Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Phenotype Characterization
2.3. Chlorophyll Content Measurement
2.4. RNA Extraction
2.5. Transcriptome Detection and Measurement
2.6. Metabolome Detection and Measurement
2.7. Statistical Analysis
3. Results
3.1. Thermotolerant and Thermosensitive Wolfberry Plant Phenotypes under High Temperature Stress
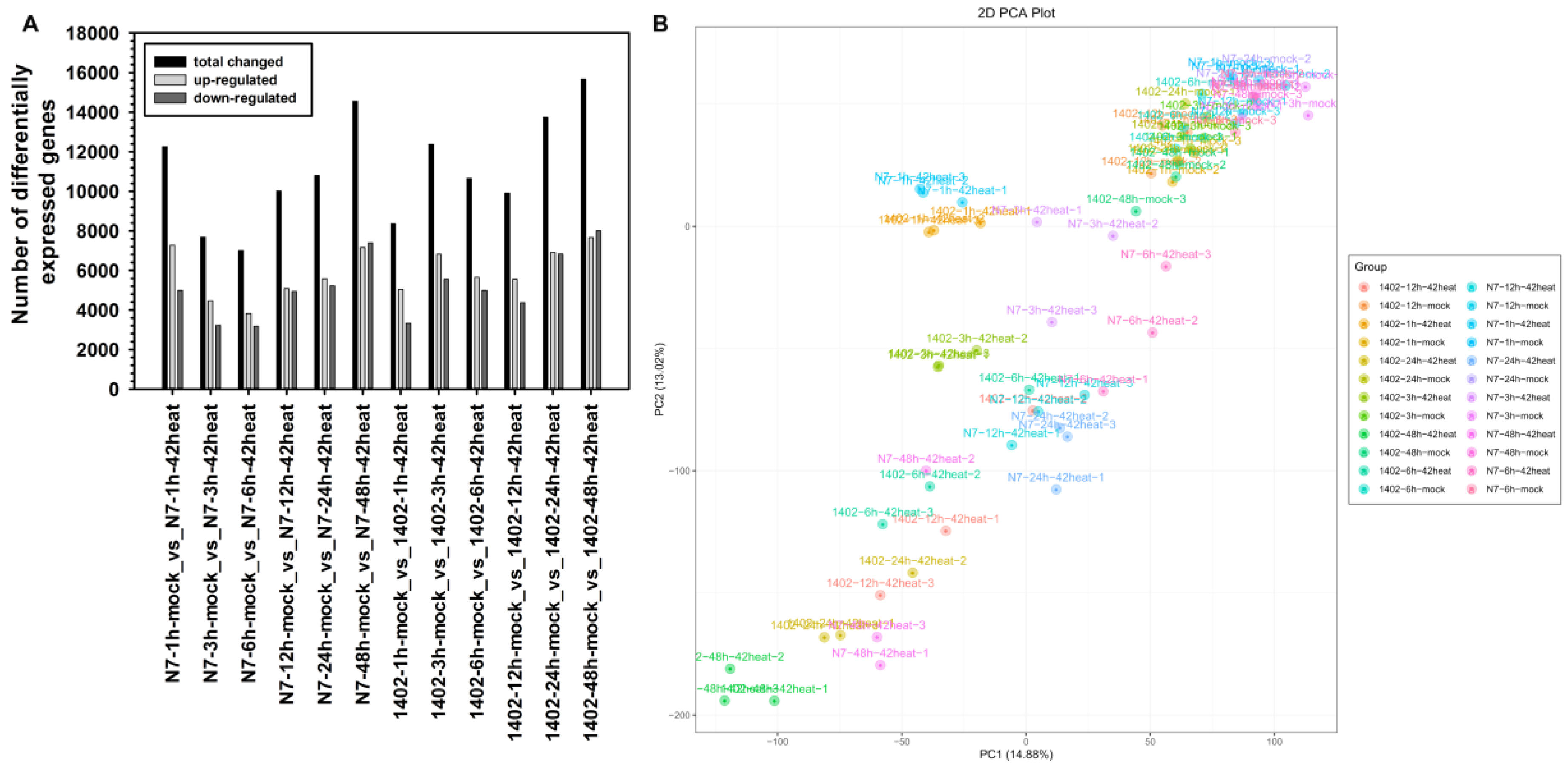
3.2. Statistical Summary of DEGs
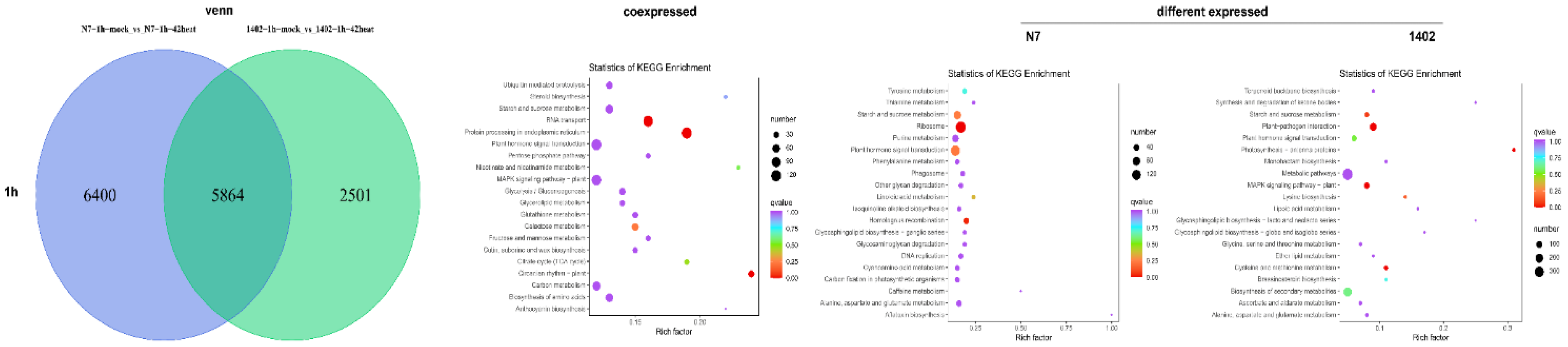
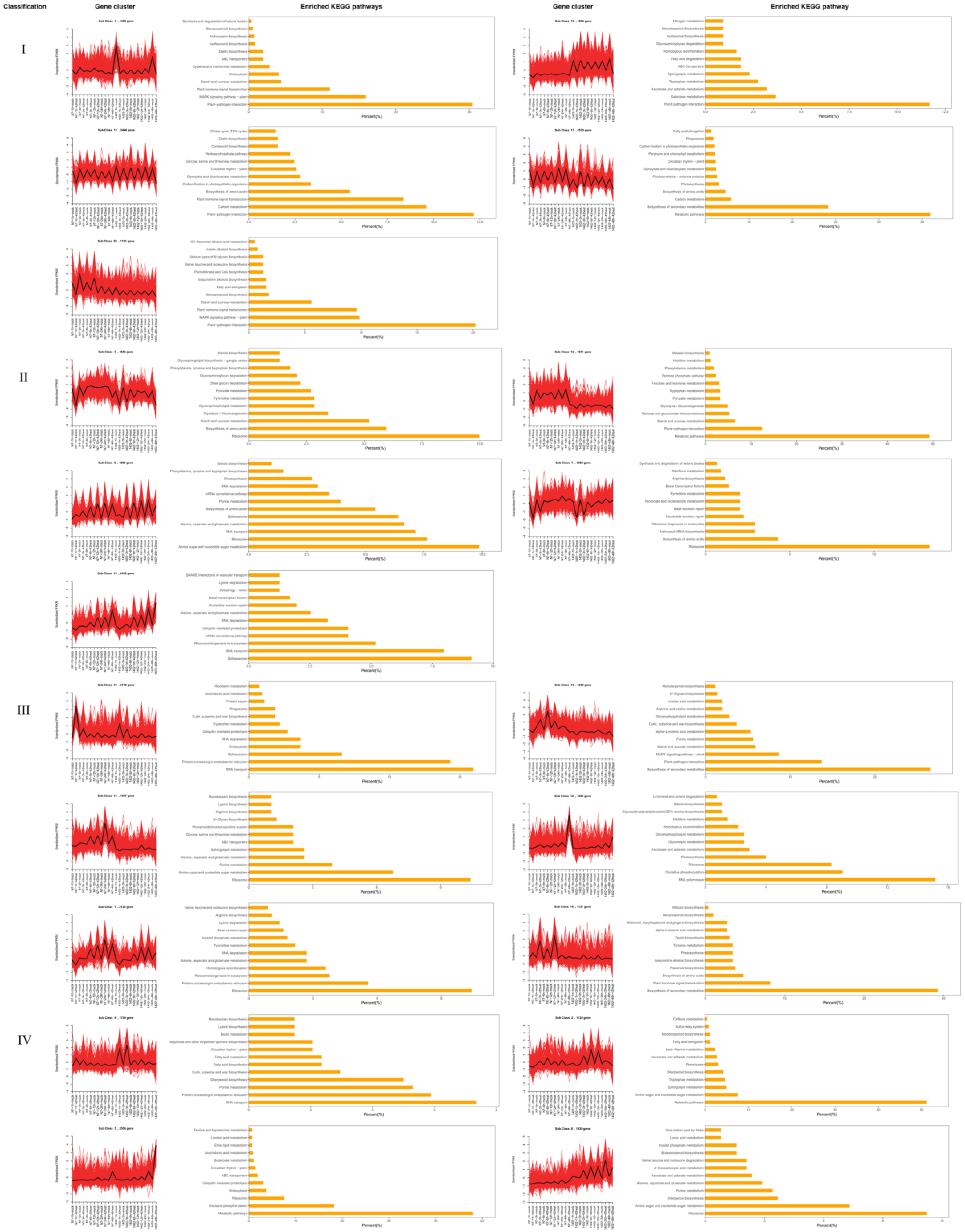
3.3. KEGG Analysis of Co- and Differentially Expressed DEGs
3.4. Dynamic Transcriptome Analysis in Response to Heat Stress
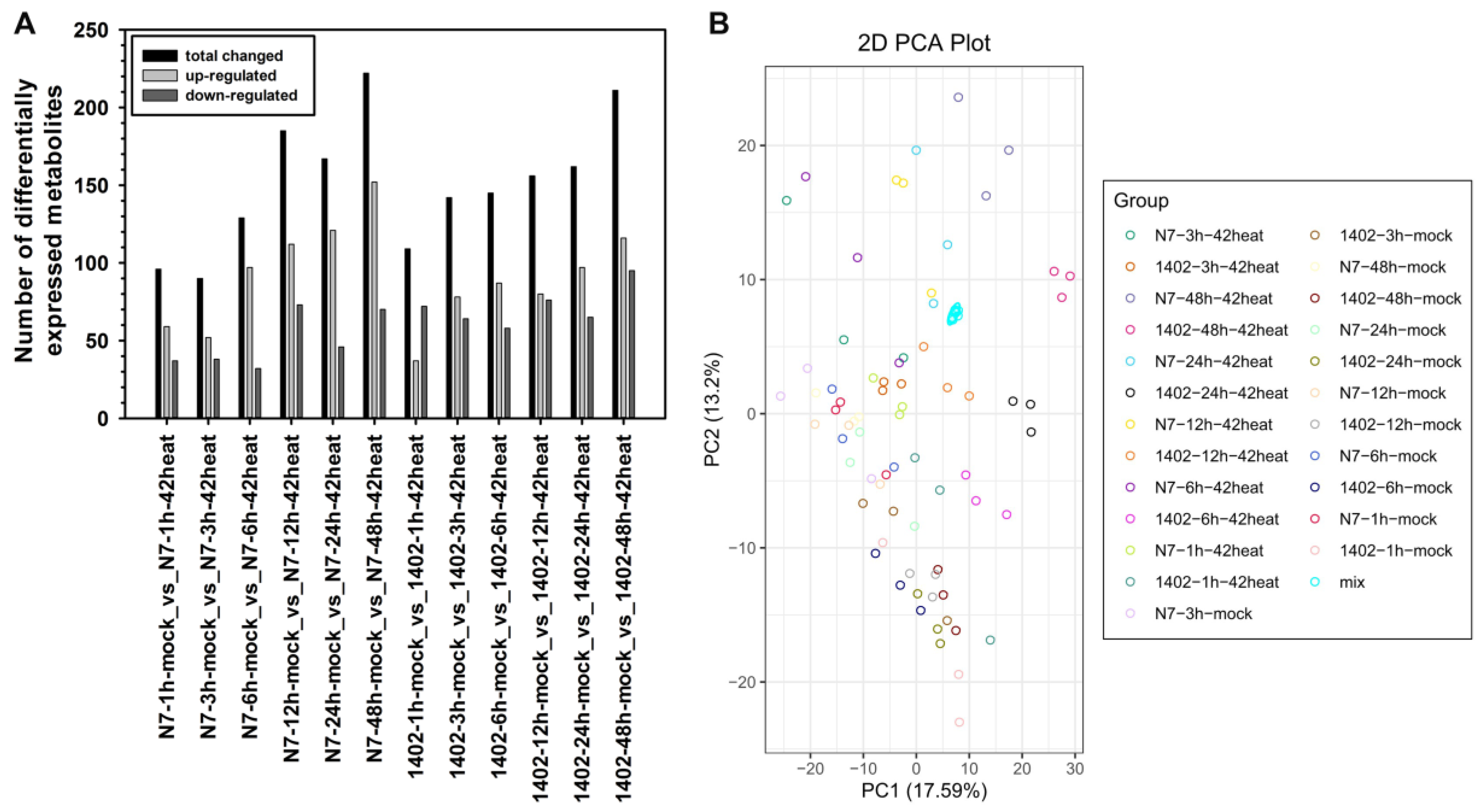
3.5. Metabolome Analysis in Response to Heat Stress
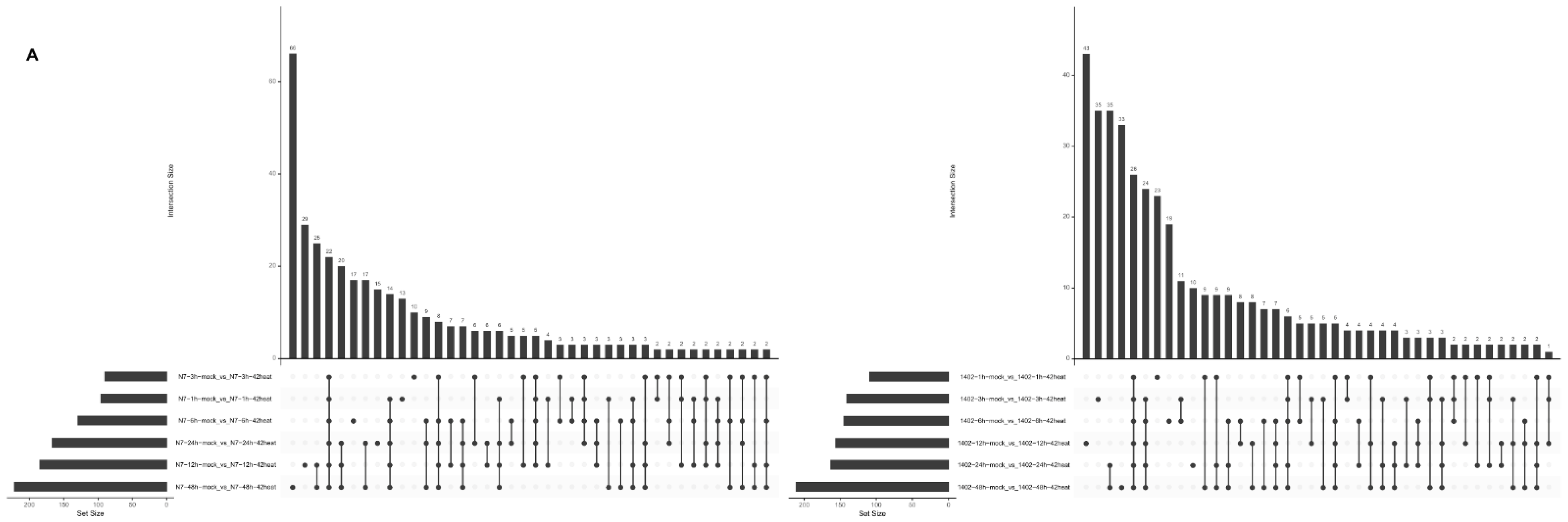
3.6. Summarized Difference Analysis in N7 and 1402 under Heat Stress
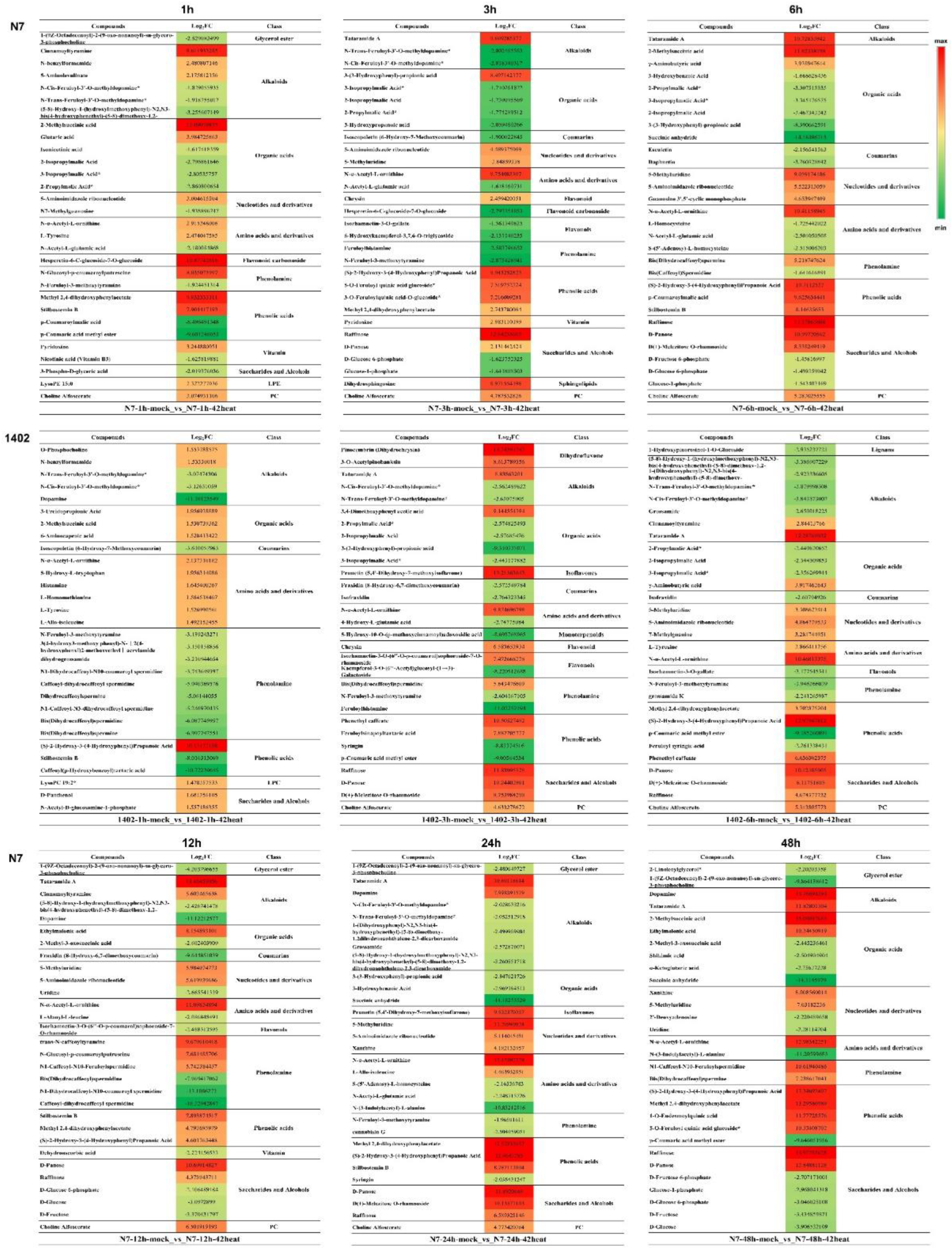
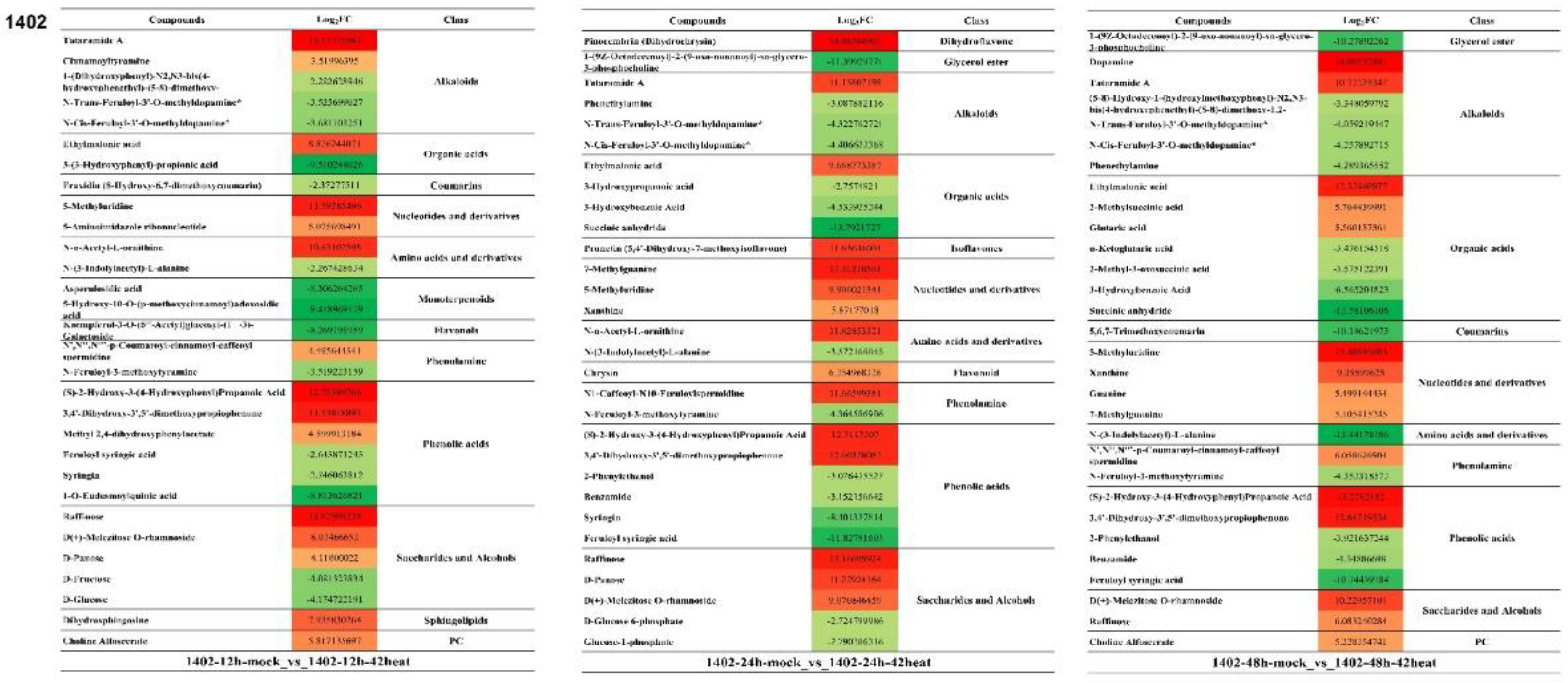
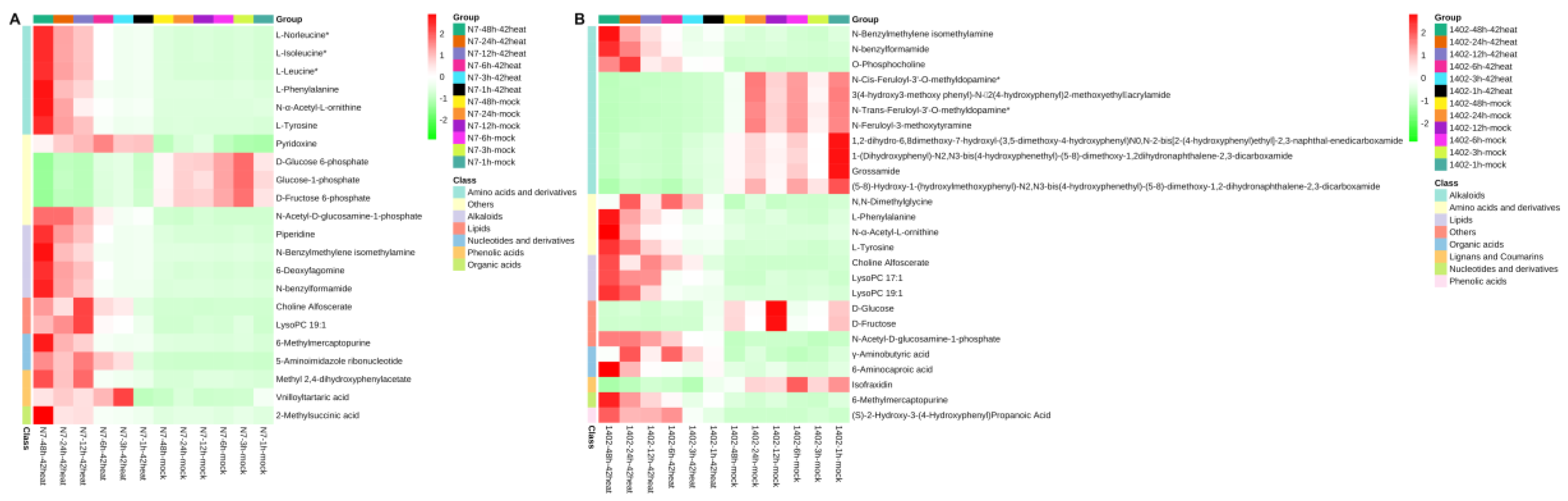
3.7. The Top 30 Differentially Expressed Metabolites in N7 and 1402
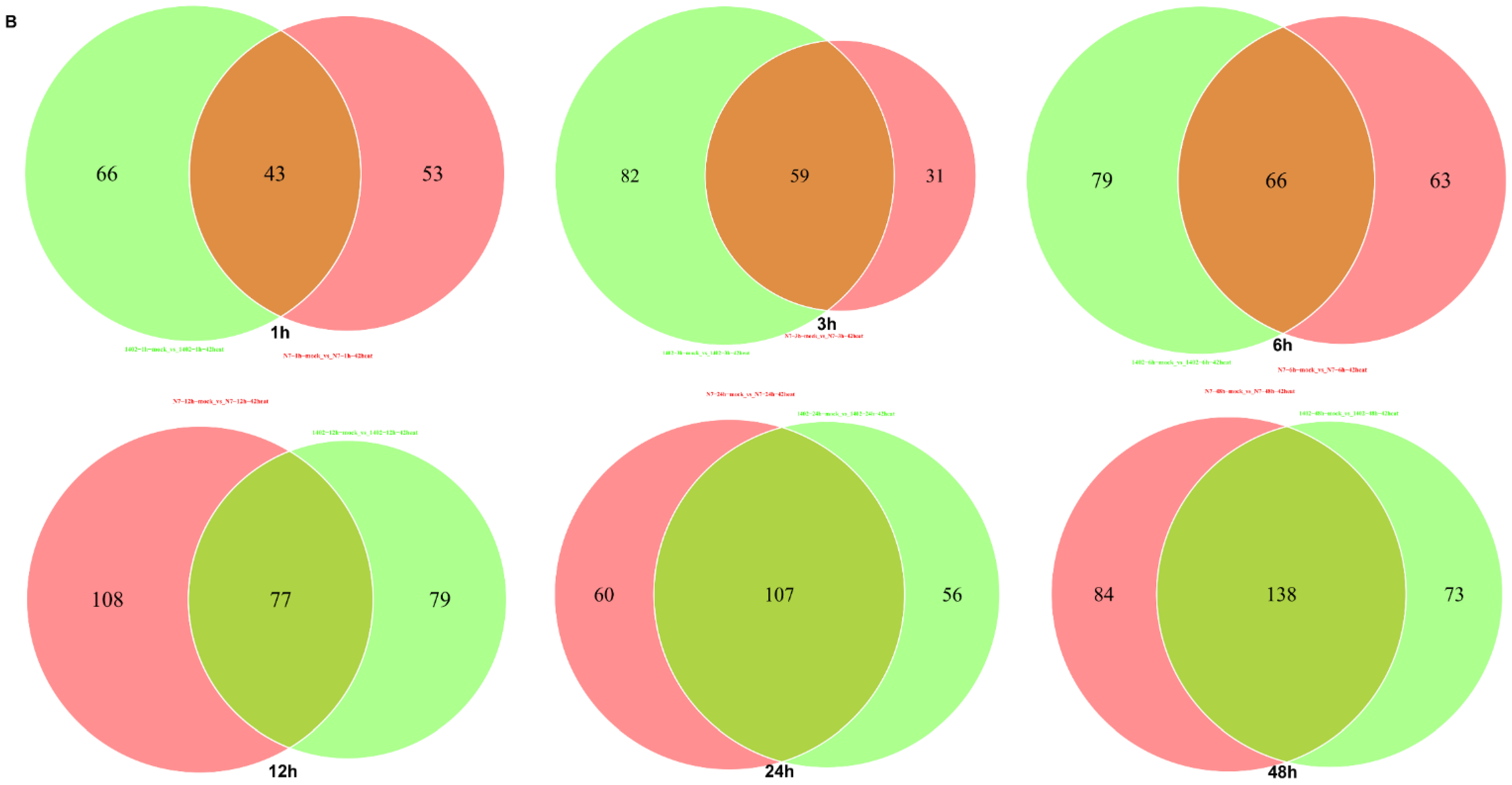
3.8. The Shared Differentially Expressed Metabolites in N7 or 1402
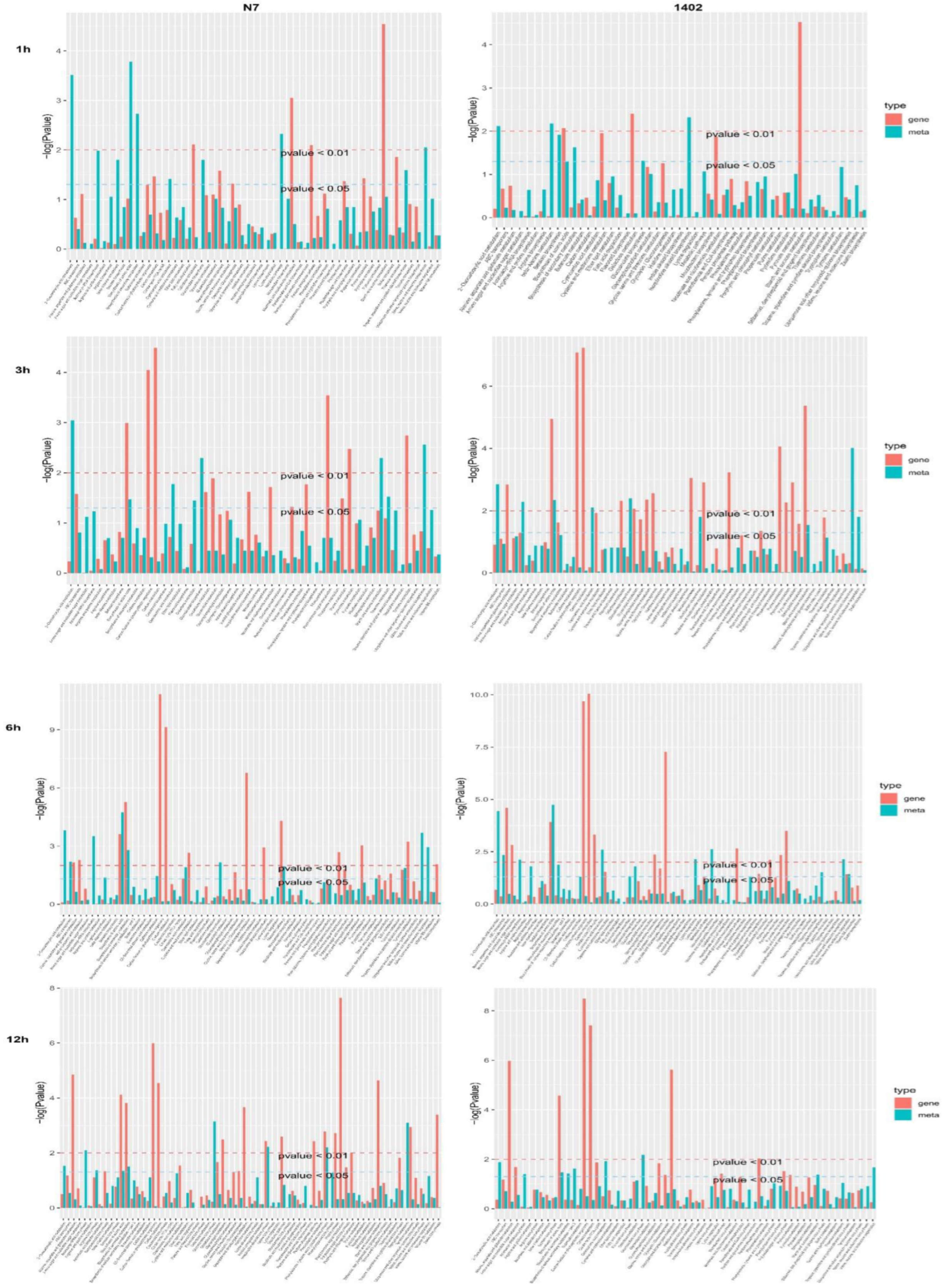
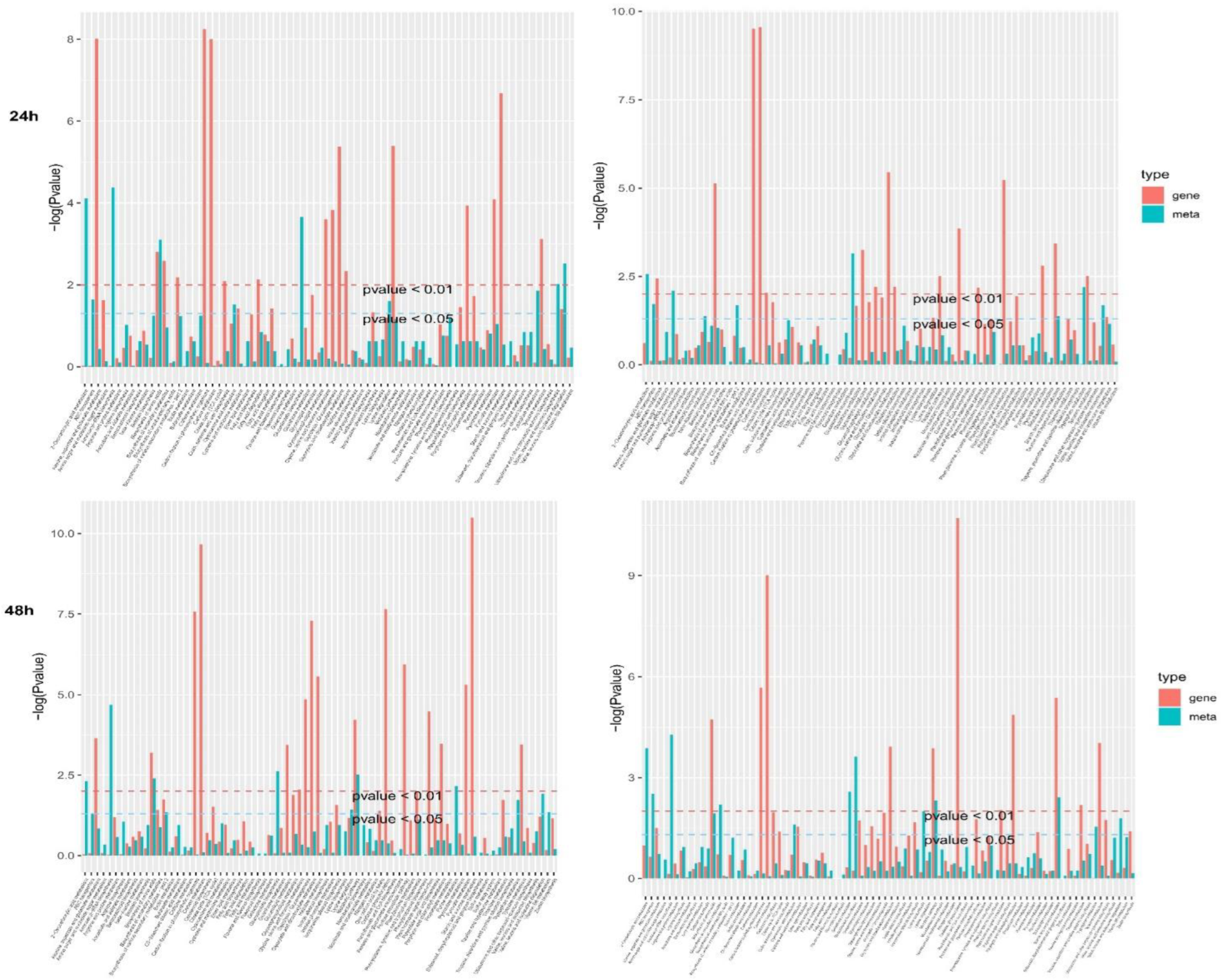
3.9. KEGG Pathway Enrichment in DEGs and DEMs in N7 and 1402
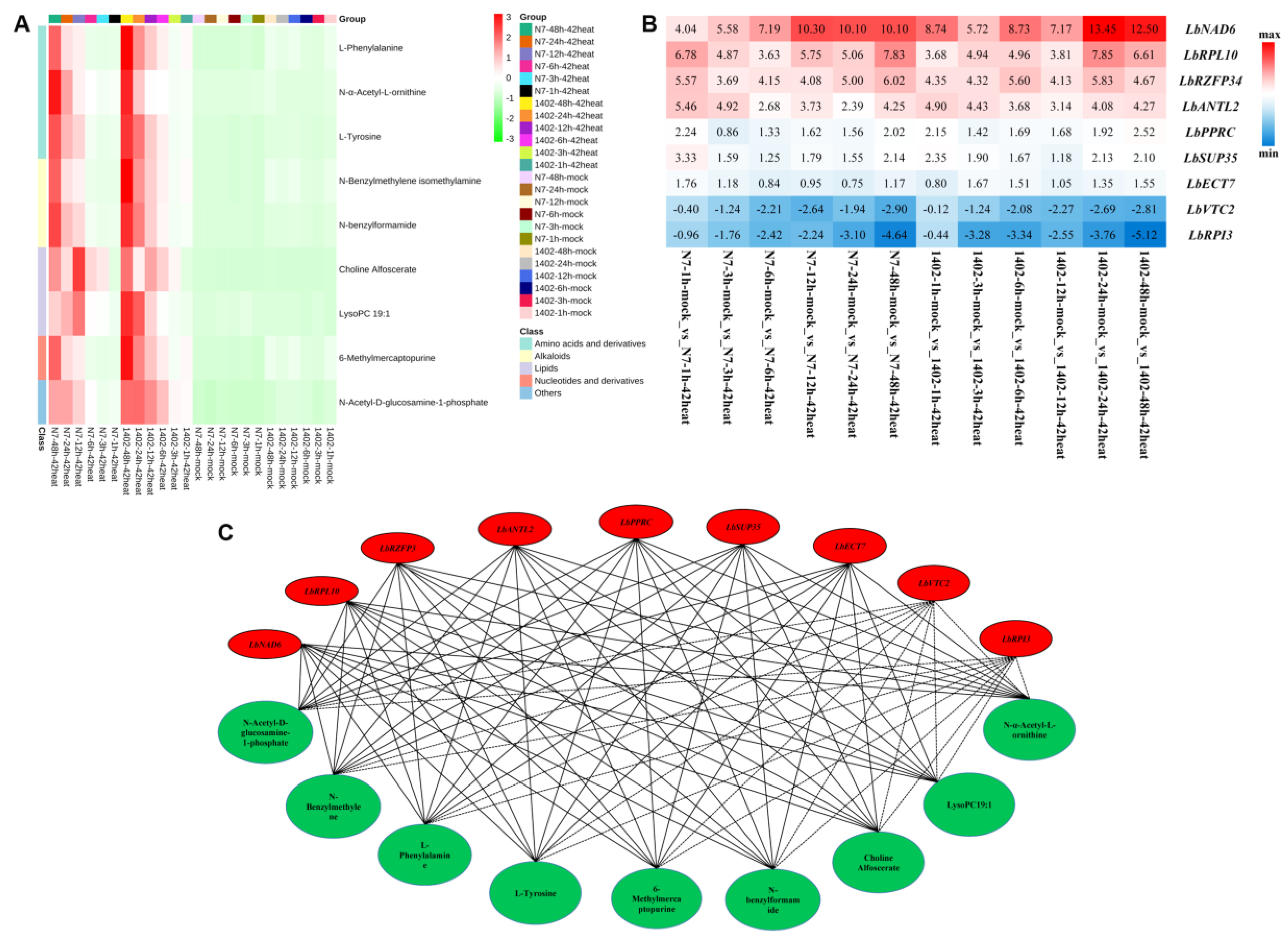
3.10. Identification of DEGs and DEMs Co-Expression in Response to Heat Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, J.; Zhou, J.; Shi, W.; Cao, X.; Luo, J.; Polle, A.; Luo, Z.B. Comparative transcriptomic analysis reveals the roles of overlapping heat-/drought-responsive genes in poplars exposed to high temperature and drought. Sci. Rep. 2017, 7, 43215. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Kakani, V.G. Screening Capsicum species of different origins for high temperature tolerance by in vitro pollen germination and pollen tube length. Sci. Hortic. 2007, 112, 130–135. [Google Scholar] [CrossRef]
- Stainforth, D.A.; Aina, T.; Christensen, C.; Collins, M.; Faull, N.; Frame, D.J.; Piani, C. Uncertainty in predictions of the climate response to rising levels of greenhouse gases. Nature 2005, 433, 403. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Bänziger, M.; Magorokosho, C.; Vivek, B. Nonlinear heat effects on Africanmaize as evidenced by historical yield trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Palak, C.; Anna, J.W.; Arindam, G.; Lenka, Z.D.; Wolfram, W.; David, H. Heat stress response mechanisms in pollen development. New Phytol. 2021, 231, 571–585. [Google Scholar]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of wild and cultivated plants under global warming conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Jagadish, S.V.K. Heat stress during flowering in cereals-Effects and adaptation strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Asjraf, M.; Foolad, M.R. Heat tolerance in plants. Environ. Exp. Botany 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Nankishore, A.; Farrell, A.D. The response of contrasting tomato genotypes to combined heat and drought stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef]
- Pareek, A.; Sopory, S.K.; Bohnert, H.J. Abiotic Stress Adaptation in Plants; Springer: Berlin/Heidelberg, Germany; University of Illinois at Urbana-USA: Champaign, IL, USA, 2009. [Google Scholar]
- Berova, M.; Stoeva, N.; Zlatko, Z.; Ganeva, D. Physiological response of some tomato genotypes (Lycopersicon esculentum L.) to high-temperature stress. J. Cent. Eur. Agric. 2013, 9, 723–732. [Google Scholar]
- Abdelrahman, M.; El-Sayed, M.; Jogaiah, S.; Burritt, D.J.; Tran, L.S. The “STAY-GREEN” trait and phytohormone signalling networks in plants under heat stress. Plant Cell Rep. 2017, 36, 1009–1025. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef]
- Krishna, K.R.; Neha, P.; Shashi, P.R. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar]
- Qu, G.Q.; Liu, X.; Zhang, Y.L.; Yao, D.; Ma, Q.M.; Yang, M.Y.; Luo, Y.B. Evidence for programmed cell death and activation of specific caspa se-like enzymes in the tomato fruit heat stress response. Planta 2009, 229, 1269–1279. [Google Scholar] [CrossRef]
- Muhammed, A.; Mahmood, T.; Richard, T.; Nabil, A. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar]
- Rhodes, D.; Hanson, A. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Patterson, B.D.; Graham, D. Temperature and metabolism. In DD Davies. Biochem. Plants A Compr. Treatise 1987, 12, 153–199. [Google Scholar]
- Randall, R.; Mayer, J.H.C.; David, R. Effects of Heat Shock on Amino Acid Metabolism of Cowpea Cells. Plant Physiol. 1990, 94, 796–810. [Google Scholar]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Ren, S.; Ma, K.; Lu, Z.; Chen, G.; Cui, J.; Tong, P.; Wang, L.; Teng, N.; Jin, B. Transcriptomic and metabolomic analysis of the heat-Stress response of Populus tomentosa Carr. Forests 2019, 10, 383. [Google Scholar] [CrossRef]
- Krishna, J.S.V.; Danielle, A.W.; Thomas, D.S. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar]
- Liam, D. Plant Evolution: An Ancient Mechanism Protects Plants and Algae from Heat Stress. Curr. Biol. 2020, 30, R263–R285. [Google Scholar]
- Zhao, L.; Jie, T.; Renu, S.; Diane, C.B.; Stephen, H.H. The Transcription Factor bZIP60 Links the Unfolded Protein Response to the Heat Stress Response in Maize. Plant Cell 2020, 32, 3559–3575. [Google Scholar]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Lee, W.Y.; Zhou, H.; Sutanto, C.N.; Lee, D.P.S.; Tan, D.; Kim, J.E. Wolfberry (Lycium barbarum) Consumption with a Healthy Dietary Pattern Lowers Oxidative Stress in Middle-Aged and Older Adults: A Randomized Controlled Trial. Antioxidants 2021, 10, 567. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Fu, C.; Duan, S.; Hu, D.; Guo, X. Genome-wide identification, transcriptome analysis and alternative splicing events of Hsf family genes in maize. Sci. Rep. 2020, 10, 8073. [Google Scholar] [CrossRef]
- Xing, F.; Li, Z.; Sun, A.; Xing, D. Reactive oxygen species promote chloroplast dysfunction and salicylic acid accumulation in fumonisin B1-induced cell death. FEBS Lett. 2013, 587, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Lachowiec, J. Roles of Brassinosteroids in Mitigating Heat Stress Damage in Cereal Crops. Int. J. Mol. Sci. 2021, 22, 2706. [Google Scholar]
- Jin, J.; Yang, L.; Fan, D.; Liu, X.; Hao, Q. Comparative transcriptome analysis uncovers different heat stress responses in heat-resistant and heat-sensitive jujube cultivars. PLoS ONE 2020, 15, e0235763. [Google Scholar] [CrossRef] [PubMed]
- Guihur, A.; Rebeaud, M.E.; Goloubinoff, P. How do plants feel the heat and survive? Trends Biochem. Sci. 2022, 22, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, T.; Tian, A.; Luo, B.; Du, C.; Zhang, S.; Huang, S.; Zhang, F.; Wang, X. Modification of Serine 1040 of SIBRI1 Increases Fruit Yield by Enhancing Tolerance to Heat Stress in Tomato. Int. J. Mol. Sci. 2020, 21, 7681. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Yang, X.; Zhang, B.; Xu, F.; Wang, Y.; Song, C.; Yi, M.; Ma, N.; Zhou, X.; et al. The Heat Stress Transcription Factor LlHsfA4 Enhanced Basic Thermotolerance through Regulating ROS Metabolism in Lilies (Lilium Longiflorum). Int. J. Mol. Sci. 2022, 23, 572. [Google Scholar] [CrossRef]
- Yu, B.; Ming, F.; Liang, Y.; Wang, Y.; Gan, Y.; Qiu, Z.; Yan, S.; Cao, B. Heat Stress Resistance Mechanisms of Two Cucumber Varieties from Different Regions. Int. J. Mol. Sci. 2022, 23, 1817. [Google Scholar] [CrossRef]
- Mo, S.; Qian, Y.; Zhang, W.; Qian, L.; Wang, Y.; Cailin, G.; Ding, H. Mitogen-activated protein kinase action in plant response to high-temperature stress: A mini review. Protoplasma 2021, 258, 477–482. [Google Scholar] [CrossRef]
- Yanglin, D.; Yiting, S.; Shuhua, Y. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar]
- Waqas, M.; Shahzad, R.; Khan, A.L.; Asaf, S.; Kim, Y.H.; Kang, S.M.; Bilal, S.; Hamayun, M.; Lee, I.J. Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Qin, B.; He, W.; Chen, Y.; Yin, Y.; Cao, Y.; An, W.; Mu, Z.; Qin, K. Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress. Sustainability 2022, 14, 12617. https://doi.org/10.3390/su141912617
Qin X, Qin B, He W, Chen Y, Yin Y, Cao Y, An W, Mu Z, Qin K. Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress. Sustainability. 2022; 14(19):12617. https://doi.org/10.3390/su141912617
Chicago/Turabian StyleQin, Xiaoya, Beibei Qin, Wei He, Yan Chen, Yue Yin, Youlong Cao, Wei An, Zixin Mu, and Ken Qin. 2022. "Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress" Sustainability 14, no. 19: 12617. https://doi.org/10.3390/su141912617
APA StyleQin, X., Qin, B., He, W., Chen, Y., Yin, Y., Cao, Y., An, W., Mu, Z., & Qin, K. (2022). Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress. Sustainability, 14(19), 12617. https://doi.org/10.3390/su141912617





