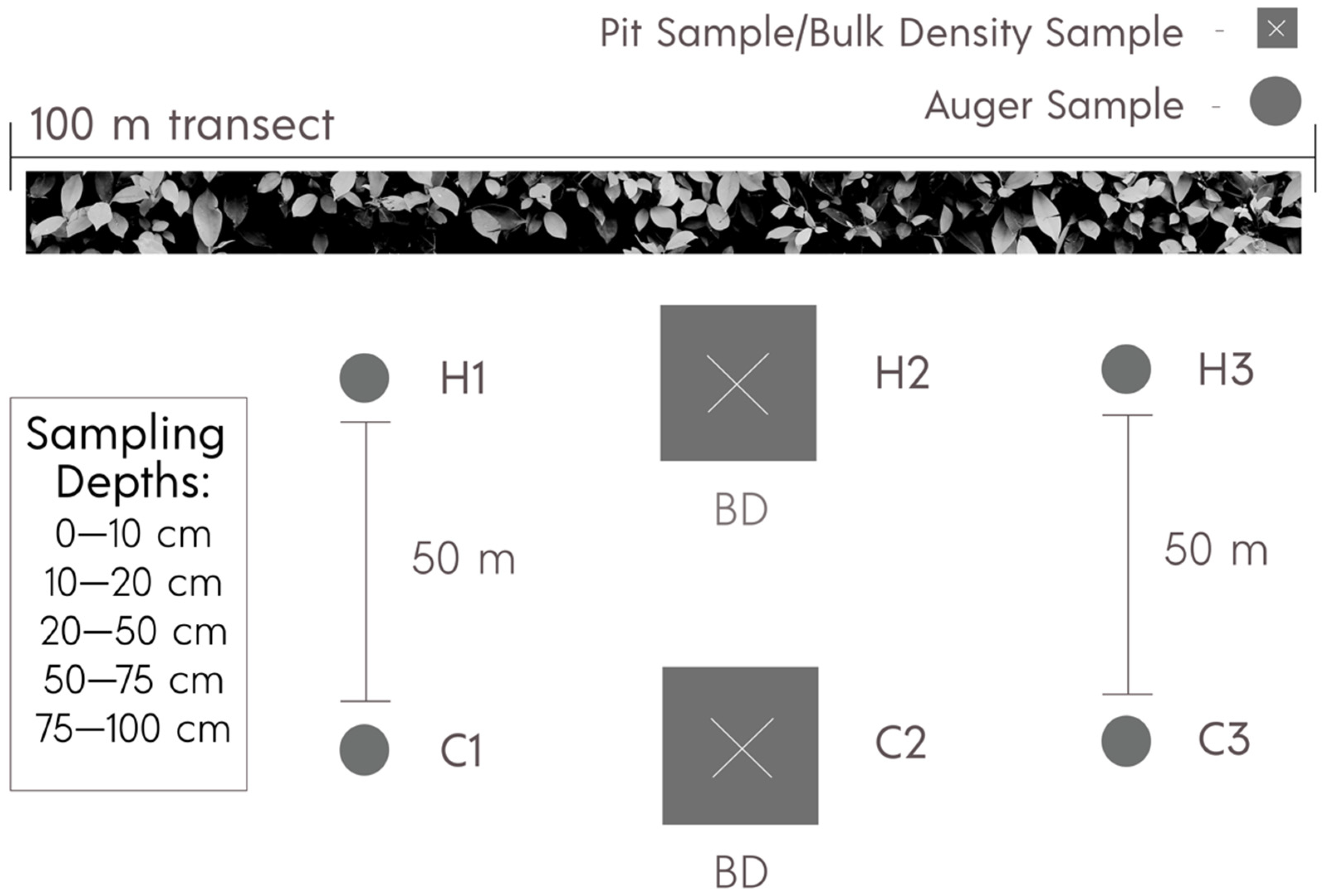
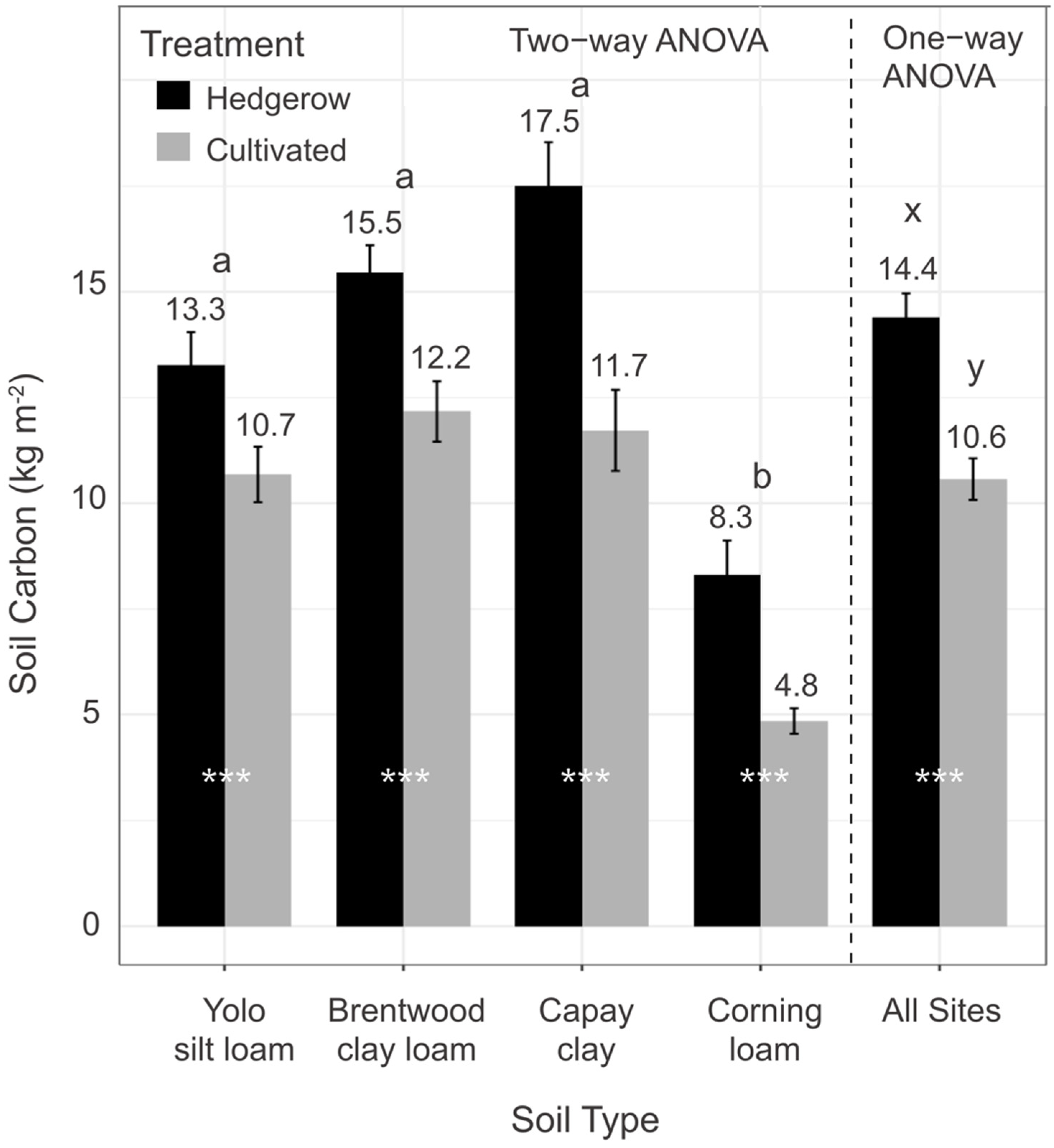
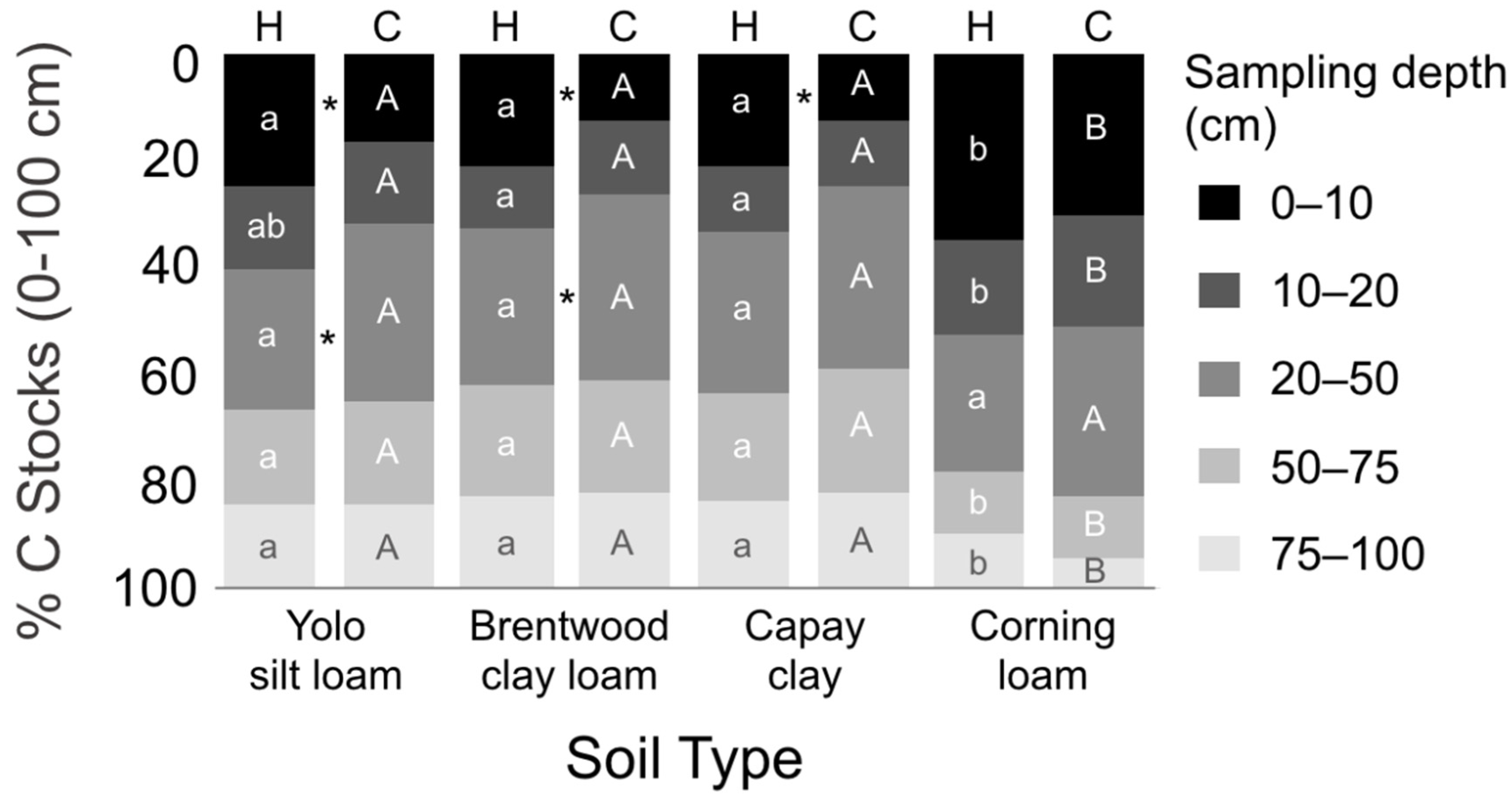
| Site | Soil Type | Soil Textural Class 1 | Hedgerow Age (yrs) | Compost (Tons ha−1 yr−1) | Crop | Cover Crop | Vegetative Cover (Months) |
|---|---|---|---|---|---|---|---|
| 1 | Yolo | Silt loam | 20 | 0 | wheat | N | 6 |
| 2 | Yolo | Silt loam | 23 | 0 | tomato | N | 7.5 |
| 3 | Yolo | Silt loam | 11 | 0 | tomato | N | 5 |
| 4 | Yolo | Silt loam | 10 | 24 | diverse 2 | Y | 10 |
| 5 | Yolo | Silt loam | 19 | 0 | tomato | N | 5 |
| 6 | Yolo | Loam | 10 | 4 | tomato | N | 5 |
| 7 | Brentwood | Clay loam | 13 | 12 | diverse | Y | 10 |
| 8 | Brentwood | Clay loam | 10 | 6 | tomato | Y | 10 |
| 9 | Brentwood | Clay loam | 10 | 6 | tomato | Y | 10 |
| 10 | Brentwood | Clay loam | 14 | 0 | tomato | N | 7.5 |
| 11 | Brentwood | Clay loam | 16 | 4 | wheat | N | 6 |
| 12 | Brentwood | Clay loam | 23 | 0 | tomato | N | 7.5 |
| 13 | Capay | Silty clay | 20 | 0 | tomato | N | 7.5 |
| 14 | Capay | Silty clay | 20 | 0 | tomato | N | 7.5 |
| 15 | Capay | Silty clay | 25 | 0 | rye | N | 9 |
| 16 | Capay | Silty clay | 25 | 0 | rye | N | 9 |
| 17 | Capay | Silty clay | 20 | 0 | wheat | N | 7.5 |
| 18 | Capay | Silty clay | 15 | 0 | wheat | N | 7.5 |
| 19 | Corning | Loam | 25 | 0 | poppies | N | 9 |
| 20 | Corning | Loam | 10 | 0 | grapes | N | 8 |
| 21 | Corning | Loam | 11 | 0 | oat hay | N | 10 |
| Soil Organic Carbon (g kg−1) | Soil Carbon Stocks (kg m−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedgerow | Cultivated | Hedgerow | Cultivated | |||||||||
| Soil Type | 0–10 cm | |||||||||||
| Yolo | 2.62 | (0.27) | 1.36 | (0.14) | *** | a | 3.27 | (0.22) | 1.74 | (0.17) | *** | a |
| Brentwood | 2.52 | (0.16) | 1.30 | (0.08) | *** | a | 3.26 | (0.17) | 1.57 | (0.08) | *** | a |
| Capay | 2.51 | (0.16) | 1.03 | (0.09) | *** | a | 3.63 | (0.21) | 1.47 | (0.15) | *** | a |
| Corning | 2.47 | (0.30) | 1.01 | (0.12) | *** | a | 3.01 | (0.35) | 1.48 | (0.20) | *** | a |
| 10–20 cm | ||||||||||||
| Yolo | 1.42 | (0.16) | 1.15 | (0.13) | ns | a | 2.05 | (0.19) | 1.66 | (0.19) | * | a |
| Brentwood | 1.29 | (0.08) | 1.20 | (0.10) | ns | a | 1.80 | (0.11) | 1.66 | (0.13) | ns | a |
| Capay | 1.29 | (0.1) | 0.90 | (0.07) | *** | a | 2.18 | (0.16) | 1.45 | (0.11) | *** | a |
| Corning | 1.01 | (0.13) | 0.70 | (0.06) | * | a | 1.53 | (0.23) | 1.03 | (0.08) | * | a |
| 20–50 cm | ||||||||||||
| Yolo | 0.82 | (0.08) | 0.77 | (0.05) | ns | a | 3.53 | (0.27) | 3.53 | (0.29) | ns | a |
| Brentwood | 0.99 | (0.07) | 0.90 | (0.06) | ns | a | 4.48 | (0.29) | 4.17 | (0.27) | ns | a |
| Capay | 1.03 | (0.07) | 0.75 | (0.07) | *** | a | 5.32 | (0.38) | 3.94 | (0.37) | *** | a |
| Corning | 0.43 | (0.02) | 0.33 | (0.02) | *** | b | 2.17 | (0.14) | 1.58 | (0.13) | *** | b |
| 50–75 cm | ||||||||||||
| Yolo | 0.64 | (0.03) | 0.54 | (0.03) | * | a | 2.34 | (0.12) | 2.01 | (0.12) | * | a |
| Brentwood | 0.88 | (0.05) | 0.72 | (0.05) | *** | a | 3.25 | (0.17) | 2.53 | (0.17) | *** | a |
| Capay | 0.77 | (0.07) | 0.60 | (0.05) | *** | a | 3.44 | (0.31) | 2.68 | (0.25) | *** | a |
| Corning | 0.24 | (0.03) | 0.14 | (0.02) | *** | b | 1.01 | (0.13) | 0.56 | (0.08) | *** | b |
| 75–100 cm | ||||||||||||
| Yolo | 0.60 | (0.04) | 0.47 | (0.03) | *** | a | 2.09 | (0.15) | 1.75 | (0.14) | * | a |
| Brentwood | 0.76 | (0.04) | 0.63 | (0.04) | *** | a | 2.68 | (0.16) | 2.25 | (0.17) | *** | a |
| Capay | 0.67 | (0.05) | 0.49 | (0.04) | *** | a | 2.93 | (0.23) | 2.19 | (0.17) | * | a |
| Corning | 0.21 | (0.06) | 0.08 | (0.01) | *** | b | 0.89 | (0.27) | 0.29 | (0.02) | *** | b |
| Adoption Rate | Soil Organic Carbon (Tg) | CO2 Equivalents (MMT) | (%) of GHG Goals |
|---|---|---|---|
| 50% | 2.96 | 10.8 | 7 |
| 80% | 4.73 | 17.3 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiartas, J.L.; Jackson, L.E.; Long, R.F.; Margenot, A.J.; O'Geen, A.T. Hedgerows on Crop Field Edges Increase Soil Carbon to a Depth of 1 meter. Sustainability 2022, 14, 12901. https://doi.org/10.3390/su141912901
Chiartas JL, Jackson LE, Long RF, Margenot AJ, O'Geen AT. Hedgerows on Crop Field Edges Increase Soil Carbon to a Depth of 1 meter. Sustainability. 2022; 14(19):12901. https://doi.org/10.3390/su141912901
Chicago/Turabian StyleChiartas, Jessica L., Louise E. Jackson, Rachael F. Long, Andrew J. Margenot, and Anthony T. O'Geen. 2022. "Hedgerows on Crop Field Edges Increase Soil Carbon to a Depth of 1 meter" Sustainability 14, no. 19: 12901. https://doi.org/10.3390/su141912901
APA StyleChiartas, J. L., Jackson, L. E., Long, R. F., Margenot, A. J., & O'Geen, A. T. (2022). Hedgerows on Crop Field Edges Increase Soil Carbon to a Depth of 1 meter. Sustainability, 14(19), 12901. https://doi.org/10.3390/su141912901







