Abstract
Eucalyptus is the main fast-growing tree for biomass production in the tropics, providing resources for pulp and paper industries and bioenergy. The potential productivity of forest sites over an eight-year rotation in Brazil was evaluated by the Physiological Principles in Predicting Growth (3-PG) model for two soils, Acrisols and Arenosols, with high and low water storage, respectively, and distinct productive potential capacity. The model was parameterized by data-sets obtained in bimonthly forest inventories performed in stands with 33, 58 and 89 months-old trees, and edaphic surveys. The average volumetric productivity of wood and biomass of the main stem determined at the 89 months-old stand was 374 m3 ha−1 (177 Mg ha−1) for Acrisols and 272.3 m3 ha−1 (130.0 Mg ha−1) for Arenosols. The estimated volumetric production in the Arenosols had a high mean annual increment up to the age of 58 months, with a significant reduction in growth rates after this time. In Acrisols, high incremental rates in wood volume up to age of 89 months (MAIVW > 50 m3 ha−1 year−1) indicate that, under ideal soil conditions, the cutting cycle may exceed 8 years with no productivity impairment. The parameterized model matched well for forest yield (r2 > 0.9) and dendrometric variables (r2 > 0.78). The expected results of lower productivity in Arenosols occurred only after 58 months, while for Acrisols productivity remained high up to 89 months. The results showed the eucalyptus cycle should be shorter in Arenosols, since the lower cutting cycle can provide higher final productivity, when using the mean annual increment to evaluate productivity.
1. Introduction
Eucalyptus plantations are a significant source of biomass and carbon storage because of the fast growth and high productivity of this short rotation forest [1], where the high carbon sequestration potential can be maximized by proper management [2]. Eucalyptus plantations are one of the main resources for energy purposes in many countries, because the woody biomass represents an important renewable energy pool [3].
The productivity of eucalyptus stands in Brazil, named as commercial forests due the intensive management, improved genetic material, and commercial purpose, has increased significantly, with an average rate of approximately 40 m3 ha−1 year−1 in the last decade [4]. Because of the great demand for raw material, mainly directed to the internal and external market of pulp and paper, many companies have invested significant resources to reach the maximum productive capacity of the genus Eucalyptus spp. The resources invested on eucalyptus commercial forests are directed to genetic enhancement studies and soil management, as soil fertilization treatment and soil tillage methods influence the initial growth of eucalyptus seedlings [5,6].
Productivity estimation and productive capacity of sites are evaluated by the use of empirical models based on easily-obtained variables, but with low power of extrapolation, mainly where climate variation is observed [7,8]. Mixed or mechanistic models, based on the whole system, are able to simulate realistic scenarios and the effect of treatments and to estimate productivity over time [9].
Models such as Forest 5 [10], TRIPLEX [11] and Physiological Principles in Predicting Growth (3-PG model) [12] have great application to simulate forest growth and carbon dynamics, as well as to evaluate the climate impacts on management plans for Eucalyptus spp. in Brazil [13]. These models have been used for analysis in management systems, predictions of attainable and potential productivity, and estimation of wood volume, biomass production, diameter at breast height and total height [14,15].
The 3-PG model is a fairly efficient option, considered relatively easy to use when compared to other physiological models, and available for free in Visual Basic Aplication (VBA) Excel. This physiologically based growth model can be used for any crop age and any desired number of years, using climatic, edaphic and stand data [14,16]. The model has been useful in Brazil for forest management due its efficiency and accuracy in estimating the growth and productivity of Eucalyptus spp. [17] and of other world species in varied climatic conditions [18,19].
The model also can be used for ecological purposes, because the outputs include the monthly or annual values of canopy leaf area index (LAI) and stem number. These are stand variables directly associated with rainfall interception and low surface runoff, and related soil degradation, soil sediment yield and soil erosion [20,21], especially in degraded sites where exotica fast-growing species as eucalyptus can be cultivated to provide site preservation.
In Brazilian eucalyptus plantations, the 3-PG model has provided good estimates of woody production, successfully representing the variability of available soil water in short rotations [22]. In the region of Atlantic Coast of Brazil, the model showed itself to be a reasonable alternative, enabling prediction of stand volume and mean tree diameter for extensive areas, where the climatic variation and drought may reduce plantations yields [23]. Because the 3-PG model has been used for a great diversity of sites within the same climatic condition, the model has a great potential for production estimation in large areas, starting from single parameterization and with changes in soil texture and soil water storage capacity. The novelty of this study is that it encompasses commercial forest sites with contrasting soils in terms of water retention and availability to plants, and considers the full cropping cycle.
Our hypothesis was that in the sites with Arenosols, the above-ground biomass and wood volume production, in all growing stages, is less than that of sites with Acrisols, with the same climatic conditions and adequate forestry practices. The objectives of the study were to estimate and compare the productivity of two cultivated areas with Eucalyptus saligna, with distinct and contrasting physical-structural soil properties and soil water storage, and to perform model parameterization under local climatic conditions, with efficient validation within the specific edaphic variability. From this scientific effort, practical recommendations for forest management can be proposed, such as cutting cycle, to improve forest productivity.
2. Materials and Methods
2.1. Study Area
The study was carried out in commercial stands of Eucalyptus saligna, located in the Brazilian southern state of Rio Grande do Sul, with geographic coordinates Latitude 30° S and Longitude 51° W. The climate of the region is of the humid subtropical “Cfa” type without drought, according to the Köppen climatic classification [24], with an average annual rainfall of 1355 mm [25], annual average temperature of 18 °C and the hot months having temperatures above 22 °C while the cold months have temperatures fluctuating between 3 and 18 °C.
Eucalyptus stands were selected to represent commercial forests of E. saligna with rotation of 8 years, in sites with high and low productivity. The sites with Acrisols [26] or Argissolo Vermelho-Amarelo Distrófico and Argissolo Vermelho-Amarelo Distrófico latossólico by the Brazilian Soil Taxonomy System [27], herein called Acrisols or ACRI, constituted areas with high productive potential capacity, while the sites on Arenosols [26] or Neossolo Quartzarênico Órtico típico [27], called Arenosols or ARE, constituted areas with low productive potential capacity.
In twelve (12) forest areas of E. saligna (clone 2864), six sites for each soil type, stands with different ages (33, 58 and 89 months-old) were selected to represent chronological stages of growth observed in an 8-years-old rotation cycle, and high and low productive sites were determined based on the soil texture and soil water storage capacity (WSC).
2.2. The 3-PG Model
The 3-PG model (3PGxl.) is an ecophysiological process model consisting of a set of empirical equations that integrate climatic variables, crop physiology, dendrometric patterns of stands, initial biomass stocks and soils properties related to soil water storage capacity and to soil nutritional status, and the model is easy to adjust and parameterize.
With the model data set such as air temperature, precipitation, insolation, vapor pressure deficit (VPD), initial soil water volume, soil texture, WSC, maximum stomatal conductance and crop production data, the 3-PG model calculates the canopy quantum efficiency, which represents the gross primary production (GPP; Mg ha−1) per absorbed photosynthetically active radiation (APAR, mol m−2). The output variables represent the growth pattern of the stands and are obtained by allometric relationships, defined as empirical equations estimating the variable of interest from easily measured variables such as the total height and the diameter at breast height.
2.3. Experimental Design and Sampling
The experimental design was composed of two conditions of productive potential (high productive potential Acrisols, and low productive potential Arenosols), which are distinct in soil properties related to soil class, texture and structure. In each condition of productive potential, forty-eight (48) permanent circular sample units (SU) of 400 m2 were installed, totaled ninety-six (96) circular sampling units for eucalyptus productivity evaluation.
In each SU, dendrometric variables of stands growth and productivity, and vegetation indexes necessary to characterize leaf dynamics and leaf area index (LAI) were collected. For the determination of granulometry, water retention curve (WRC), water storage capacity (WSC), bulk density (BD) and plant available water (PAW), a total of 504 soil samples were collected from 24 sampling points distributed between the SU, 12 sampling points in the Acrisols and 12 in the Arenosols sites. The soil samples were taken from 0.00–0.10, 0.10–0.20, 0.20–0.40, 0.40–0.60, 0.60–0.80, 0.80–1.00 and 1.00–1.20 m soil layers, which were located in excavation trenches of 1.5 m depth.
2.4. Growth, Dendrometric Characterization and Aboveground Biomass Stock
Two-month forest inventories were carried out for the evaluation of forest growth. The forest inventories were taken in 96 permanent SU and contemplate the measuring of the circumference at breast height (cbh; 1.3 m) of each tree within each SU, the total height of the first ten trees (mean height; h), and total height of the nine trees with the highest cbh (dominant height; hdom). The total height of the other trees was estimated by exponential equations adjusted as a function of the diameter at breast height (dbh) and age of the stands.
Individual wood volume was obtained by the cubed Smalian method, of 48 trees of quadratic mean diameter (dg; cm), selected for each pair of SUs, covering 24 trees in the Acrisols and 24 in the Arenosols sites. The estimation of the volume per hectare for each SU, plot and condition of productive potential was carried out from two equations adjusted as a function of dbh, total height (h) and tree age (r2 ≥ 0.98 and CV ≤ 12%). The same trees cubed by the Smalian method were sectioned to determine the basic wood density (Bwd; g cm−3) and the wood biomass (wb), from samples of the main stem, and the leaf biomass partition per tree. The wood biomass production per hectare (WB; Equation (1)), bark and branches biomass (BBB, Equation (2)), and leaf biomass (LB; Equation (3)) were estimated by equations adjusted based on h, dbh, stand density (number of trees per hectare; Nh), and stand age (A).
where WB is the wood biomass, BBB is the branch biomass, LB is the leaf biomass, dbh is the diameter at breast height, Nh is the number of trees per hectare and A is stand age.
Litter biomass was determined from weighing the material deposited in two square samplers installed in the line planting and between planting rows of each SU. Final litter biomass (Mg ha−1 month−1) was estimated by considering the surface area of each square sampler (0.25 m2), the number of days between set-up and collection, and the decomposition litter rate of 0.0072 kg day−1 [28].
Leaf area index was calculated by techniques of Remote Sensing, from analysis of orbital digital images of Landsat 8 satellite, orbit/point 218/72, with spatial resolution of 30 m, available from the American Geological Survey (USGS Earth Explorer).
The 3-PG parameters as effective depth of the root system (DRS) and canopy quantum efficiency (αC), were defined based on literature references. The DRS for Acrisols was defined as 2.5 m depth, as suggested for the standard parameterization process of the 3-PG model, while for the Arenosols sites, where trees tend to develop and lengthen their root system due to lower nutrient and water availability of soils [29,30], the DRS used was 4.0 m, defined as a mean value based on the results obtained in other studies [30,31,32]. The αC, which ranges between 0.5 and 0.8 molC molPAR−1 for eucalyptus [28,33,34], was defined based on results of the research carried out with the same genetic material and in the same climatic and edaphic condition of the Acrisols sites [35], in which the value of αC = 0.074 molC molPAR−1.
2.5. Edaphic Properties
Disturbed and undisturbed soil samples were taken from 0.00–0.10, 0.10–0.20, 0.20–0.40, 0.40–0.60, 0.60–0.80, 0.80–1.00 and 1.00–1.20 m soil layers at 24 sampling points, where 12 were allocated in Arenosols sites, and 12 in Acrisols sites. Undisturbed soil samples were collected by core samplers, 0.057 m diameter and 0.04 m height, with three replicates, to determine soil bulk density (BD).
Disturbed soil samples were air-dried, 2-mm sieved and used to analyze particle size distribution with a dispersion procedure [36], and to estimate water content at 1500 kPa water tension using a psychrometer [37]. Gravimetric water content obtained from the psychrometer was multiplied by soil bulk density to obtain volumetric water content.
Soil field capacity (FC) and permanent wilting point (PWP) were calculated as volumetric water content at 10 and 1500 kPa water tension, respectively. Plant available water (PAW) for each soil layer was estimated as the difference between volumetric water content at FC and PWP. The soil water storage capacity (mm m−1 of soil) was estimated considering the effective depth of the root system (DRS) and the total soil water availability in the soil profile (Equation (4)). Soil WSC (Equation (5)) was determined based on the soil bulk density (BD; g cm−3) and the water content at 10 kPa tension, corresponding to the field capacity (FC), and 1500 kPa, corresponding to permanent wilting point (PWP).
where: PAW = plant availability water (mm m−2); FC = field capacity (%); PWP = permanent wilting point (%); BD = soil bulk density (g cm−3); WSC = water storage capacity (mm m−1); DRS = effective depth of root system (cm);
2.6. Parameterization, Calibration and Validation of the 3-PG Model
The calibration procedure was performed by manual optimization of sensitive parameters of the model by comparing the observed and calculated values of growth and productivity variables, based on graphical analysis. Thus, model parameters such as canopy quantum efficiency, maximum canopy conductance, and soil moisture modifier, among others, were manually changed, based on model output and on parameter available in other 3-PG studies. These adjustment steps were performed only in areas with adequate water and nutrients supply, as observed in Acrisols sites, determined based on SWC, soil class and crop fertilization performed by the company.
Model validation was performed after model calibration. With the calibrated model the variables of interest as dbh, h, wood volume, and woody biomass (bark, branches, and main stem wood) were estimated for plots not included in the calibration database, and were compared with the estimated results with the observed database. The validation step was developed to evaluate the statistical accuracy and efficiency of the model by graphical analysis and by the statistical indicators as determination coefficient (r2), Nash–Sutcliffe efficiency coefficient (NSE), percent of BIAS (PBIAS), Willmot concordance coefficient (W), Pearson correlation coefficient (r) and confidence coefficient (c) tests.
3. Results
3.1. Parameterization of the 3-PG Model
The allometric relationships of the mean total height, volume per hectare and woody biomass (main stem, bark and branches) as a function of dbh, adjusted to obtain the allometric constants (aS, aV and aH) and allometric power functions (nS, nV and nH), presented results within the range defined as biologically correct for the species, as observed on reference values of analogous species. The functions had high statistical precision (Figure 1), according to the high values of r2 (>0.92) and to the low residuals sum of squares (RSS).
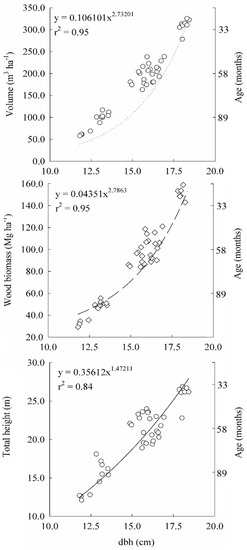
Figure 1.
Mean height (h), stem biomass (SB) and wood volume versus diameter at breast height (dbh; 1.3 m) at sites with high productive potential capacity (ACRI—Acrisols) and with low productive potential capacity (ARE—Arenosols), evaluated at 33 (ACRI33 and ARE33), 58 (ACRI58 and ARE58) and 89 (ACRI89 and AREa89) months age of the stands.
The soil water storage capacity (WSC), which represents the volume of water available between the FC and the PWP, was significantly different between the Acrisols and Arenosols, being 172 and 40 mm m−1, respectively. Based on the rooting depth (DRS) values of 1.6 and 4.0 m for Acrisols and Arenosols, the WSC determined for each site was 275 and 158 mm.
The relationship between the leaf biomass (LF) and wood biomass (WB) partitions, estimated with reference to main stem diameter of 2.0 (pfS2) and 20.0 cm (pfS20), which represents the ages of the stands, had distinct results for stands on Arenosols (PfS2 = 0.95, pfS20 = 0.24) and Acrisols (pfS2 = 0.67, pfS20 = 0.20). In the parameterization process of the 3-PG model, unique parameters for the two sites were estimated as pfS2 = 2.43 and pfS20 = 0.01.
The fraction of bark and branches for adult stands (fracBB1) was defined as 0.15, based on field values, while for young stands (fracBB0) a value of 0.29 was used, based on the values available in the literature. The monthly litter rates were estimated as 0.25 (ℽFx), for the adult stands, and 0.001 (ℽF0), which represents a standard value for deposition in young stands [38].
3.2. Efficiency and Validation of the Model
The model efficiency, evaluated by the simple regression analysis of the estimated values of dbh, h, main stem wood volume, and woody biomass as a function of the observed values, showed high r2 values and coefficients of variation between 5 and 10%. The Nash–Sutcliffe efficiency coefficient [39] shows the model is more efficient for the prediction of h and WB, and inefficient for the estimation of dbh. By the Willmott’s concordance coefficient [40], the analysis presented similar behavior but with better results for dbh estimates (Table 1). Even though the model presented low efficiency for dbh estimation, the under and overestimated values were lower than 12%, based on the comparison between the estimated and observed values (PBIAS) (Table 1).

Table 1.
Main parameter values of 3-PG model after calibration for Eucalyptus saligna in Acrisols and Arenosols sites.
The parameterized and calibrated model accurately estimated the pattern of growth and productivity of stands as a function of time, during the validation of the model. Results from Nash–Sutcliffe and Willmott, correlation analysis (r) and PBIAS were satisfactory to validate the model, which presented relative sensitivity to estimate the dbh variable (Table 2). The 3-PG model was more accurate in the Acrisols sites, with the h (m) and WB (Mg ha−1) variables more accurate, while for the dbh variable, in both Acrisols and Arenosols sites, the values estimated by the model were lower than those observed. The validation of the model showed that the best estimates were obtained in the Acrisols sites, with emphasis on h (m) and WB (Mg ha−1), while for the dbh variable, in both Acrisols and Arenosols sites, the results showed a tendency of underestimation. The percentage error, called the root mean square error (RMSE) calculated based on the relative difference between the observed and estimated values, had values of approximately 5% for both dbh and h and 17% and 18% for WB and V, respectively.

Table 2.
Statistical indicators for height (h), diameter at the breast height (dbh; 1.3 m), volume (V) and wood biomass (WB) estimative at sites with high productive potential capacity (ACRI—Acrisols) and with low productive potential capacity (ARE—Arenosols).
In model validation, the precision and efficiency tests presented similar results to those obtained with the observed data, with larger errors in the dbh estimates, while the highest precision was verified in the estimation of h (Table 3). Underestimation errors were not indicated by the PBIAS test; on the other hand, the tendency of overestimations was less than 10% for dbh and less than or equal to 5% for WB, V and h (Table 3). Dispersion of the observed and estimated values showed that, for the group of variables considered, the model tended to overestimate the results, mainly above 80 months-old.

Table 3.
Statistical indicators for height (h), diameter at the breast height (dbh; 1.3 m), volume (V) and wood biomass (WB) estimate obtained in model validation process.
3.3. Growth, Dendrometric Characterization and Aboveground Biomass Stock
Considering means including both ACRI and ARE sites, forest stands at 33 months-old presented dbh and total height of 0.13 m and 13.9 m, respectively, where wood volumetric productivity (VWB) of 75.7 m3 ha−1 represented a main stem biomass stock (WBT) of 27.5 Mg ha−1 (Table 4). At 89 months-old of age, the stands had a mean dbh of 0.178 m and mean total height of 25.4 m (Table 4), which resulted in an estimated mean volumetric stock of wood (VWB) of 323.1 m3 ha−1 (Table 4). The individual evaluations of the different productive potential conditions indicated distinct development patterns, with higher growth (dbh and h) and productivity (VWB and WBT) in Acrisols sites at the end of the cutting cycle (Table 4).

Table 4.
Mean height (h), diameter at breast height (dbh; 1.3 m), volume of wood without bark (VWB) and wood biomass of the trunk (WBT) in sites named ACRI—Acrisols and ARE—Arenosols, evaluated at 33 (ACRI33 and ARE33), 58 (ACRI58 and ARE58) and 89 (ACRI89 and ARE89) months age of the stands.
The coefficient of variation (CV%) indicated that Acrisols sites have lower variability in the diameter-height relationship, with greater homogeneity between the trees, which impacts the final production due to the lower occurrence of suppressed trees.
The mean annual volume of wood without bark increment (MAIVW) had an average rate of 43.7 m3 ha−1 year−1 at 96 months-old, while at 33 months-old the MAIVW was greater than 29 m3 ha−1 year−1. The production of biomass of the main stem at the end of rotation was 20.8 Mg ha−1 year−1, representing a significant increase from the 10.1 Mg ha−1 year−1 observed at 33 months-old.
The Arenosols sites had MAIVW rates described by an exponential function with asymptote at 58 months-old and, consequently, there were smaller increments in older stands. In the Acrisols, the MAIVW decreased until the age of cut, with an increasing linear behavior as a function of age resulting in incremental rates of approximately 34 and 52 m3 ha−1 year−1, at 33 and 89 months-old (Figure 2a). The increase in main stem biomass for Acrisols sites, at 89 months-old, was 24.6 Mg ha−1 year−1; this rate represented an increase close to 50% in relation to that observed at 58 months. The MAIWS in the Arenosols sites reached a maximum value at 58 months and remained practically constant until the end of the cycle, when a rate of 16.5 Mg ha−1 year−1 was observed (Figure 2b).
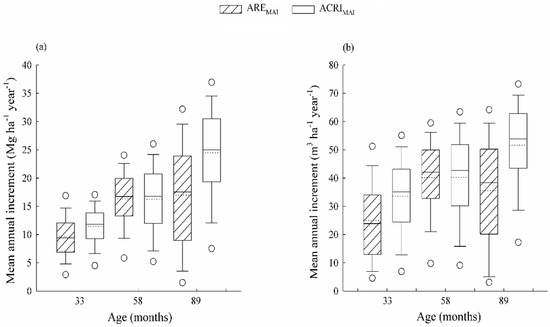
Figure 2.
Mean annual volume of wood without bark increment (a), and mean annual biomass of main stem increment (b), at sites with high productive potential capacity (ACRI—Acrisols) and with low productive potential capacity (ARE—Arenosols), evaluated at 33 (ACRI33 and ARE33), 58 (ACRI58 and ARE58) and 89 (ACRI89 and AREa89) months age of the stands.
The rise in increment rates as function of the stand age provided a mean aboveground biomass stock, based on mean dbh at 83 month-age, of 145.3 Mg ha−1, which represented the biomass partitioning in main stem wood (121.8 Mg ha−1), bark (14.2 Mg ha−1), leaves (3.4 Mg ha−1) and branches (5.9 Mg ha−1), at 83 months-old.
In biomass estimates per hectare, where stands density is associated with productivity, an increase of approximately 10% was observed in the production of woody biomass from 33 to 83 months-old, with a significant decrease in the proportion of bark and branches.
The individual biomass partitions determined at 33 months-old showed approximately 70% of the aboveground biomass was woody biomass, while the leaves were close to 8%. With stand age increase, these relationships changed and the proportion of the woody partition represented more than 84% of the aboveground biomass, with a biomass of leaves of less than 2.5%.
Biomass production per hectare gave, at 83 months-old, an accumulated residue, consisting of bark, leaves and branches, of approximately 30% of the aboveground biomass. The two sites showed significant differences in the productivity of each partition, mainly at 33 and 83 months, at which age a total aboveground biomass of 205.7 Mg ha−1 quantified in the Acrisols sites represented approximately 40% more than Arenosols sites aboveground biomass production.
Aboveground woody biomass stocks were higher in Acrisols sites over the whole growing cycle (Figure 3). As observed in dbh and h behavior, the difference between woody biomass stocks for Acrisols and Arenosols augemented with increased stand age (Figure 3). At 36 months-old, the woody biomass stock in Acrisols sites (61 Mg ha−1) represented about 32% more than Arenosols woody biomass productivity (46 Mg ha−1). This difference represents the Arenosols site productivity limitations, which at 72 and 96 months-old had woody biomass stocks 35% and 38% less than the Acrisols site. The highest productive capacity of the Acrisols sites was more evident in first three years of growth, but annually the Acrisols sites produced about 2% more than the Arenosols site, leading to woody biomass stocks of 189 and 137 Mg ha−1, respectively, at 96 months old (Figure 3).
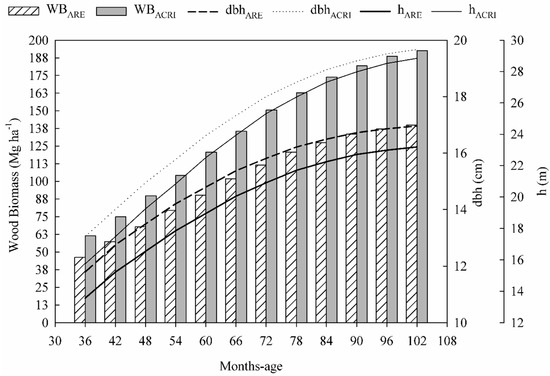
Figure 3.
Total height (h), diameter at breast height (dbh) and aboveground woody biomass stocks at sites with high productive potential capacity (Acrisols) and with low productive potential capacity (Arenosols), over the whole growing cycle.
4. Discussion
4.1. Model Parameterization
When fitting the allometric ratios for volume, woody biomass and total height, as a function of dbh, the statistical accuracy (r2 > 0.84) was higher than the results obtained by Londero et al. [41] and Klippel [35], in forest of E. saligna. In the model 3-PG, the biomass productivity as a function of the dbh considers the relationship between the dbh and the age of the stands, since for a given volume the aboveground wood biomass may present a significant variation as a function of the wood basic density (WDb), which tends to increase as age increases. This effect stems mainly from the decrease in growth rates, which, when very high in younger stands, tend to result in low density biomass production; due the rapid growth rates, eucalyptus species tend to produce low woody biomass [2].
In leaf biomass estimation, the maximum rate was 0.81 Mg ha−1 month−1; (ℽF0) and 0.25 for the maximum rate (ℽF1), the first defined as standard [12] and the second adjusted from values available in the literature [41]. The age at which the average litterfall rate was observed was defined as 12 months-old [12]. High values obtained in litter sampling may have occurred due to the influence of the El Niño phenomenon, which in the period of 2015–2016 caused high temperatures and rainfall above average. This climatic pattern maximizes litter deposition, as evidenced by studies that identified higher deposition rates in regions with higher rainfall indexes [42].
Deposition of litter presents a close relationship with the leaf dynamics (LAI and SLA). During the year, climatic variation due to seasonality provided higher values of LAI, SLA and litter deposition in the spring and summer periods, which corroborates with other results [43]. Specific leaf area (SLA) had estimated values of 7.6 m2 kg−1 for adult stands (SLA1), similar to the value obtained by Klippel [35] but lower than the value of 8.5 m2 kg−1 obtained by Lemos [17], while for young stands the specific leaf area (SL0) was 5.6 m2 kg−1. The increase of SLA with age does not corroborate with the expectations of the foliar dynamics, since it is expected that the SLA presents a lower value in older stands due to the competition and the reallocation of photoassimilates from the canopy to the woody portions and due to changes in leaf pattern, as leaves become more coriaceous with age [44,45].
Another important 3-PG parameter associated with leaf dynamics is the canopy quantum efficiency (αC), which, along with the specific leaf area for mature leaves (SLA1), provides significant changes to model results during the model parameterization process. Other authors [46] identify stomatal response to vapor pressure deficit, canopy quantum efficiency, extinction coefficient for the absorption of PAR by canopy, as well as allometric relationship and partitioning power and constant in the stem mass vs. diameter relationship maximum fraction of NPP to roots, and minimum fraction of net primary productivity to roots as sensitive parameters for 3-PG results.
Adverse results may be associated with SLA being obtained in different stands at each age, which may have compromised the determination of leaf dynamics over time. However, evaluations related to SLA and MAI had a high positive correlation when considering the local factor, which may justify the pattern observed in the foliar dynamics of the stands [47].
The site also influenced the belowground biomass, because in soils of low productive capacity and low soil water storage capacity the plants tend to increase their root system to maximize the abstraction of water and nutrients. The soil water storage capacity (WSC) was determined separately for each site, given the great difference observed between them. For Acrisols the 172 mm m−1 WSC represented a value similar to the observed in soils of medium and clayey texture [48,49,50]. The 40 mm m−1 WSC for the Arenosols site was higher than the value obtained in eucalyptus stands on sandy soils in the state of Minas Gerais, where the WSC was 30 mm m−1 [51].
In the determination of the weight of branch and bark biomass in relation to WB, the values observed and adjusted by the equation proposed by the 3-PG model (fracBB0 = 0.31; fracBB1 = 0.11) were adjusted in the parameterization process and had their final values defined as 0.29 and 0.15. These results indicate that about 15% of the woody biomass is allocated to the branches and bark partitions, which corroborates with the estimates presented in different studies with eucalyptus [12,52]. However, the value of fracBB0, which indicates that approximately 30% of the woody biomass corresponds to the branch and bark fraction, is below that observed by the same authors, who described the fracBB0 between 0.6 and 0.75. The value of fracBB0 = 0.30 was similar to other results, where fracBB0 values ranged between 0.30 and 0.31 in E. globulus forests, hybrid E. grandis × E. urophylla, and E. urophylla [52,53].
The parameterized model provided good estimates of production stands, with a lower accuracy in the determination of dbh. The statistical efficiency evaluated by the Nash–Sutcliffe test (NSE) had results higher than 0.7 for the dbh, 0.8 for the volume and woody biomass, and higher than 0.9 for the variable h, in the different locations. The results are higher than those obtained in the 3-PG estimates in commercial forests in the state of São Paulo, where the NSE had a value lower than 0.85 for main stem biomass [17]. Analyzing the Willmott concordance coefficient (WCC) only for the dbh variable of the Arenosols sites gave a value lower than 0.8. The h had the highest level of agreement (WCC > 0.89). In an evaluation of WCC’s 3-PG estimates, Rascon [52] obtained better results for wood volume (WCC = 0.97), but lower WCC values for dbh and h than the results obtained in this study.
The coefficient of determination (r2) was higher than 0.9 for all variables, except for the estimated dbh at the Arenosols site. The values of r2 are very similar to the results obtained by Klippel [35] in the stands of E. saligna in Eldorado do Sul, RS, and characterize the model as having high statistical precision. Higher sub and overestimate trends of the model were related to the WB and V variables, which according to the PBIAS test had a negative tendency lower than 10% in the Arenosols sites and 12% in the Acrisols sites. The estimates obtained for independent plots, which aimed to evaluate the extrapolation capacity of the parameterized 3-PG, were closely related to the values observed in the inventories, and the NSE and WCI tests were higher than 0.9, except for the results obtained for the dbh.
4.2. Eucalyptus Productivity
Average initial growth in the present study (dbh = 13.0 cm; h = 13.9 m), evaluated at 33 months-old, presented ae higher growth pattern than results observed in the state of São Paulo for stands of the hybrid E. urophylla × E. grandis, evaluated at 36 months-old at different densities, which presented dbh of 9.3 cm and h of 11.2 m [54]. In commercial forests of E. grandis grown under different residue management, Rocha [55] observed that the dendrometric pattern at 36 months-old, in second rotation, had an average dbh of approximately 10.0 cm and h close to 16.0 m. The values observed in this study were below the results observed in forest sites in the northwest of Minas Gerais, cultivated with the hybrid E. camaldulensis × E. grandis, where at 33 months-old the average dbh was 13.4 cm and mean total height was 16.6 m [56].
Although Arenosols sites present low productivity soils, the average growth pattern observed in this study was within the average productivity margin of the commercial forests in different regions of Brazil. The regions where Acrisols and Arenosols sites are located have, as a climatic characteristic, absence of drought. This water availability associated with proper pest control and fertilization may explain our results.
Volumetric productivity of wood without bark, quantified at 33 months-old as 73.5 m3 ha−1, was similar to the obtained in commercial E. grandis forests in the Brazilian states of Espírito Santo and São Paulo [14,57], but much higher than other results [58], which showed values lower than 50 m3 ha−1 in forests of E. nitens cultivated in Australia. The total production of stands with age close to 8 years-old (44 m3 ha−1 year−1) was higher than the last Brazilian national estimate [4], and similar to results obtained in other regions of the country [33].
In the most productive areas (ACRI89) the main stem wood stock (374 m3 ha−1; 177 Mg ha−1), obtained from average incremental rates (51 m3 ha−1 year−1, 26.9 Mg ha−1 year−1), exceeded the production of 331 m3 ha−1 year−1 observed in stands of E. grandis × E. urophylla at 7 years-old, distributed in seven different productive areas in the state of Minas Gerais [59]. For E. saligna stands located in the same region as our study, namely in Vera Cruz and Eldorado do Sul in southern Brazil, the volume of wood without bark was 371 and 438 m3 ha−1, respectively [35,60].
Areas of higher productivity had good structure and higher clay and organic matter contents. These soil properties have shown a high correlation with the rates of increment of the sites, mainly due to their relationship with soil water storage capacity, because the low soil water deficit during the eucalyptus growth allows the trees to develop to their maximum growth potential [61]. In this context, other growth responses can be related to the availability of boron micronutrient, originated from the mineralization of the organic matter [62,63,64]. The maintenance of organic residue can provide an increase of about 71% of over-bark trunk volume [65]. Changes in soil structure significantly interfere with soil water movement due to changes in soil hydraulic properties [66].
Associated with edaphic factors, Acrisols sites had soil water storage capacity (WSC) of 42.9 mm m−1, which represents almost three times the storage capacity of the Arenosols sites. This higher storage capacity positively affects crop productivity, since water directly influences nutrient availability in the soil solution, as well as the growth and production of eucalyptus stands [33,65,67].
The properties of Arenosols related to the dynamics and availability of water characterize them as highly drainable and susceptible to water deficits due to their coarse texture and poor structure, characteristic of sandy soils [68,69]. In these soils the available water for the plants was only 25 mm, which is equivalent to approximately 20% of the value observed in Acrisols.
The difference in the productive capacity of the two sites, Acrisols and Arenosols, may also be associated with crown development dynamics, with an increasing LAI behavior as a function of age in Acrisols stands, which corroborates the higher rates of increase in response to the increase of the photosynthetic capacity of the trees [70,71]. The evolution of LAI as a function of age has a direct relationship with soil properties, because sites with higher nutritional and water availability tend to present higher biomass allocation in the aerial part, to the detriment of the root system [72,73].
In forests harvested under operational fertilization rates, significant gains in productivity are obtained by increasing the water supply, with or without fertilization, which makes soil water storage capacity a major limiting factor for growth [28]. In soils with greater productive capacity, this nutritional contribution and the high availability of water provided the maintenance of the high current annual increment (CAI) and, consequently, the delay in the decrease in the MAI and the technical age of cut, defined as the point where there are no differences in the rates of MAI and CAI [33].
Aboveground biomass production (177.3 Mg ha−1) quantified at 89 months-old was higher than that observed in populations of E. globulus (153 Mg ha−1) evaluated at 108 months-old [74], while the mean annual increment rate (23.8 Mg ha−1 year−1) was higher than the values of 13.0 and 21.0 Mg ha−1 year−1 measured in stands evaluated at 60 and 77 months-old, respectively [75,76]. The increment rates observed in this study are within the productivity range of the commercial stands of Brazil, where the eucalyptus plantations are managed with nutritional, genetic and weed controls [33].
The observed relationship between aboveground biomass and main stem wood biomass (84%), branches and bark biomass (14%) and foliar biomass (2.3%) was similar to the results obtained in stands of different species of eucalyptus grown in different regions and evaluated at 66 months-old [77,78,79]. Other studies found divergent values in the proportions of woody biomass partitions of the main stem and leaf, with rates around 75% and 10%, respectively [74,80]. This variation in the biomass distribution may be related to soil and climatic properties, or to the initial density of stands [81].
The distinct aboveground woody biomass stocks observed in Arenosols and Acrisols sites reflect the site limitations related to Arenosols properties such as high permeability [82] and low water retention and availability to plants [83]. Furthermore, this soil can present low biotic activity and low nutrient cycling [84], which reduce the availability of nutrients and the stand productivity [65]. Aboveground woody biomass production had a direct relationship with the soil production capacity because in poor soils the increase of belowground biomass results in a less aboveground biomass stock [73]. Wood stock at 96-month-old of 137 Mg ha−1 for Arenosols was less than the production of 199 Mg ha−1 obtained at 120-month-old by Viera et al. [85], because Arenosols presented the lowest wood productivity, but the results were higher than the aboveground stock of 125 Mg ha−1 obtained at 102-month-old in Eucalyptus globulus plantations [74].
5. Conclusions
In the more productive areas (Acrisols), the maintenance of the high incremental rates up to 89 months-old, with mean annual volume of wood without bark increment higher than 50 m3 ha−1 year−1, suggests that under ideal soil conditions, the eucalyptus cutting cycle may exceed 8 years-old with no impairment of the mean annual increment rate and aboveground woody biomass stocks. Even though Arenosols sites had limitations in water retention and availability, the biomass production was similar in Acrisols and in Arenosols up to 58 months-old. Only at 89 months-old, significant decrease in productivity rates provided deficit in productivity at the end of the cutting cycle for Arenosols.
The 3-PG model was efficient in estimating biomass aboveground production in both Acrisols and Arenosols. The parameterized 3-PG model showed high statistical efficiency, capable of describing more than 90% of the variance of the main productive variables observed in Acrisols and Arenosols, with greater sensitivity and less precision when related to diameter at breast height. The satisfactory results in the validation process confirmed the ability to extrapolate the 3-PG model to other areas within the same climatic condition, although new validation is recommended in more distinct areas of soil properties.
The expected observed results of lower productivity in Arenosols occurred only after 58 months-old, which partially confirms our hypothesis that Arenosols sites are less productive than Acrisols in the overall rotation. For Acrisols, productivity remained high up to 89 months-old. This behavior is adequately represented by the model, and shows that the eucalyptus cycle should be shorter in Arenosols, so that the lower cutting cycle in Arenosols can provide higher final woody biomass productivity. However, in Acrisols, the cutting cycle should be higher because productivity increases with age up to 89 months (close to 8 years age).
Author Contributions
Conceptualization, J.P.C. and J.M.R.; methodology, J.P.C. and E.F.d.A.; validation, J.P.C.; formal analysis, J.P.C. and J.M.R.; investigation, J.P.C. and E.F.d.A.; data curation, J.P.C.; writing—original draft preparation, J.P.C.; writing—review and editing, all authors; supervision, J.M.R.; project administration, J.M.R.; funding acquisition, J.M.R. and E.F.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
Study partially funded by Coordination for the Improvement of Higher Education Personnel (Capes)—Finance code 001, National Council for Scientific and Technological Development (CNPq), and Celulose Riograndense Company (CMPC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data will be made available upon request.
Acknowledgments
The authors also thank Miriam Rodrigues for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Razakamanarivo, R.H.; Razakavololona, A.; Razafindrakoto, M.; Vieilledent, G.; Albrecht, A. Below-ground biomass production and allometric relationships of eucalyptus coppice plantation in the central highlands of Madagascar. Biomass Bioenerg. 2012, 45, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Song, M. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. For. Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Djomo, S.N.; Ac, A.; Zenone, T.; De Groote, T.; Bergante, S.; Facciotto, G.; Sixto, H.; Ciria, P.; Weger, J.; Ceulemans, R. Energy performances of intensive and extensive short rotation cropping systems for woody biomass production in the EU. Renew. Sustain. Energy Rev. 2015, 41, 845–854. [Google Scholar] [CrossRef]
- IBÁ—Indústria Brasileira de Árvores. Relatório 2015. Ano Base 2014. Brasília. 2015. Available online: https://iba.org/images/shared/iba_2015.pdf (accessed on 21 July 2022).
- França, J.S.; Reichert, J.M.; Holthusen, D.; Rodrigues, M.F.; Araújo, E.F. Subsoiling and mechanical hole-drilling tillage effects on soil physical properties and initial growth of eucalyptus after eucalyptus on steeplands. Soil Tillage Res. 2021, 207, 104860. [Google Scholar] [CrossRef]
- Reichert, J.M.; Morales, C.A.S.; Lima, E.M.; Bastos, F.; Sampietro, J.A.; Araújo, E.F.; Srinivasan, R. Best tillage practices for early-growth of clonal eucalyptus in soils with distinct granulometry, drainage and profile depth. Soil Tillage Res. 2021, 212, 105038. [Google Scholar] [CrossRef]
- Weiskittel, A.R.; Maguire, D.A.; Monserud, R.A.; Johnson, G.P. A hybrid model for intensively managed Douglas-fir plantations in the Pacific Northwest, USA. Eur. J. For. Res. 2009, 129, 325–338. [Google Scholar] [CrossRef]
- Wang, W.; Peng, C.; Zhang, S.Y.; Zhoua, X.; Larocquec, G.R.; Kneeshawa, D.D.; Leia, X. Development of TRIPLEX-Management model for simulating the response of forest growth to pre-commercial thinning. Ecol. Modell. 2011, 222, 2249–2261. [Google Scholar] [CrossRef]
- Pretzsch, H.; Grote, R.; Reineking, B.; Rotzeri, T.H.; Seifert, S.T. Models for forest ecosystem management: A European perspective. Ann. Bot. 2008, 101, 1065–1087. [Google Scholar] [CrossRef]
- Robinson, A.P.; Ek, A.R. Description and validation of a hybrid model of forest growth and stand dynamics for the Great Lakes region. Ecol. Modell. 2003, 170, 73–104. [Google Scholar] [CrossRef]
- Peng, C.; Liu, J.; Dang, Q.; Apps, M.J.; Jiang, H. TRIPLEX: A generic hybrid model for predicting forest growth and carbon and nitrogen dynamics. Ecol. Modell. 2002, 153, 109–130. [Google Scholar] [CrossRef]
- Sands, P.J.; Landsberg, J.J. Parameterisation of 3-PG for plantation grown Eucalyptus grobulus. For. Ecol. Manag. 2002, 163, 273–292. [Google Scholar] [CrossRef]
- Palma, J.H.N.; Hakamada, R.; Moreira, G.G.; Nobre, S.; Rodrigues, L.C.E. Using 3PG to assess climate change impacts on management plan optimization of Eucalyptus plantations. A case study in Southern Brazil. Sci. Rep. 2021, 11, 2708. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Landsberg, J.J.; Sands, P.J. Parameterisation of 3-PG model for fast-growing Eucalyptus grandis plantations. For. Ecol. Manag. 2004, 193, 179–195. [Google Scholar] [CrossRef]
- Zhao, M.; Xiang, W.; Deng, X.; Tian, D.; Huang, Z.; Zhou, X.; Yu, G.; He, H.; Pen, C. Application of TRIPLEX model for predicting Cinninghamia lanceolata and Pinus massoniana forest stand production in Hunan Province, southern China. Ecol. Modell. 2013, 250, 58–71. [Google Scholar] [CrossRef]
- Landsberg, J.; Sands, P. Physiological Ecology of Forest Production: Principles, Processes and Models; Academic Press: London, UK, 2010. [Google Scholar]
- Lemos, C.C.Z. Aprimoramentos, Teste e Uso do Modelo 3-PG em Plantios Clonais de Eucalyptus no Nordeste do Estado de São Paulo. Ph.D. Thesis, Universidade de São Paulo, Piracibaba, Brasil, 2012. [Google Scholar]
- Nolè, A.; Law, B.E.; Magnani, F.; Matteucci, G.; Ferrara, A.; Ripullone, F.; Borghetti, M. Application of the 3-PGS model to assess carbon accumulation in forest ecosystems at a regional level. Can. J. For. Res. 2009, 39, 1647–1661. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Jokela, E.J.; Cropper, W.P., Jr.; Bracho, R.; Leduc, D.J. Parameterisation of the 3-PG model for Pinus elliottii stands using alternative methods to estimate fertility rating, biomass partitioning and canopy closure. For. Ecol. Manag. 2014, 327, 55–75. [Google Scholar] [CrossRef]
- Valente, M.L.; Reichert, J.M.; Cavalcante, R.B.L.; Minella, J.P.G.; Evrard, O.; Srinivasan, R. Afforestation of degraded grasslands reduces sediment transport and may contribute to streamflow regulation in small catchments in the short-run. Catena 2021, 204, 105371. [Google Scholar] [CrossRef]
- Ebling, E.D.; Reichert, J.M.; Pelaez, J.J.Z.; Rodrigues, M.F.; Valente, M.L.; Cavalcante, R.B.L.; Reggiani, P.; Srinivasan, R. Event-based hydrology and sedimentation in paired watersheds under commercial eucalyptus and grasslands in the Brazilian Pampa biome. Int. Soil Water Conserv. Res. 2021, 9, 180–194. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Eucalyptus production and the supply use and the efficiency of use of water, light and nitrogen across a geographic gradient in Brazil. For. Ecol. Manag. 2004, 193, 17–31. [Google Scholar] [CrossRef]
- Almeida, A.C.; Siggins, A.; Batista, T.R.; Beadle, C.; Fonseca, S.; Loos, R. Mapping the effect of spatial and temporal variation in climate and soils on Eucalyptus plantation production with 3-PG, a process-based growth model. For. Ecol. Manag. 2010, 259, 1730–1740. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Goncalves, J.L.M.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Zeitschrif 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Moreno, J.A. Clima do Rio Grande do Sul; Secretaria da Agricultura: Porto Alegre, Brazil, 1961.
- IUSS Working Group WRB. World Reference Base for Soil Resources 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Cunha, T.J.F.; Oliveira, J.B. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2013. [Google Scholar]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For. Ecol. Manag. 2008, 255, 920–930. [Google Scholar] [CrossRef]
- Leite, F.P.; Silva, I.R.; Novais, R.F.; Barros, N.F.; Neves, J.C.L.; Villani, E.M.A. Nutrient relations during a eucalyptus cycle at different population densities. Rev. Bras. Cienc. Solo 2011, 35, 949–959. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Laclau, J.P.; Arnaud, M.; Bouillet, J.P.; Ranger, J. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: Relationships with the ability of the stand to take up water and nutrients. Tree Physiol. 2001, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Christina, M.; Laclau, J.P.; Goncalves, J.L.M.; Jourdan, C.; Nouvellon, Y.; Bouillet, J.P. Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forest. Ecosphere 2011, 2, 27–30. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.A.; et al. The Brazilian Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Baesso, R.C.E. Modelagem do Comportamento Ecofisiológico de Plantios de Eucalipto Submetidos a Aumentos na Concentração de CO2. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2011. [Google Scholar]
- Klippel, V.H. Modelagem Ecofisiológica de Cultivos de Eucalipto em Regiões Subtropicais do Brasil. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2015. [Google Scholar]
- Suzuki, L.E.A.S.; Reichert, J.M.; Albuquerque, J.A.; Reinert, D.J.; Kaiser, D.R. Dispersion and flocculation of Vertisols, Alfisols and Oxisols in Southern Brazil. Geoderma Reg. 2015, 5, 64–70. [Google Scholar] [CrossRef]
- Gubiani, P.I.; Reichert, J.M.; Campbell, C.; Reinert, D.J.; Gelain, N.S. Assessing errors and accuracy in dew-point potentiometer and pressure plate extractor measurements. Soil Sci. Soc. Am. J. 2013, 77, 19–24. [Google Scholar] [CrossRef]
- Sands, P.J. Adaptation of 3-PG to Novel Species: Guidelines for Data Collection and Parameter Assignment; Cooperative Research Center for Sustainable Production Forestry: Hobart, Australia, 2004. [Google Scholar]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. Am. Soc. Agric. Biol. Eng. 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Willmott, C.J. On the validation of models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Londero, E.K.; Schumacher, M.V.; Szymczak, D.A.; Araújo, E.F. Calibração do modelo 3-PG para Eucalyptus saligna Smith na Região de Guaíba—RS. Cienc. Florest. 2015, 25, 293–305. [Google Scholar] [CrossRef]
- Inkotte, J.; Mafra, A.L.; Rios, P.D.A. Deposição de serapilheira em reflorestamentos de eucalipto e florestas nativas nas regiões Planalto e Oeste do Estado de Santa Catarina. Sci. For. 2015, 43, 261–270. [Google Scholar]
- Eyles, A.; Worledge, D.; Sands, P.; Ottenschlaeger, M.L.; Paterson, S.C.; Mendham, D.; O’Grady, A.P. Ecophysiological responses of a young blue gum (Eucalyptus globulus) plantation to weed control. Tree Physiol. 2012, 32, 1008–1020. [Google Scholar] [CrossRef]
- England, J.R.; Attiwill, P.M. Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees Struct. Funct. 2006, 20, 79–90. [Google Scholar] [CrossRef]
- Raich, J.W.; Clark, D.A.; Schwendenmann, L.; Wood, T.E. Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS ONE 2014, 9, e100275. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, X.; Ren, Y. Parameter optimization of the 3PG model based on sensitivity analysis and a Bayesian method. Forests 2020, 11, 1369. [Google Scholar] [CrossRef]
- Landsberg, J.J.; Johnsen, K.H.; Albaugh, T.J.; Allen, H.L.; McKeand, S.E. Applying 3-PG, a simple process-based model designed to produce practical results, to data from loblolly pine experiments. For. Sci. 2001, 47, 43–51. [Google Scholar] [CrossRef]
- Vaz, C.M.P.; Freitas Iossi, M.; Mendonça Naime, J.; Macedo, A.; Reichert, J.M.; Reinert, D.J.; Cooper, M. Validation of the Arya and Paris water retention model for Brazilian soils. Soil Sci. Soc. Am. J. 2005, 69, 577–583. [Google Scholar] [CrossRef]
- Reichert, J.M.; Albuquerque, J.A.; Kaiser, D.R.; Reinert, D.J.; Urach, F.L.; Carlesso, R. Estimation of water retention and availability for Rio Grande do Sul soils. Rev. Bras. Cienc. Solo 2009, 33, 1547–1560. [Google Scholar] [CrossRef]
- Reichert, J.M.; Albuquerque, J.A.; Solano Peraza, J.E.; Costa, A. Estimating water retention and availability in cultivated soils of southern Brazil. Geoderma Reg. 2020, 21, e00277. [Google Scholar] [CrossRef]
- Guimarães, D.P.; Guenther, G.; Silva, C.; Sans, L.M.A.; Leite, F.P. Uso do modelo de crescimento 3-PG para o zoneamento do potencial produtivo do eucalipto no estado de Minas Gerais. Rev. Bras. Agrometeorol. 2006, 15, 192–197. [Google Scholar]
- Rascon, N.J.L. Modelagem Ecofisiológica do Desenvolvimento do Eucalipto na Amazônia. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2012. [Google Scholar]
- Almeida, A.C. Needs and opportunities for using a process-based productivity model as a practical tool in Eucalyptus plantations. For. Ecol. Manag. 2004, 193, 167–177. [Google Scholar] [CrossRef]
- Ferreira, D.H.A.A.; Leles, P.S.S.; Machado, E.C.; Abreu, A.H.M.; Abilio, F.M. Crescimento de clone de Eucalyptus urophylla x E. grandis em diferentes espaçamentos. Floresta 2014, 44, 431–440. [Google Scholar] [CrossRef]
- Rocha, J.H.T. Reflexos do Manejo de Resíduos Florestais na Produtividade, Nutrição e Fertilidade do Solo em Plantações de Eucalyptus grandis. Ph.D. Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2014. [Google Scholar]
- Fontan, I.C.I.; Reis, G.G.; Reis, M.G.F.; Leite, H.G.; Monte, M.A.; Ramos, D.C.; Souza, F.C. Growth of pruned eucalypt clone in an agroforestry system in southeastern Brazil. Agrofor. Syst. 2011, 83, 121–131. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Gonçalves, J.L.M.; Gava, J.L.; Godinho, T.O.; Melo, E.A.S.C.; Bazani, J.H.; Hubner, A.; Junior, J.C.A.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Machado, R.R.; Conceição, S.V.; Leite, H.G.; Souza, A.L.; Wolff, E. Evaluation of forest growth and carbon stock in forestry projects by system dynamics. J. Clean. Prod. 2015, 96, 520–530. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Interactive effects of simultaneously applied thinning, pruning and fertilizer application treatments on growth, biomass production and crown architecture in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2012, 267, 104–116. [Google Scholar] [CrossRef]
- Schumacher, M.V.; Witschoreck, R.; Calil, F.N. Biomassa em povoamentos de Eucalyptus spp. de pequenas propriedades rurais de Vera Cruz, RS. Cienc. Florest. 2011, 21, 17–22. [Google Scholar] [CrossRef]
- Reichert, J.M.; Prevedello, J.; Gubiani, P.I.; Vogelmann, E.S.; Reinert, D.J.; Consensa, C.O.B.; Soares, J.C.W.; Srinivasan, R. Eucalyptus tree stockings effect on water balance and use efficiency in subtropical sandy soil. For. Ecol. Manag. 2021, 497, 119473. [Google Scholar] [CrossRef]
- Gonçalves, J.L.D.M.; Alvares, C.A.; Gonçalves, T.D. Mapeamento de solos e da produtividade de plantações de Eucalyptus grandis, com uso de sistema de informação geográfica. Sci. For. 2012, 40, 187–201. [Google Scholar]
- Gonçalves, J.L.D.M.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.B.; Lima, W.P.; Brancalion, P.H.S.; Hubnera, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Stape, J.L.; Laclau, J.P.; Bouillet, J.P.; Ranger, J. Assessing the effects of early silvicultural management on long-term site productivity of fast-growing eucalypt plantations: The Brazilian experience. South. For. 2008, 70, 105–118. [Google Scholar] [CrossRef]
- Laclau, J.P.; Ranger, J.; Gonçalves, J.L.M.; Maquère, V.; Krusche, A.V.; M’Bouf, A.T.; Nouvellon, Y.; Saint-Andre, L.; Bouillet, J.P.; Piccolo, M.C.; et al. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations. Main features shown by intensive monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Alaoui, A.; Lipiec, J.; Gerke, H.H. A review of the changes in the soil pore system due to soil deformation. Soil Tillage Res. 2011, 115–116, 1–15. [Google Scholar] [CrossRef]
- Balieiro, F.C.; Oliveira, W.C.; Pereira, M.G.; Anjos, L.H.C.; Piccolo, M.C.; Jaccoud, C.F. Fertilidade e carbono do solo e uso da água pelo eucalipto numa topossequência em Seropédica, RJ. Rev. Árvore 2008, 32, 153–162. [Google Scholar] [CrossRef]
- Reichert, J.M.; Corcini, A.L.; Awe, G.O.; Reinert, D.J.; Albuquerque, J.A.; García Gallarreta, C.C.; Docampo, R. Onion-forage cropping systems on a Vertic Argiudoll in Uruguay: Onion yield and soil organic matter, aggregation, porosity and permeability. Soil Tillage Res. 2022, 216, 105229. [Google Scholar] [CrossRef]
- Ferreto, D.O.C.; Reichert, J.M.; Lopes Cavalcante, R.B.; Srinivasan, R. Water budget fluxes in catchments under grassland and Eucalyptus plantations of different ages. Can. J. For. Res. 2021, 51, 513–523. [Google Scholar] [CrossRef]
- Horn, D.; Ernani, P.R.; Sangoi, L.; Schweitzer, C.; Cassol, P.C. Parâmetros cinéticos e morfológicos da absorção de nutrientes em cultivares de milho com variabilidade genética contrastante. Rev. Bras. Cienc. Solo 2006, 30, 77–85. [Google Scholar] [CrossRef]
- Chmura, D.J.; Rahman, M.S.; Tjoelker, M.G. Crown structure and biomass allocation patterns modulate aboveground productivity in young loblolly pine and slash pine. For. Ecol. Manag. 2007, 243, 219–230. [Google Scholar] [CrossRef]
- Reis, G.G.; Reis, M.D.G.F.; Fontan, I.D.C.I.; Monte, M.A.; Gomes, A.N.; Oliveira, C.H.R. Crescimento de raízes e da parte aérea de clones de híbridos de Eucalyptus grandis × Eucalyptus urophylla e de Eucalyptus camaldulensis × Eucalyptus spp submetidos a dois regimes de irrigação no campo. Rev. Árvore 2006, 30, 921–931. [Google Scholar] [CrossRef]
- Epron, D.; Laclau, J.P.; Almeida, J.C.R.; Gonçalves, J.L.M.; Ponton, S.; Sette Jr, C.R.; Delgado-Rojas, J.S.; Bouillet, J.P.; Nouvellon, Y. Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations? Tree Physiol. 2011, 32, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, M.; Olsson, M.; Verwijst, T. Above-ground biomass production and allometric relations of Eucalyptus globulus Labill. coppice plantations along a chronosequence in the central highlands of Ethiopia. Biomass Bioenerg. 2009, 33, 421–428. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Gonçalves, J.L.M.; Voigtlaender, M.; Gavad, J.L.; Leite, F.P.; Hakamadaf, R.; Mareschal, L.; Mabiala, A.; Tardy, F.; et al. Eucalyptus and Acacia tree growth over entire rotation in single- and mixed-species plantations across five sites in Brazil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Santos, F.M.; Balieiro, F.C.; Ataíde, D.H.S.; Diniz, A.R.; Chaer, G.M. Dynamics of aboveground biomass accumulation in monospecific and mixed-species plantations of Eucalyptus and Acacia on a Brazilian sandy soil. For. Ecol. Manag. 2016, 363, 86–97. [Google Scholar] [CrossRef]
- Paixão, F.A.; Soares, C.P.B.; Jacovine, L.A.G.; Silva, M.L. ; Leite, H.G.; Silva, G.F. Quantificação do estoque de carbono e avaliação econômica de diferentes alternativas de manejo em um plantio de eucalipto. Rev. Árvore 2006, 30, 411–420. [Google Scholar] [CrossRef]
- Muñoz, F.; Rubilar, R.; Espinosa, M.; Cancino, J.; Toro, J.; Herrera, M. The effect of pruning and thinning on above ground aerial biomass of Eucalyptus nitens (Deane & Maiden) Maiden. For. Ecol. Manag. 2008, 255, 365–373. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Soares, C.P.B.; Fehrmann, L.; Jacovine, L.A.G.; Von Gadow, K. Aboveground and belowground biomass and carbon estimates for clonal eucalyptus trees in southeast Brazil. Rev. Árvore 2015, 39, 353–363. [Google Scholar] [CrossRef]
- Kuyah, S.; Dietz, J.; Muthuri, C.; Van Noordwijk, M.; Neufeldt, H. Allometry and partitioning of above- and below-ground biomass in farmed eucalyptus species dominant in Western Kenyan agricultural landscapes. Biomass Bioenerg. 2013, 55, 276–284. [Google Scholar] [CrossRef]
- Pinkard, E.; Neilsen, W. Crown and stand characteristics of Eucalyptus nitens in response to initial spacing: Implications for thinning. For. Ecol. Manag. 2003, 172, 215–227. [Google Scholar] [CrossRef]
- Reichert, J.M.; Bervald, C.M.P.; Rodrigues, M.F.; Kato, O.R.; Reinert, D.J. Mechanized land preparation in eastern Amazon in fire-free forest-based fallow systems as alternatives to slash-and-burn practices: Hydraulic and mechanical soil properties. Agric. Ecosyst. Environ. 2014, 192, 47–60. [Google Scholar] [CrossRef]
- Reichert, J.M.; Rodrigues, M.F.; Bervald, C.M.P.; Kato, O.R. Fire-free fallow management by mechanized chopping of biomass for sustainable agriculture in eastern Amazon: Effects on soil compactness, porosity, and water retention and availability. Land Degrad. Dev. 2016, 27, 1403–1412. [Google Scholar] [CrossRef]
- Reichert, J.M.; Amado, T.J.C.; Reinert, D.J.; Rodrigues, M.F.; Suzuki, L.E.A.S. Land use effects on subtropical, sandy soil under sandyzation/desertification processes. Agric. Ecosyst. Environ. 2016, 233, 370–380. [Google Scholar] [CrossRef]
- Viera, M.; Schumacher, M.V.; Araújo, E.F.; Corrêa, R.S.; Caldeira, M.V.W. Deposição de serapilheira e nutrientes em plantio de Eucalyptus urophylla × E. globulus. Flor. Amb. 2014, 21, 327–338. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).