Effect of Laser Biostimulation on Germination of Sub-Optimally Stored Flaxseeds (Linum usitatissimum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
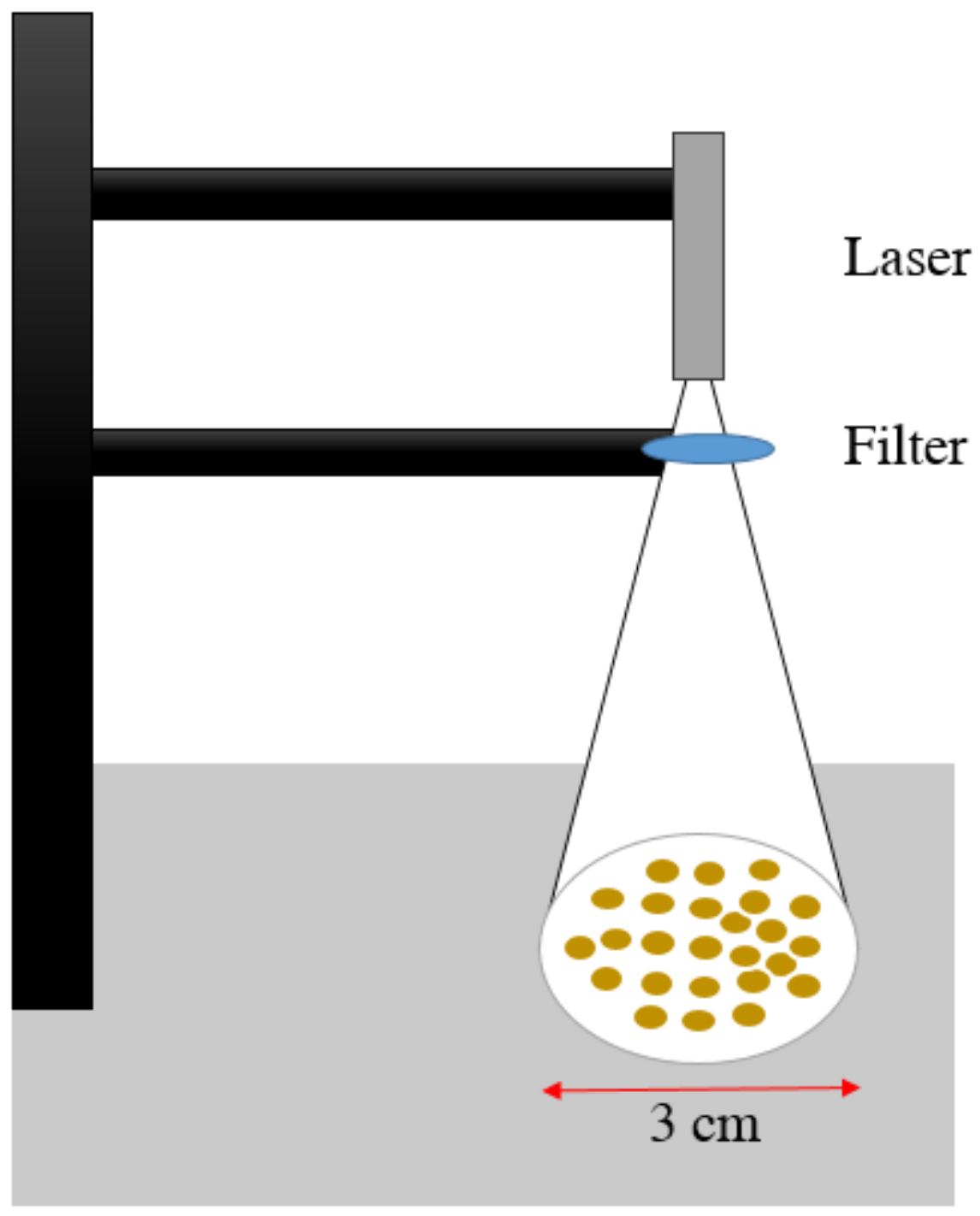
2.2. Laser Treatment
2.3. Germination Parameters
2.4. Statistical Analysis
3. Results
3.1. Healthy Flaxseeds
3.2. Artificially Degraded Flaxseeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakumar, C.; Chaudhry, M.M.A.; Nadimi, M.; Paliwal, J.; Courcelles, J. Characterization of Roller and Ferkar-Milled Pulse Flours Using Laser Diffraction and Scanning Electron Microscopy. Powder Technol. 2022, 409, 117803. [Google Scholar] [CrossRef]
- Erkinbaev, C.; Nadimi, M.; Paliwal, J. A Unified Heuristic Approach to Simultaneously Detect Fusarium and Ergot Damage in Wheat. Meas. Food 2022, 7, 100043. [Google Scholar] [CrossRef]
- Nadimi, M.; Brown, J.M.; Morrison, J.; Paliwal, J. Examination of Wheat Kernels for the Presence of Fusarium Damage and Mycotoxins Using Near-Infrared Hyperspectral Imaging. Meas. Food 2021, 4, 100011. [Google Scholar] [CrossRef]
- Li, X.; Guillermic, R.M.; Nadimi, M.; Paliwal, J.; Koksel, F. Physical and Microstructural Quality of Extruded Snacks Made from Blends of Barley and Green Lentil Flours. Cereal Chem. 2022, 99, 1112–1123. [Google Scholar] [CrossRef]
- Sabzi, S.; Nadimi, M.; Abbaspour-Gilandeh, Y.; Paliwal, J. Non-Destructive Estimation of Physicochemical Properties and Detection of Ripeness Level of Apples Using Machine Vision. Int. J. Fruit Sci. 2022, 22, 628–645. [Google Scholar] [CrossRef]
- Kheiralipour, K.; Nadimi, M.; Paliwal, J. Development of an Intelligent Imaging System for Ripeness Determination of Wild Pistachios. Sensors 2022, 22, 7134. [Google Scholar] [CrossRef]
- Metwally, S.A.; Abou Leila, B.H.; Gaballah, M.S. Laser Application in Agriculture and Its Physiological Effect on Plant: A Review. Plant Arch. 2020, 20, 9535–9543. [Google Scholar]
- Nadimi, M.; Sun, D.W.; Paliwal, J. Recent Applications of Novel Laser Techniques for Enhancing Agricultural Production. Laser Phys. 2021, 31, 053001. [Google Scholar] [CrossRef]
- Hernandez, A.C.; Dominguez, P.A.; Cruz, O.A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R. Laser in Agriculture. Int. Agrophys. 2010, 24, 407–422. [Google Scholar]
- Abu-Elsaoud, A.M.; Tuleukhanov, S.T.; Abdel-Kader, D.Z. Effect of Infra-Red Laser on Wheat (Triticum aestivum) Germination. Int. J. Agric. Res. 2008, 3, 433–438. [Google Scholar] [CrossRef][Green Version]
- Jamil, Y.; Perveen, R.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He-Ne Laser-Induced Changes in Germination, Thermodynamic Parameters, Internal Energy, Enzyme Activities and Physiological Attributes of Wheat during Germination and Early Growth. Laser Phys. Lett. 2013, 10, 045606. [Google Scholar] [CrossRef]
- Awaad, H.A. Role of Helium-Neon Laser in Improving Wheat Grain Yield Potentiality. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer: Cham, Switzerland, 2021; pp. 391–408. [Google Scholar]
- Podlesna, A.; Gładyszewska, B.; Podlesny, J.; Zgrajka, W. Changes in the Germination Process and Growth of Pea in Effect of Laser Seed Irradiation. Int. Agrophys. 2015, 29, 485–492. [Google Scholar] [CrossRef]
- Muthusamy, A.; Kudwa, P.P.; Prabhu, V.; Mahato, K.K.; Babu, V.S.; Rao, M.R.; Gopinath, P.M.; Satyamoorthy, K. Influence of Helium-Neon Laser Irradiation on Seed Germination In Vitro and Physico-Biochemical Characters in Seedlings of Brinjal (Solanum melongena L.) Var. Mattu Gulla. Photochem. Photobiol. 2012, 88, 1227–1235. [Google Scholar] [CrossRef]
- Swathy, P.S.; Rupal, G.; Prabhu, V.; Mahato, K.K.; Muthusamy, A. In Vitro Culture Responses, Callus Growth and Organogenetic Potential of Brinjal (Solanum melongena L.) to He-Ne Laser Irradiation. J. Photochem. Photobiol. B Biol. 2017, 174, 333–341. [Google Scholar] [CrossRef]
- Swathy, S.P.; Kiran, K.R.; Rao, M.S.; Mahato, K.K.; Rao, M.R.; Satyamoorthy, K.; Muthusamy, A. Responses of He-Ne Laser Irradiation on Agronomical Characters and Chlorogenic Acid Content of Brinjal (Solanum melongena L.) Var. Mattu Gulla. J. Photochem. Photobiol. B Biol. 2016, 164, 182–190. [Google Scholar] [CrossRef]
- Muszyński, S.; Gładyszewska, B. Representation of He-Ne Laser Irradiation Effect on Radish Seeds with Selected Germination Indices. Int. Agrophys. 2008, 22, 151–157. [Google Scholar]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He-Ne Laser-Induced Improvement in Biochemical, Physiological, Growth and Yield Characteristics in Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Asghar, T.; Iqbal, M.; Jamil, Y.; Haq, Z.U.; Nisar, J.; Shahid, M. Comparison of He-Ne Laser and Sinusoidal Non-Uniform Magnetic Field Seed Pre-Sowing Treatment Effect on Glycine Max (Var 90-I) Germination, Growth and Yield. J. Photochem. Photobiol. B Biol. 2017, 166, 212–219. [Google Scholar] [CrossRef]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Haq, Z.U.; Abbas, M. Laser Light and Magnetic Field Stimulation Effect on Biochemical, Enzymes Activities and Chlorophyll Contents in Soybean Seeds and Seedlings during Early Growth Stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of Laser Light Treatment on Some Biochemical and Physiological Processes in Seeds and Seedlings of White Lupine and Faba Bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Álvarez, A.; Ramírez, R.; Chávez, L.; Camejo, Y.; Licea, L.; Porras, E.; García, B. Efect of Seed Treatment with Low Potency Laser on the Growth and Yield in Tomato Plants (Solanum lycopersicum L.). Inf. Téc. Econ. Agrar. 2011, 107, 290–299. [Google Scholar]
- Dziwulska-Hunek, A.; Kornarzyńska-Gregorowicz, A.; Niemczynowicz, A.; Matwijczuk, A. Influence of Electromagnetic Stimulation of Seeds on the Photosynthetic Indicators in Medicago sativa L. Leaves at Various Stages of Development. Agronomy 2020, 10, 594. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Li, J.T.; Zhang, M.M.; Bi, Z.Z.; Li, Z.L. He-Ne Laser Pretreatment Protects Wheat Seedlings against Cadmium-Induced Oxidative Stress. Ecotoxicol. Environ. Saf. 2013, 88, 135–141. [Google Scholar] [CrossRef]
- Qiu, Z.; Yuan, M.; He, Y.; Li, Y.; Zhang, L. Physiological and Transcriptome Analysis of He-Ne Laser Pretreated Wheat Seedlings in Response to Drought Stress. Sci. Rep. 2017, 7, 6108. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; He, Y.; Zhang, Y.; Guo, J.; Wang, L. Characterization of MiRNAs and Their Target Genes in He-Ne Laser Pretreated Wheat Seedlings Exposed to Drought Stress. Ecotoxicol. Environ. Saf. 2018, 164, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.A.; Mohamed, S.L.M.; Abou-leila, B.H.; Aly, M.S. Effect of Drought Stress and Helium Neon (He-Ne) Laser Rays on Growth, Oil Yield and Fatty Acids Content in Caster Bean (Ricinus communis L.). Agric. For. Fish. 2014, 3, 203–208. [Google Scholar] [CrossRef]
- Gao, L.M.; Li, Y.F.; Han, R. He-Ne Laser Preillumination Improves the Resistance of Tall Fescue (Festuca Arundinacea Schreb.) Seedlings to High Saline Conditions. Protoplasma 2015, 252, 1135–1148. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Shen, Z.; Han, R. Responses of He-Ne Laser on Agronomic Traits and the Crosstalk between UVR8 Signaling and Phytochrome B Signaling Pathway in Arabidopsis thaliana Subjected to Supplementary Ultraviolet-B (UV-B) Stress. Protoplasma 2018, 255, 761–771. [Google Scholar] [CrossRef]
- AbuElsaoud, A.; Shahda, R. Role of He-Ne Laser Pre-Treatment in Protecting Zea Mays against the Deleterious Effects of Ultraviolet Radiations. Egypt. Soc. Exp. Biol. 2017, 13, 403–422. [Google Scholar] [CrossRef]
- Siyami, R.; Mirshekari, B.; Farahvash, F.; Rashidi, V.; Tarinejad, A. The Effect of Physical Priming of Seed on Traits and Yield of Corn (Zea mays L.) under Water Deficit Conditions in Iran. Appl. Ecol. Environ. Res. 2018, 16, 617–627. [Google Scholar] [CrossRef]
- Ali, S.I.; Gaafar, A.A.; Metwally, S.A.; Habba, I.E.; Abdel khalek, M.R. The Reactive Influences of Pre-Sowing He-Ne Laser Seed Irradiation and Drought Stress on Growth, Fatty Acids, Phenolic Ingredients, and Antioxidant Properties of Celosia Argentea. Sci. Hortic. 2020, 261, 108989. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P. Effect of Long-Term of He-Ne Laser Light Irradiation on Selected Physiological Processes of Triticale. Plants 2020, 9, 1703. [Google Scholar] [CrossRef]
- Hasan, M.; Hanafiah, M.M.; Aeyad Taha, Z.; AlHilfy, I.H.H.; Said, M.N.M. Laser Irradiation Effects at Different Wavelengths on Phenology and Yield Components of Pretreated Maize Seed. Appl. Sci. 2020, 10, 1189. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Szymanek, M.; Stadnik, J. Impact of Pre-Sowing Red Light Treatment of Sweet Corn Seeds on the Quality and Quantity of Yield. Agriculture 2020, 10, 165. [Google Scholar] [CrossRef]
- Hasan, M.; Hanafiah, M.M.; Alhilfy, I.H.H.; Taha, Z.A. Comparison of the Effects of Two Laser Photobiomodulation Techniques on Bio-Physical Properties of Zea mays L. Seeds. PeerJ 2021, 9, e10614. [Google Scholar] [CrossRef]
- Thorat, S.A.; Poojari, P.; Kaniyassery, A.; Kiran, K.R.; Satyamoorthy, K.; Mahato, K.K.; Muthusamy, A. Red Laser-Mediated Alterations in Seed Germination, Growth, Pigments and Withanolide Content of Ashwagandha [Withania somnifera (L.) Dunal]. J. Photochem. Photobiol. B Biol. 2021, 216, 112144. [Google Scholar] [CrossRef]
- Dudareva, L.; Tarasenko, V.; Rudikovskaya, E. Involvement of Photoprotective Compounds of a Phenolic Nature in the Response of Arabidopsis thaliana Leaf Tissues to Low-Intensity Laser Radiation. Photochem. Photobiol. 2020, 96, 1243–1250. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Dłużniewska, J.; Ślizowska, A.; Dobrowolski, J.W. Impact of Coherent Laser Irradiation on Germination and Mycoflora of Soybean Seeds—Innovative and Prospective Seed Quality Management. Agriculture 2020, 10, 314. [Google Scholar] [CrossRef]
- Nadimi, M.; Sun, D.W.; Paliwal, J. Effect of Laser Biostimulation on Germination of Wheat. Appl. Eng. Agric. 2022, 38, 77–84. [Google Scholar] [CrossRef]
- Nadimi, M.; Loewen, G.; Paliwal, J. Assessment of Mechanical Damage to Flaxseeds Using Radiographic Imaging and Tomography. Smart Agric. Technol. 2022, 2, 100057. [Google Scholar] [CrossRef]
- Mundhada, S.; Chaudhry, M.M.A.; Erkinbaev, C.; Paliwal, J. Development of Safe Storage Guidelines for Prairie-Grown Flaxseed. J. Stored Prod. Res. 2022, 97, 101965. [Google Scholar] [CrossRef]
- Aligholizadeh Moghadam, P.; Alaei, Y. Evaluation of Important Germination Traits of Soybean Genotypes through Factor Analysis in Osmotic Drought Stress Conditions. Environ. Pharmacol. Life Sci 2014, 3, 5–8. [Google Scholar]
- Erkinbaev, C.; Morrison, J.; Paliwal, J. Assessment of Seed Germinability of Mechanically-Damaged Soybeans Using Near-Infrared Hyperspectral Imaging. Available online: https://library.csbe-scgab.ca/all-publications/2751-assessment-of-seed-germinability-of-mechanically-damaged-soybeans-using-near-infrared-hyperspectral-imaging (accessed on 31 December 2021).
- Nithya, U.; Chelladurai, V.; Jayas, D.S.; White, N.D.G. Safe Storage Guidelines for Durum Wheat. J. Stored Prod. Res. 2011, 47, 328–333. [Google Scholar] [CrossRef]
- Sathya, G.; Jayas, D.S.; White, N.D.G. Safe Storage Guidelines for Canola as the Seeds Slowly Dry. Can. Biosyst. Eng. 2009, 51, 29–38. [Google Scholar]
- Sathya, G.; Jayas, D.S.; White, N.D.G. Safe Storage Guidelines for Rye. Can. Biosyst. Eng. 2008, 50, e3. [Google Scholar]
- Rani, P.R.; Chelladurai, V.; Jayas, D.S.; White, N.D.G.; Kavitha-Abirami, C.V. Storage Studies on Pinto Beans under Different Moisture Contents and Temperature Regimes. J. Stored Prod. Res. 2013, 52, 78–85. [Google Scholar] [CrossRef]
- Ri, P.; Choe, S.; Jang, Y. Study on Laser Pre-Sowing Treatment of Rice Seeds by Free-Falling Transport Method. Inf. Process. Agric. 2019, 6, 515–521. [Google Scholar] [CrossRef]
- Nadimi, M.; Major, A. Continuous-Wave Dual-Wavelength Operation of a Diode-Pumped Nd:GdVO4 Laser at the 1063 & 1071 Nm, 1063 & 1083 Nm and 1083 &1086 Nm Wavelength Pairs. Laser Phys. 2018, 28, 95001. [Google Scholar] [CrossRef]
- Sedaghati, Z.; Nadimi, M.; Major, A. Efficient Continuous-Wave Nd:YLF Laser in-Band Diode-Pumped at 908 Nm and Its Thermal Lensing. Laser Phys. Lett. 2019, 16, 125002. [Google Scholar] [CrossRef]
- Nadimi, M.; Waritanant, T.; Major, A. High Power and Beam Quality Continuous-Wave Nd:GdVO_4 Laser in-Band Diode-Pumped at 912 Nm. Photonics Res. 2017, 5, 346. [Google Scholar] [CrossRef]
- Nadimi, M.; Onyenekwu, C.; Major, A. Continuous-Wave Dual-Wavelength Operation of the in-Band Diode-Pumped Nd:GdVO4/Nd:YVO4 Composite Laser with Controllable Spectral Power Ratio. Appl. Phys. B Lasers Opt. 2020, 126, 75. [Google Scholar] [CrossRef]
- Nadimi, M.; Waritanant, T.; Major, A. Discrete Multi-Wavelength Tuning of a Continuous Wave Diode-Pumped Nd:GdVO4 Laser. Laser Phys. Lett. 2018, 15, 055002. [Google Scholar] [CrossRef]
- Nadimi, M.; Waritanant, T.; Major, A. Passively Mode-Locked High Power Nd:GdVO4 Laser with Direct in-Band Pumping at 912 Nm. Laser Phys. Lett. 2018, 15, 015001. [Google Scholar] [CrossRef]
- Nadimi, M.; Waritanant, T.; Major, A. Thermal Lensing in Nd:GdVO4 Laser with Direct in-Band Pumping at 912 Nm. Appl. Phys. B Lasers Opt. 2018, 124, 170. [Google Scholar] [CrossRef]
- Nadimi, M.; Waritanant, T.; Major, A. A Simple Approach to Estimate Thermal Lensing in Nd-Ion Doped Vanadate Laser Crystals. In Solid State Lasers XXX: Technology and Devices; SPIE: Bellingham, WA, USA, 2021; Volume 11664, p. 38. [Google Scholar] [CrossRef]
- Howlader, C.; Nadimi, M.; Waritanant, T.; Fedorova, K.; Sokolovskii, G.; Rafailov, E.; Major, A. High Power Nd:YVO-KGW Conical Refraction Laser. In Laser Resonators, Microresonators, and Beam Control XXI; SPIE: Bellingham, WA, USA, 2019; Volume 10904, p. 69. [Google Scholar] [CrossRef]
| Dual-Wavelength Laser | |||||||
| Exposure Time (Min) | Germination Percentage | Mean Germination Time (Days) | Germination Speed (Number of Germinated Seeds/Day) | Germination Rate Index (%/Day) | Primary Root Length (mm) | Wet Weight (mg) | Dry Weight (mg) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 87.0 ± 7.4 | 2.1 ± 0.2 | 11.3 ± 1.0 | 45.0 ± 4.1 | 55.0 ± 4.3 | 24.7 ± 5.6 | 3.5 ± 0.3 |
| 5 | 79.5 ± 8.4 * | 2.2 ± 0.2 | 9.6 ± 1.3 *** | 38.3 ± 5.2 *** | 52.1 ± 3.9 | 25.5 ± 5.0 | 3.5 ± 0.2 |
| 10 | 88.0 ± 4.3 | 2.0 ± 0.3 | 11.3 ± 0.7 | 45.1 ± 2.7 | 69.3 ± 3.7 * | 30.3 ± 3.1 * | 3.8 ± 0.3 * |
| 15 | 90.5 ± 4.2 | 2.3 ± 0.2 | 10.5 ± 0.8 | 41.9 ± 3.3 | 64.6 ± 4.7 | 28.7 ± 4.3 | 3.7 ± 0.3 |
| Test of significance | KW | CA | CA | CA | CA | CA | CA |
| Red Laser | |||||||
| Exposure Time (Min) | Germination Percentage | Mean Germination Time (Days) | Germination Speed (Number of Germinated Seeds/Day) | Germination Rate Index (%/Day) | Primary Root Length (mm) | Wet Weight (mg) | Dry Weight (mg) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 87.0 ± 7.4 | 2.1 ± 0.2 | 11.3 ± 1.0 | 45.0 ± 4.1 | 55.0 ± 4.3 | 24.7 ± 5.6 | 3.5 ± 0.3 |
| 5 | 79.0 ± 5.6 ** | 2.2 ± 0.3 | 9.7 ±1.3 ** | 38.9 ± 5.3 ** | 48.8 ± 12.5 | 23.1 ± 7.1 | 3.4 ± 0.5 |
| 10 | 89.0 ± 5.6 | 2.8 ± 0.6 *** | 8.6 ± 1.6 *** | 34.5 ± 6.2 *** | 47.3 ± 16.1 | 24.9 ± 7.4 | 3.8 ± 0.4 |
| 15 | 87.5 ± 5.8 | 2.3 ± 0.2 | 10.1 ± 0.7 | 40.3 ± 2.7 | 53.9 ± 9.3 | 22.9 ± 5.1 | 3.7 ± 0.4 |
| Test of significance | CA | KW | CA | CA | KW | CA | CA |
| Dual-Wavelength Laser | |||||||
| Exposure Time (Min) | Germination Percentage | Mean Germination Time (Days) | Germination Speed (Number of Germinated Seeds/Day) | Germination Rate Index (%/Day) | Primary Root Length (mm) | Wet Weight (mg) | Dry Weight (mg) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 49.5 ± 11.3 | 4.4 ± 0.3 | 3.1 ± 0.8 | 12.3 ± 3.2 | 33.1 ± 3.6 | 17.9 ± 4.0 | 2.8 ± 0.6 |
| 5 | 64.0 ± 12.5 ** | 3.7 ± 0.5 ** | 3.8 ± 0.8 * | 15.3 ± 3.3 * | 32.0 ± 7.2 | 20.7 ± 2.2 | 3.4 ± 0.4 |
| 10 | 56.5 ± 10.8 | 4.2 ± 0.4 | 3.1 ± 0.6 | 12.5 ± 2.6 | 26.6 ± 4.7 * | 18.8 ± 3.8 | 3.2 ± 0.8 |
| 15 | 42.0 ± 8.6 | 3.6 ± 0.7 *** | 2.2 ± 0.5 * | 8.9 ± 1.8 * | 29.2 ± 6.7 | 14.3 ± 4.2 | 2.3 ± 0.8 |
| Test of significance | CA | CA | CA | CA | CA | CA | CA |
| Red Laser | |||||||
| Exposure Time (Min) | Germination percentage | Mean Germination Time (Days) | Germination Speed (Number of Germinated Seeds/Day) | Germination Rate Index (%/Day) | Primary Root Length (mm) | Wet Weight (mg) | Dry Weight (mg) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 49.5 ± 11.3 | 4.4 ± 0.3 | 3.1 ± 0.8 | 12.3 ± 3.2 | 33.1 ± 3.6 | 17.9 ± 4.0 | 2.8 ± 0.6 |
| 5 | 56.5 ± 13.8 | 3.8 ± 0.2 ** | 3.4 ± 1.0 | 13.7 ± 3.9 | 31.3 ± 9.1 | 20.4 ± 9.7 | 3.0 ± 1.4 |
| 10 | 61.0 ± 16.0 | 4.6 ± 1.6 | 3.4 ± 1.1 | 13.6 ± 4.3 | 25.6 ± 4.7 *** | 23.7 ± 13.2 | 4.1 ± 2.3 |
| 15 | 42.0 ± 14.0 | 4.0 ± 0.9 | 2.3 ± 0.7 | 9.1 ± 2.8 | 24.9 ± 6.2 ** | 17.7 ± 9.0 | 2.9 ± 1.7 |
| Test of significance | CA | KW | CA | CA | WA | WA | CA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadimi, M.; Loewen, G.; Bhowmik, P.; Paliwal, J. Effect of Laser Biostimulation on Germination of Sub-Optimally Stored Flaxseeds (Linum usitatissimum). Sustainability 2022, 14, 12183. https://doi.org/10.3390/su141912183
Nadimi M, Loewen G, Bhowmik P, Paliwal J. Effect of Laser Biostimulation on Germination of Sub-Optimally Stored Flaxseeds (Linum usitatissimum). Sustainability. 2022; 14(19):12183. https://doi.org/10.3390/su141912183
Chicago/Turabian StyleNadimi, Mohammad, Georgia Loewen, Pankaj Bhowmik, and Jitendra Paliwal. 2022. "Effect of Laser Biostimulation on Germination of Sub-Optimally Stored Flaxseeds (Linum usitatissimum)" Sustainability 14, no. 19: 12183. https://doi.org/10.3390/su141912183
APA StyleNadimi, M., Loewen, G., Bhowmik, P., & Paliwal, J. (2022). Effect of Laser Biostimulation on Germination of Sub-Optimally Stored Flaxseeds (Linum usitatissimum). Sustainability, 14(19), 12183. https://doi.org/10.3390/su141912183








