Reduction of Graphene Oxide Using Citrus hystrix Peels Extract for Methylene Blue Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
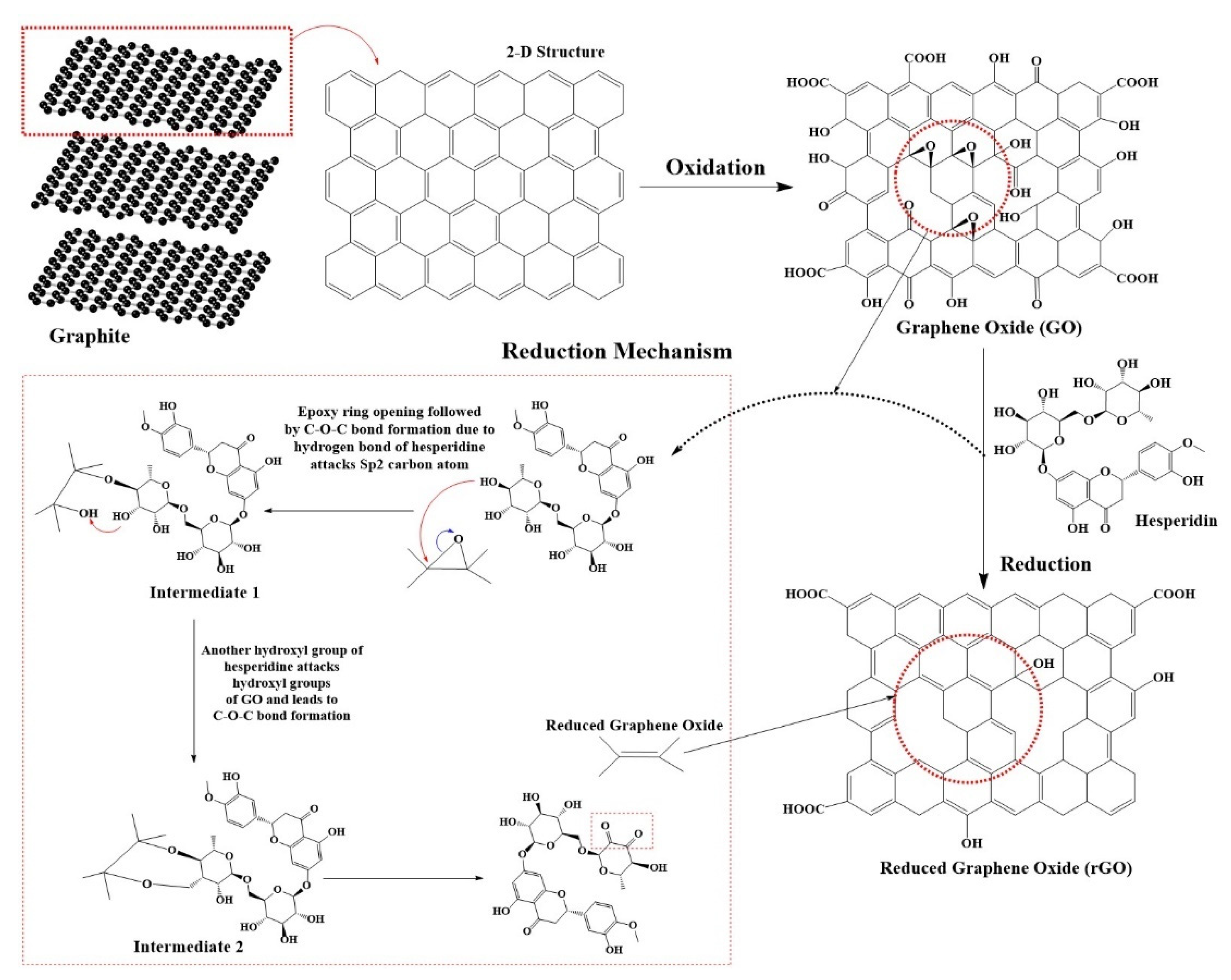
2.2. Preparation of rGO
2.3. Adsorption Experiment
2.4. Characterization
3. Results and Discussion
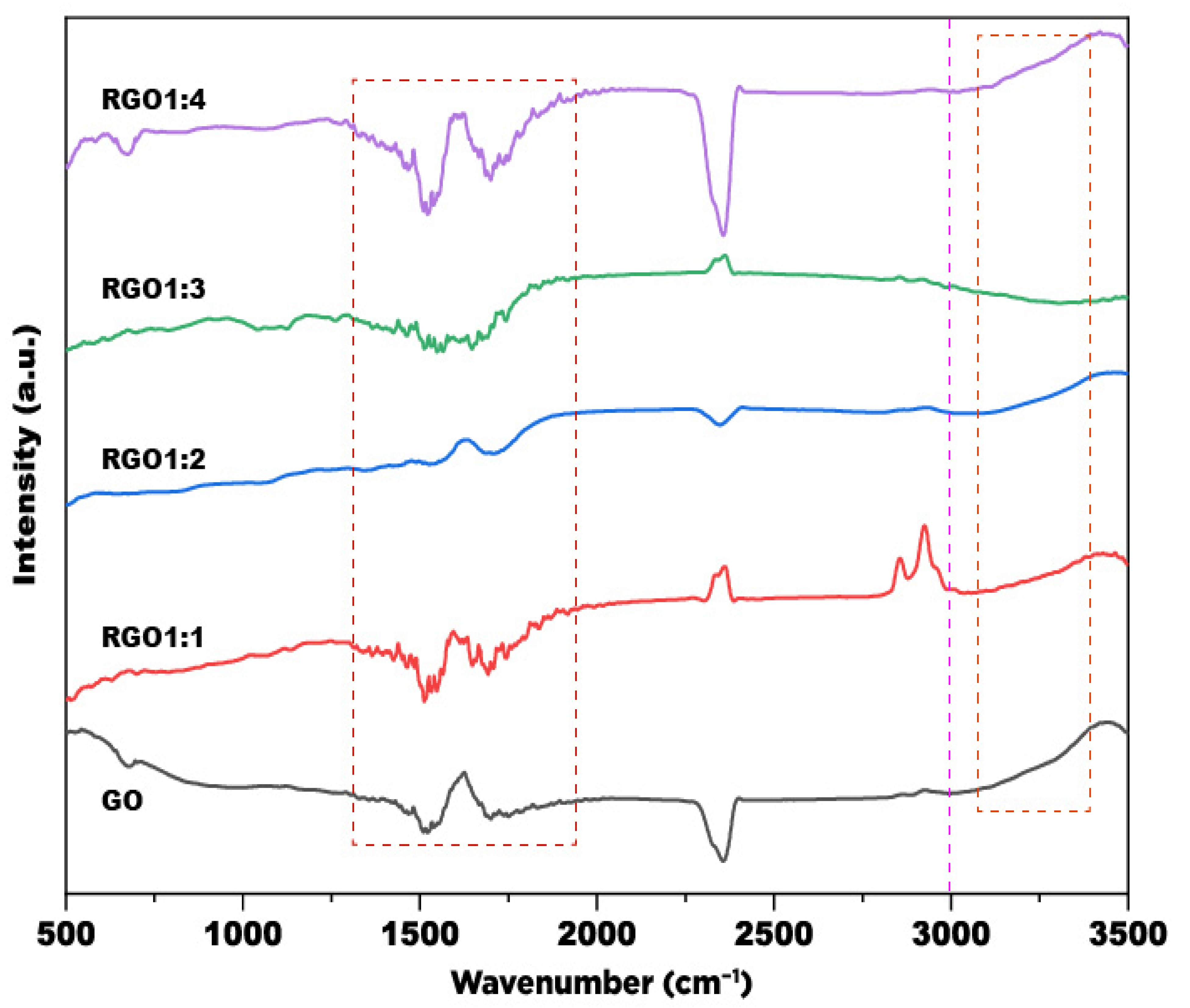
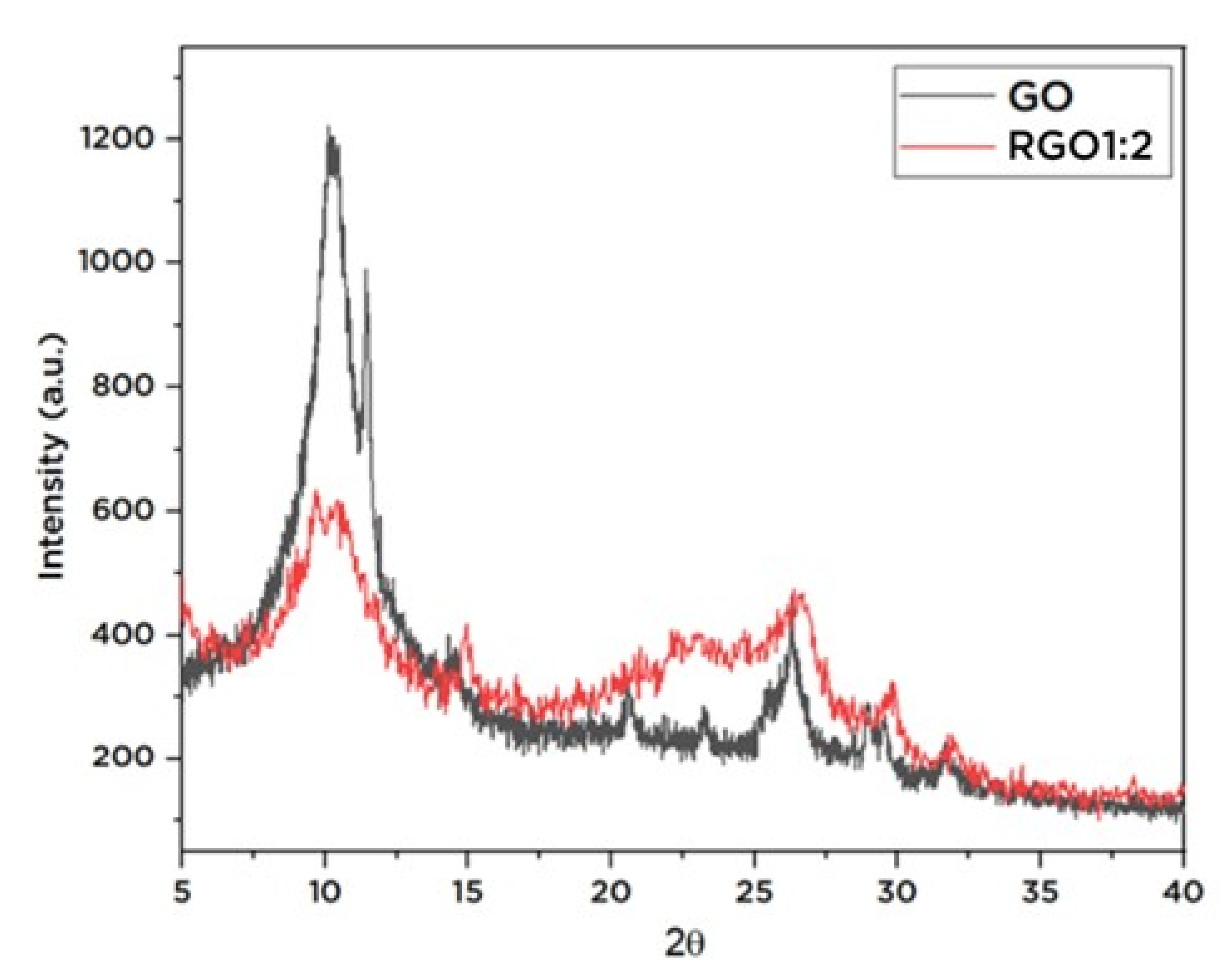
3.1. Structural Characteristics
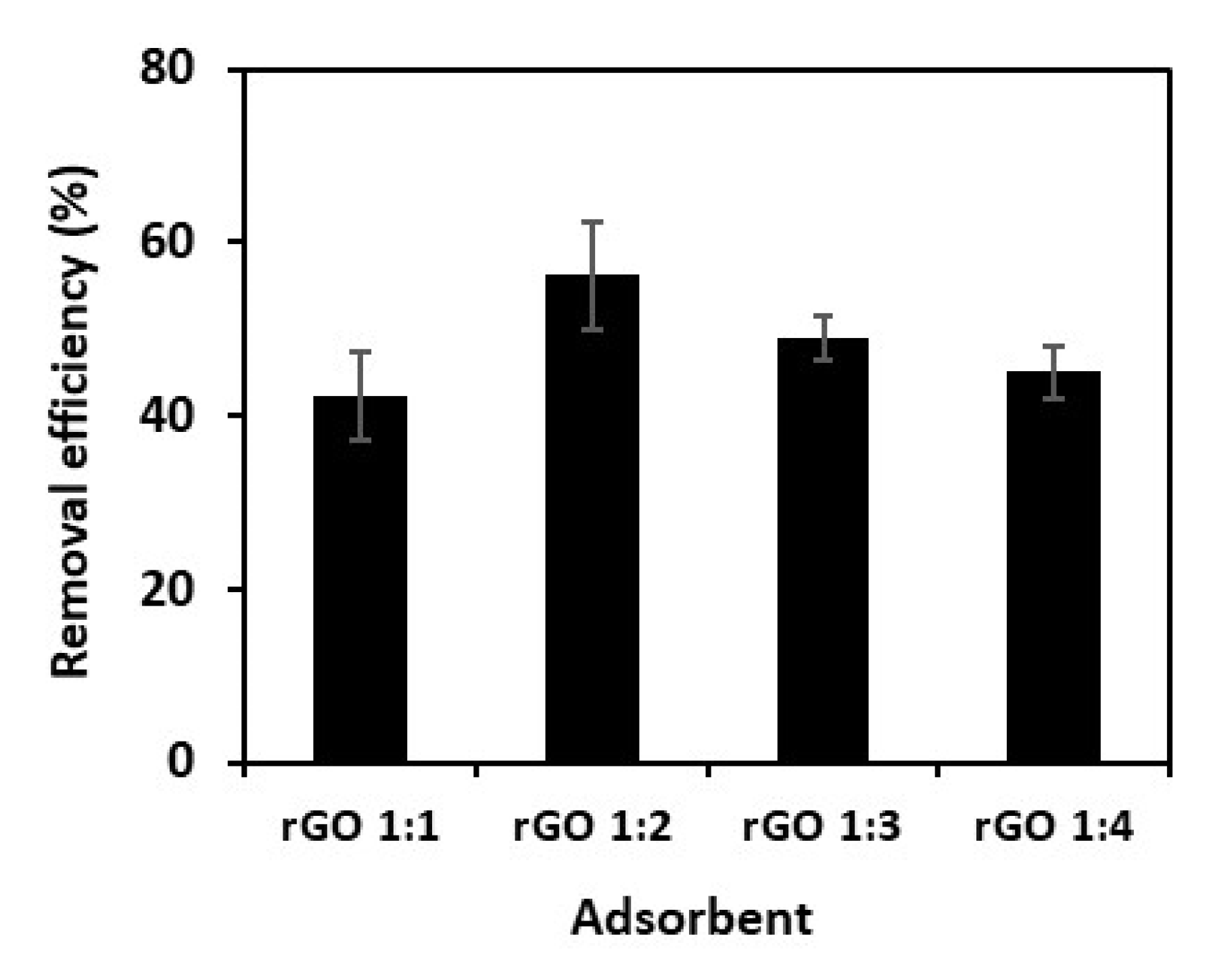
3.2. Adsorption Studies
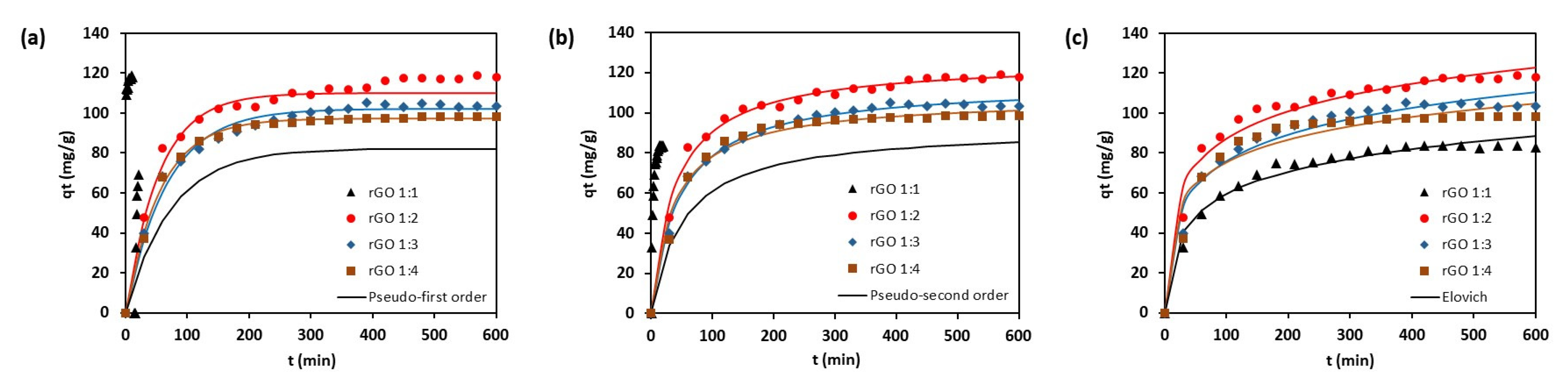
3.2.1. Adsorption Kinetics
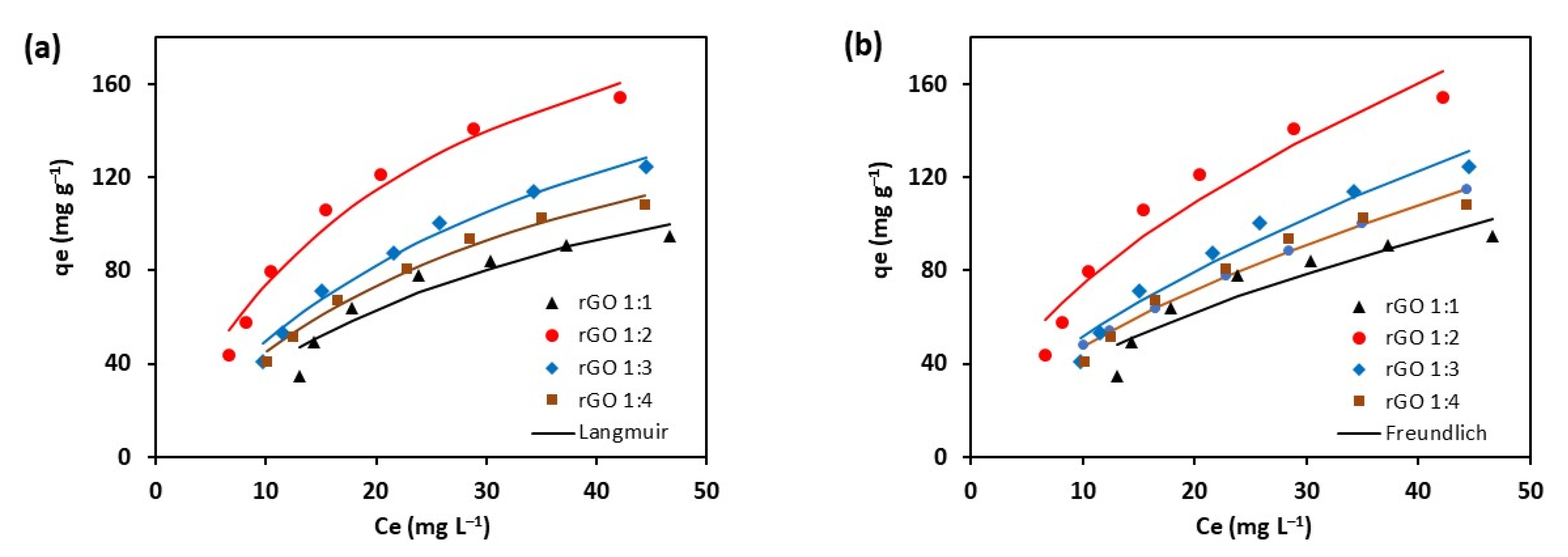
3.2.2. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Mi, X.; Li, Y.; Zhan, S. 3D Graphene-Based Macrostructures for Water Treatment. Adv. Mater. 2020, 32, e1806843. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Yan, Y.; Chen, D.; Huang, L.; Zhang, J.; Ke, Y.; Tan, S. The utilization of a three-dimensional reduced graphene oxide and montmorillonite composite aerogel as a multifunctional agent for wastewater treatment. RSC Adv. 2018, 8, 4239–4248. [Google Scholar] [CrossRef]
- Ye, F.; Wang, Z.; Mi, Y.; Kuang, J.; Jiang, X.; Huang, Z.; Luo, Y.; Shen, L.; Yuan, H.; Zhang, Z. Preparation of reduced graphene oxide/titanium dioxide composite materials and its application in the treatment of oily wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124251. [Google Scholar] [CrossRef]
- Gao, C.; Dong, Z.; Hao, X.; Yao, Y.; Guo, S. Preparation of Reduced Graphene Oxide Aerogel and Its Adsorption for Pb(II). ACS Omega 2020, 5, 9903–9911. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.-S.; Choi, Y.-L.; Chang, Y.-Y.; Yang, J.-K.; Karri, R.R.; Koduru, J.R. Process modeling and optimization of an iron oxide immobilized graphene oxide gadolinium nanocomposite for arsenic adsorption. J. Mol. Liq. 2020, 299, 112261. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Kumar, R.T.R. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and p-p interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the Oxygen Content of Graphite and Reduced Graphene Oxide for Specific Applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef]
- Ramanathan, S.; Moorthy, S.; Ramasundaram, S.; Rajan, H.K.; Vishwanath, S.; Selvinsimpson, S.; Durairaj, A.; Kim, B.; Vasanthkumar, S. Grape Seed Extract Assisted Synthesis of Dual-Functional Anatase TiO2 Decorated Reduced Graphene Oxide Composite for Supercapacitor Electrode Material and Visible Light Photocatalytic Degradation of Bromophenol Blue Dye. ACS Omega 2021, 6, 14734–14747. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—dependence on the reducing agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Brunauer–Emmett–Teller (BET) specific surface area analysis of different graphene materials: A comparison to their structural regularity and electrical properties. Solid State Commun. 2020, 320, 114004. [Google Scholar] [CrossRef]
- Lehner, B.A.E.; Janssen, V.A.E.C.; Spiesz, E.M.; Benz, D.; Brouns, S.J.J.; Meyer, A.S.; Zant, H.S.J.v.d. Creation of conductive graphene materials by bacterial reduction using Shewanella oneidensis. Chem. Open 2019, 8, 888–895. [Google Scholar] [CrossRef]
- Siong, V.L.E.; Lee, K.M.; Juan, J.C.; Lai, C.W.; Tai, X.H.; Khe, C.S. Removal of methylene blue dye by solvothermally reduced graphene oxide: A metal-free adsorption and photodegradation method. RSC Adv. 2019, 9, 37686–37695. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, J.; Wang, Z.; Xing, B. New insight into the photo-transformation mechanisms of graphene oxide under UV-A, UV-B and UV-C lights. J. Hazard. Mater. 2021, 403, 123683. [Google Scholar] [CrossRef]
- Tajiki, A.; Abdouss, M.; Sadjadi, S.; Mazinani, S.; Ramakrishna, S. Photo-induced green synthesis of bimetallic Ag/Pd nanoparticles decorated reduced graphene oxide/nitrogen-doped graphene quantum dots nanocomposite as an amperometric sensor for nitrite detection. Anal. Bioanal. Chem. 2021, 413, 6289–6301. [Google Scholar] [CrossRef]
- Park, O.-K.; Kim, N.H.; Lee, J.H. Rapid effective reduction by microwave-irradiated thermal reaction for large-scale production of high-quality reduced graphene oxide. Carbon 2021, 187, 330–337. [Google Scholar] [CrossRef]
- Lee, W.-J.; Jang, H.-R.; Kim, M.-J.; Kim, H.-M.; Oh, J.-M.; Paek, S.-M. Microwave-irradiated reduced graphene oxide nanosheets for highly reversible and ultrafast sodium storage. J. Alloy. Compd. 2018, 778, 382–390. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Tayade, U.S.; Borse, A.U.; Meshram, J.S. Green reduction of graphene oxide and its applications in band gap calculation and antioxidant activity. Green Mater. 2019, 7, 143–155. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Gan, L.; Li, B.; Chen, Y.; Yu, B.; Chen, Z. Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: A waste-to-resource supply chain. Chemosphere 2019, 219, 148–154. [Google Scholar] [CrossRef]
- Li, B.; Jin, X.; Lin, J.; Chen, Z. Green reduction of graphene oxide by sugarcane bagasse extract and its application for the removal of cadmium in aqueous solution. J. Clean. Prod. 2018, 189, 128–134. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene oxide—Based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renew. Energy 2020, 151, 731–739. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, Tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Wijaya, Y.A.; Widyadinata, D.; Irawaty, W.; Ayucitra, A. Fractionation of phenolic and flavonoid compounds from kaffir lime (Citrus hystrix) peel extract and evaluation of antioxidant activity. Reaktor 2017, 17, 111–117. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Pham, V.H.; Balasubramaniyan, R.; Varshney, S.; Jang, J.; Hur, S.H.; Choi, W.M.; Kumar, M.; Dhawan, S.K.; et al. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against lectromagnetic pollution. Nanoscale 2013, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Srifuengfung, S.; Bunyapraphatsara, N.; Satitpatipan, V.; Tribuddharat, C.; Junyaprasert, V.B.; Tungrugsasut, W.; Srisukh, V. Antibacterial oral sprays from kaffir lime (Citrus hystrix DC.) fruit peel oil and leaf oil and their activities against respiratory tract pathogens. J. Tradit. Complement. Med. 2020, 10, 594–598. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Ochiai, B. Lemon Juice Assisted Green Synthesis of Reduced Graphene Oxide and Its Application for Adsorption of Methylene Blue. Technologies 2021, 9, 96. [Google Scholar] [CrossRef]
- Weng, X.; Wu, J.; Ma, L.; Owens, G.; Chen, Z. Impact of synthesis conditions on Pb(II) removal efficiency from aqueous solution by green tea extract reduced graphene oxide. Chem. Eng. J. 2019, 359, 976–981. [Google Scholar] [CrossRef]
- Mindivan, F.; Göktaş, M. Rosehip-Extract-Assisted Green Synthesis and Characterization of Reduced Graphene Oxide. ChemistrySelect 2020, 5, 8980–8985. [Google Scholar] [CrossRef]
- Alshamsi, H.A.; Ali, S.K.; Altaa, S.H.A. Green Synthesis and Characterization of Reduced Graphene Oxide (RGO) using Sabdarriffa L extract and its Solubility Property. J. Phys. Conf. Ser. 2020, 1664, 012058. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Purkait, M.K. A brief review on graphene oxide Nanoscrolls: Structure, Synthesis, characterization and scope of applications. Chem. Eng. J. 2021, 420, 129914. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Bundjaja, V.; Kurniawan, A.; Yuliana, M.; Hsieh, C.-W.; Go, A.W.; Cheng, K.-C.; Soetaredjo, F.E.; Ismadji, S. Investigation of the influence of crosslinking activation methods on the physicochemical and Cu(II) adsorption characteristics of cellulose hydrogels. J. Environ. Chem. Eng. 2022, 10, 106971. [Google Scholar] [CrossRef]


| Particle | Area | C–C/C–O | |
|---|---|---|---|
| GO | C–C C–OH C–O–C C=O C–OOH | 1410.58 258.95 721.21 107.44 580.91 | 0.85 |
| rGO 1: 1 | C–C C–OH C–O–C C=O C–OOH | 1968.00 1148.46 426.01 37.77 220.83 | 1.07 |
| rGO 1: 2 | C–C C–OH C–O–C C=O C–OOH | 1988.42 352.98 408.23 20.59 152.43 | 2.13 |
| rGO 1: 3 | C–C C–OH C–O–C C=O C–OOH | 1971.51 859.74 579.85 152.00 135.84 | 1.14 |
| rGO 1: 4 | C–C C–OH C–O–C C=O C–OOH | 2189.70 1058.62 251.25 126.17 154.84 | 1.38 |
| Particle | Surface Area (m2 g–1) |
|---|---|
| rGO 1: 1 | 402 |
| rGO 1: 2 | 765 |
| rGO 1: 3 | 582 |
| rGO 1: 4 | 604 |
| Model and Parameters | Unit | rGO 1:1 | rGO 1:2 | rGO 1:3 | rGO 1:4 |
|---|---|---|---|---|---|
| Pseudo-first-order | |||||
| q1e | mg g–1 | 82.165 | 110.095 | 102.219 | 97.278 |
| k1 | min–1 | 0.014 | 0.019 | 0.0150 | 0.0177 |
| R2 (Adj.) | 0.989 | 0.977 | 0.988 | 0.983 | |
| SSE | 104.595 | 515.209 | 174.394 | 200.425 | |
| Pseudo-second-order | |||||
| q2e | mg g–1 | 92.666 | 125.885 | 114.337 | 107.275 |
| k2 × 103 | g mg–1 min–1 | 0.208 | 0.202 | 0.189 | 0.148 |
| R2 (Adj.) | 0.997 | 0.992 | 0.994 | 0.996 | |
| SSE | 30.511 | 131.617 | 76.496 | 43.897 | |
| Elovich | |||||
| α | mg g–1 min–1 | 5.958 | 15.761 | 9.521 | 15.999 |
| β | g mg–1 | 0.061 | 0.050 | 0.052 | 0061 |
| RE | 0.196 | 0.168 | 0.185 | 0.167 | |
| R2 (Adj.) | 0.975 | 0.970 | 0.968 | 0.937 | |
| SSE | 216.908 | 479.425 | 427.335 | 749.385 |
| Model and Parameters | Unit | rGO 1:1 | rGO 1:2 | rGO 1:3 | rGO 1:4 |
|---|---|---|---|---|---|
| Langmuir | |||||
| qmax | mg g–1 | 177.580 | 251.879 | 237.239 | 198.186 |
| KL | L mg–1 | 0.028 | 0.042 | 0.026 | 0.029 |
| RL | 0.417 | 0.322 | 0.435 | 0.408 | |
| R2 (Adj.) | 0.913 | 0.975 | 0.981 | 0.987 | |
| SSE | 272.185 | 290.399 | 119.412 | 56.832 | |
| Freundlich | |||||
| KF | (mg g–1)(mg L–1)–n | 10.668 | 20.585 | 12.336 | 12.099 |
| n | 1.702 | 1.793 | 1.605 | 1.685 | |
| R2 (Adj.) | 0.875 | 0.932 | 0.956 | 0.963 | |
| SSE | 382.503 | 726.612 | 257.608 | 148.853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priliana, V.; Sucitro, C.; Wijaya, R.; Lunardi, V.B.; Santoso, S.P.; Yuliana, M.; Gunarto, C.; Angkawijaya, A.E.; Irawaty, W. Reduction of Graphene Oxide Using Citrus hystrix Peels Extract for Methylene Blue Adsorption. Sustainability 2022, 14, 12172. https://doi.org/10.3390/su141912172
Priliana V, Sucitro C, Wijaya R, Lunardi VB, Santoso SP, Yuliana M, Gunarto C, Angkawijaya AE, Irawaty W. Reduction of Graphene Oxide Using Citrus hystrix Peels Extract for Methylene Blue Adsorption. Sustainability. 2022; 14(19):12172. https://doi.org/10.3390/su141912172
Chicago/Turabian StylePriliana, Veronika, Clarissa Sucitro, Ronald Wijaya, Valentino Bervia Lunardi, Shella Permatasari Santoso, Maria Yuliana, Chintya Gunarto, Artik Elisa Angkawijaya, and Wenny Irawaty. 2022. "Reduction of Graphene Oxide Using Citrus hystrix Peels Extract for Methylene Blue Adsorption" Sustainability 14, no. 19: 12172. https://doi.org/10.3390/su141912172
APA StylePriliana, V., Sucitro, C., Wijaya, R., Lunardi, V. B., Santoso, S. P., Yuliana, M., Gunarto, C., Angkawijaya, A. E., & Irawaty, W. (2022). Reduction of Graphene Oxide Using Citrus hystrix Peels Extract for Methylene Blue Adsorption. Sustainability, 14(19), 12172. https://doi.org/10.3390/su141912172







