Assessment of the Pollution of Soil Heavy Metal(loid)s and Its Relation with Soil Microorganisms in Wetland Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and the Determination of Soil HMs
2.2. A Comprehensive Assessment of HM Pollution
2.3. Illumina MiSeq Sequencing and the Processing of Raw Data
2.4. Data Analysis
3. Results
3.1. Characteristics of HMs in Different Utilized Types of Wetland Soils
3.2. Assessment of HM Pollution in AS, NWS, and RWS
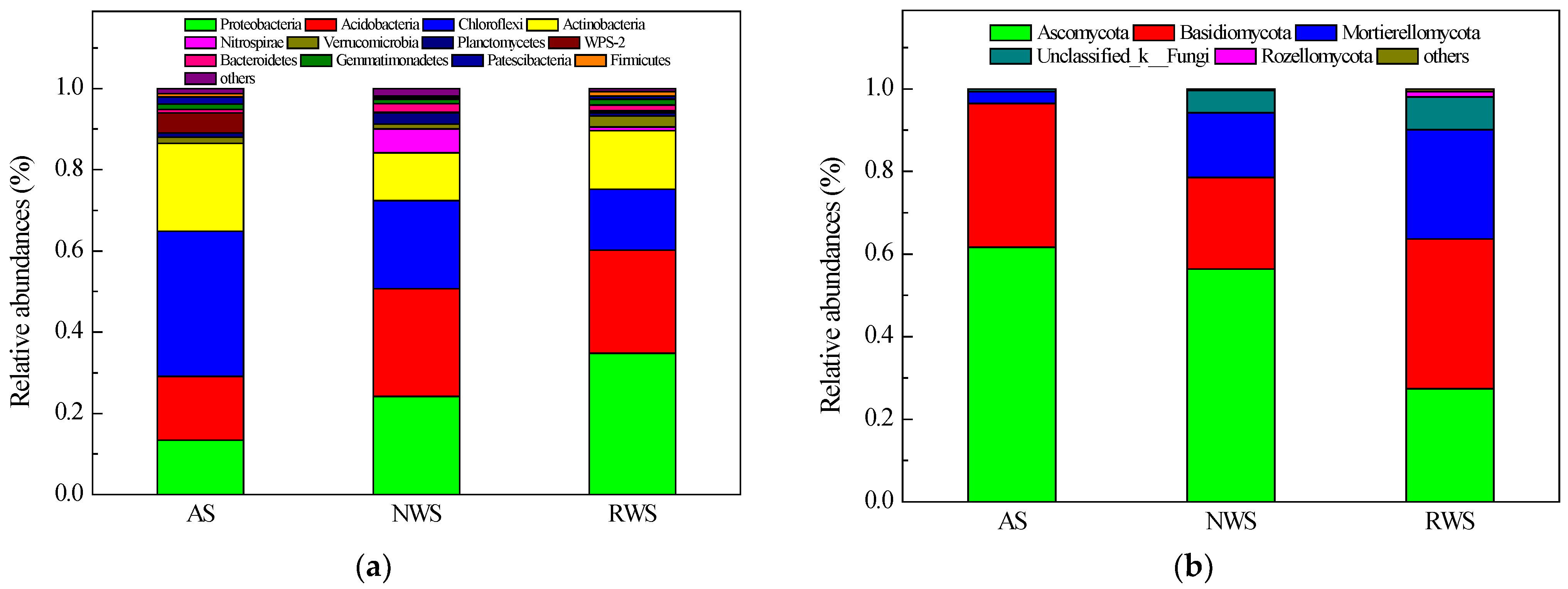
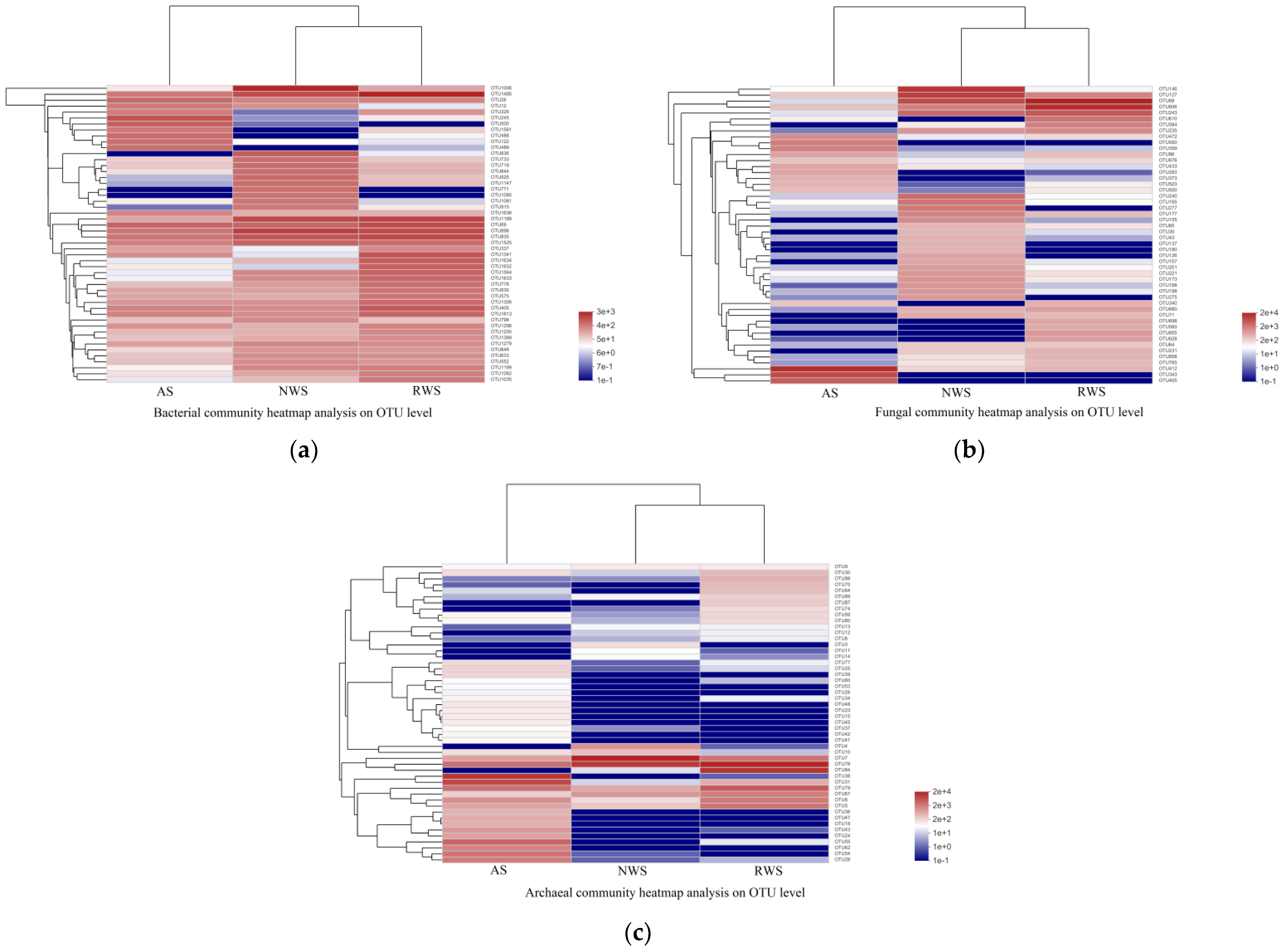
3.3. Analyses of Microbial Community Compositions in AS, NWS, and RWS
3.4. Co-Occurrence Network of Soil Microbiomes
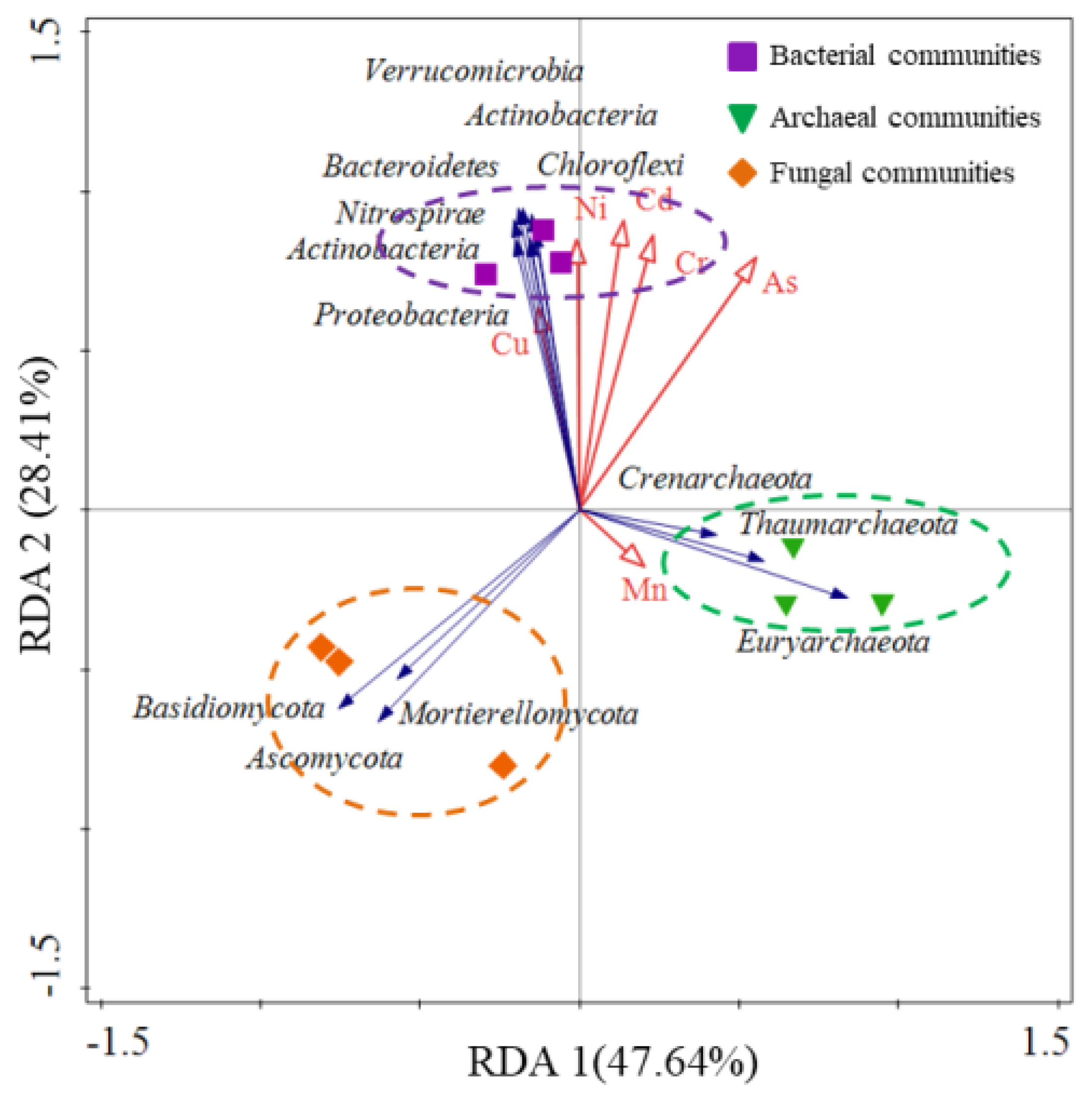
3.5. Relationships between Microbial Community Compositions and HMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, D.; Wang, G.; Li, X.; Wang, S.; Zhao, Y. Pollution, sources and environmental risk assessment of heavy metals in the surface AMD water, sediments and surface soils around unexploited Rona Cu deposit, Tibet, China. Chemosphere 2020, 248, 125988. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Akinola, M.O.; Njoku, K.L.; Ekeifo, B.E. Determination of lead, cadmium and chromium in the tissue of an economically important plant grown around a textile industry at Ibeshe, Ikorodu area of Lagos State, Nigeria. Adv. Environ. Biol. 2008, 2, 25–31. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment. Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Bungau, S.; Tit, D.M.; Fodor, K.; Cioca, G.; Agop, M.; Iovan, C.; Cseppento, D.C.N.; Bumbu, A.; Bustea, C. Aspects regarding the pharmaceutical waste management in Romania. Sustainability 2018, 10, 2788. [Google Scholar] [CrossRef]
- Samuel, A.D.; Brejea, R.; Domuta, C.; Bungau, S.; Cenusa, N.; Tit, D.M. Enzymatic indicators of soil quality. J. Environ. Prot. Ecol. 2017, 18, 871–878. [Google Scholar]
- Samuel, A.D.; Tit, D.M.; Melinte, C.E.; Iovan, C.; Purza, L.; Gitea, M.; Bungau, S. Enzymological and physicochemical evaluation of the effects of soil management practices. Rev. Chim. 2017, 68, 2243–2247. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, C.; Hong, X.; Kang, G.; Qin, M.; Yang, D.; Pang, B.; Li, Y.; He, J.; Dick, R.P. Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci. Total Environ. 2018, 633, 1136–1147. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Uddin, M.; Zaman, S.; Begum, Y.; Ashraf, G.M.; Bin-Jumah, M.N.; Bungau, S.G.; Mousa, S.A.; Abdel-Daim, M.M. Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 2021, 58, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–790. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Huang, J.; Wang, H.; Tang, D. Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. Res. 2020, 27, 20215–20226. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Al-Mamun, M.H.; Islam, S.M.S. Sources and ecological risks of heavy metals in soils under different land uses in Bangladesh. Pedosphere 2019, 29, 665–675. [Google Scholar] [CrossRef]
- Bai, J.; Yang, Z.; Cui, B.; Gao, H.; Ding, Q. Some heavy metals distribution in wetland soils under different land use types along a typical plateau lake, China. Soil Tillage Res. 2010, 106, 344–348. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, M.; Zuo, X.; Yao, Y. Comparative investigation of bacterial, fungal, and archaeal community structures in soils in a typical oilfield in Jianghan, China. Arch. Environ. Contam. Toxicol. 2017, 72, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xue, K.; Wei, P.; Jia, Y.; Zhang, Y.; Chen, S. Soil microbial distribution and assembly are related to vegetation biomass in the alpine permafrost regions of the Qinghai-Tibet Plateau. Sci. Total Environ. 2022, 834, 155259. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gong, H.; Guo, X. Rhizosphere bacterial community of Typha angustifolia L. and water quality in a river wetland supplied with reclaimed water. Appl. Microbiol. Biotechnol. 2015, 99, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Abd Hesham, E.L.; Qiao, M.; Rehman, S.; He, Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef]
- Luo, L.Y.; Xie, L.L.; Jin, D.C.; Mi, B.B.; Wang, D.H.; Li, X.F.; Dai, X.Z.; Zou, X.X.; Zhang, Z.; Ma, Y.Q.; et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Appl. Soil Ecol. 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Wu, H.; Guan, Q.; Ma, H.; Xue, Z.; Yang, M.; Batzer, D.P. Effects of saline conditions and hydrologic permanence on snail assemblages in wetlands of Northeastern China. Ecol. Indic. 2019, 96, 620–627. [Google Scholar] [CrossRef]
- Wang, Z.; Song, K.; Ma, W.; Ren, C.; Zhang, B.; Liu, D.; Chen, J.M.; Song, C. Loss and fragmentation of marshes in the Sanjiang Plain, Northeast China, 1954–2005. Wetlands 2011, 31, 945–954. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wu, Y.; Song, Y.; Liu, R.; Cao, Z.; Cui, Y. Shifts of the nirS and nirK denitrifiers in different land use types and seasons in the Sanjiang Plain, China. J. Basic Microbiol. 2019, 59, 1040–1048. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Guo, J.; Cui, Y. Effects of Cr (VI)-reducing bacteria on the behaviour of Cr (VI) adsorption by goethite and haematite: Speciation and distribution. J. Soils Sediments 2020, 20, 3733–3741. [Google Scholar] [CrossRef]
- Li, F.; Shao, L.; Chen, Y.; Zhang, G.; Nie, Q.; Jin, Z. Leaching characteristic of potentially toxic metals of artificial soil made from municipal sludge compost. Chemosphere 2021, 270, 128632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Q.; Yang, J.; Ma, J.; Chen, G.; Chen, T.; Zhu, G.; Wang, J.; Zhang, G.; Wang, X.; et al. Comparison of chelates for enhancing Ricinus communis L. phytoremediation of Cd and Pb contaminated soil. Ecotoxicol. Environ. Saf. 2016, 133, 57–62. [Google Scholar] [CrossRef]
- Barkett, M.O.; Akün, E. Heavy metal contents of contaminated soils and ecological risk assessment in abandoned copper mine harbor in Yedidalga, Northern Cyprus. Environ. Earth Sci. 2018, 77, 378. [Google Scholar] [CrossRef]
- Wang, F.; Guan, Q.; Tian, J.; Lin, J.; Yang, Y.; Yang, L.; Pan, N. Contamination characteristics, source apportionment, and health risk assessment of heavy metals in agricultural soil in the Hexi Corridor. Catena 2020, 191, 104573. [Google Scholar] [CrossRef]
- CNEMC (China National Environmental Monitoring Center). The Background Concentrations of Soil Elements of China; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Yang, H.; Wang, F.; Yu, J.; Huang, K.; Zhang, H.; Fu, Z. An improved weighted index for the assessment of heavy metal pollution in soils in Zhejiang, China. Environ. Res. 2021, 192, 110246. [Google Scholar] [CrossRef]
- Chi, Q.; Yan, M. Handbook on Applied Geochemistry Elements Abundance Data; Geology Publishing House: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zhang, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Liang, Y.; Su, X.; Song, T.; Zhou, T.; He, J. Geo-statistics Spatial Characteristics and Source Analysis of Soil Heavy Metals Elements: A Case Study of One Farming Area in Sanjiang Plain. Sci. Technol. Eng. 2017, 17, 1671–1815. (In Chinese) [Google Scholar]
- Wang, C.; Hou, X.; Islam, Z.U.; Zhang, Z.; Zhu, B.; Yang, T. Driving Factors of Microbial Community Abundance and Structure in Typical Forest Soils of Sanjiang Plain, Northeast China. Sustainability 2022, 14, 8040. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Z.; Guo, Y.; Yang, G.; Zhao, F.; Wei, G.; Han, X.; Feng, L.; Feng, Y.; Ren, G. Contrasting patterns of microbial community and enzyme activity between rhizosphere and bulk soil along an elevation gradient. Catena 2021, 196, 104921. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S. Identifying the biogeographic patterns of rare and abundant bacterial communities using different primer sets on the loess plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef]
- Yi, X.; Yang, Y.; Yuan, H.; Chen, Z.; Duan, G.; Zhu, Y. Coupling metabolisms of arsenic and iron with humic substances through microorganisms in paddy soil. J. Hazard. Mater. 2019, 373, 591–599. [Google Scholar] [CrossRef]
- Chang, J.; Duan, Y.; Dong, J.; Shen, S.; Si, G.; He, F.; Yang, Q.; Chen, J. Bioremediation of Hg-contaminated soil by combining a novel Hg-volatilizing Lecythophora sp. fungus, DC-F1, with biochar: Performance and the response of soil fungal community. Sci. Total Environ. 2019, 671, 676–684. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.M.; Zhao, G. Thiocyanate increases the nitrous oxide formation process through modifying the soil bacterial community. J. Sci. Food Agric. 2022, 102, 2321–2329. [Google Scholar] [CrossRef]
- Zheng, H.; Wei, P.; Zhang, G.; Xu, W.; Li, Y. The impact of different Saccharomyces cerevisiae strains on microbial composition and quality of Chinese rice wine fermentations. Yeast 2021, 38, 147–156. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, W.; Zu, X.; Xie, H.; Li, H.; Li, Y.; Zhang, W. Integrative Metabolic and Microbial Profiling on Patients with Spleen-yang-deficiency Syndrome. Sci. Rep. 2018, 8, 6619. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, H.; Liu, D.; Han, G.; Zhao, P.; Kang, Y. Crab Bioturbation Significantly Alters Sediment Microbial Composition and Function in an Intertidal Marsh. Estuar. Coast. Shelf Sci. 2021, 249, 107116. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef] [PubMed]
- GB15618-1995; Environmental Quality Standard for Soils. Standards Press of China: Beijing, China, 1997.
- CCME (Canadian Council of Ministers of the Environment). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; National Guidelines and Standards Office: Quebec, QC, Canada, 2007. [Google Scholar]
- Yan, C.; Zhuang, T.; Bai, J.; Wen, X.; Lu, Q.; Zhang, L. Assessment of As, Cd, Zn, Cu and Pb pollution and toxicity in river wetland sediments and artificial wetland soils affected by urbanization in a Chinese delta. Wetlands 2020, 40, 2799–2809. [Google Scholar] [CrossRef]
- Bai, J.; Huang, L.; Yan, D.; Wang, Q.; Gao, H.; Xiao, R.; Huang, C. Contamination characteristics of heavy metals in wetland soils along a tidal ditch of the Yellow River Estuary, China. Stoch. Environ. Res. Risk Assess. 2011, 25, 671–676. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, D.; Xue, Z.; Wu, H.; Jiang, M. Identification of anthropogenic contributions to heavy metals in wetland soils of the Karuola Glacier in the Qinghai-Tibetan Plateau. Ecol. Indic. 2019, 98, 678–685. [Google Scholar] [CrossRef]
- Yang, P.; Drohan, P.J.; Yang, M.; Li, H. Spatial variability of heavy metal ecological risk in urban soils from Linfen, China. Catena 2020, 190, 104554. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221, 82–90. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Ma, F.; You, Y.; Ju, C. Multi-level methods to quantify risk assessment, source apportionment and identifying key risk areas of soil toxic elements in Ashi River watershed, China. Sci. Total Environ. 2021, 800, 149385. [Google Scholar] [CrossRef]
- Shen, F.; Liao, R.; Ali, A.; Mahar, A.; Guo, D.; Li, R.; Sun, X.; Kumar, M. Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County, China. Ecotoxicol. Environ. Saf. 2017, 139, 254–262. [Google Scholar] [CrossRef]
- Xiao, H.; Shahab, A.; Li, J.; Xi, B.; Sun, X.; He, H.; Yu, G. Distribution, ecological risk assessment and source identification of heavy metals in surface sediments of Huixian karst wetland, China. Ecotoxicol. Environ. Saf. 2019, 185, 109700. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, Y.; Li, F.; Yin, X.; Li, R.; Wu, Z.; Liang, X.; Li, Z. Phosphate fertilizers facilitated the Cd contaminated soil remediation by sepiolite: Cd mobilization, plant toxicity, and soil microbial community. Ecotoxicol. Environ. Saf. 2022, 234, 113388. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; Chen, L.; Wang, D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ. Pollut. 2019, 252, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Hintemann, T.; Kramarewa, T.; Katayama, A.; Yasuta, T.; Marschner, P.; Kandeler, E. Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol. Biochem. 2006, 38, 1430–1437. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Yang, Y.; Zhong, X.; Lv, J. Dynamics of fungal and bacterial communities in different types of soil ageing with different dosages of cadmium. Ecotoxicol. Environ. Saf. 2022, 242, 113860. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chou, M.; Jean, J.; Yang, H.; Kim, P.J. Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pteris vittata. J. Hazard. Mater. 2017, 325, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tang, S.; Shi, X.; Liang, X.; Liu, K.; Huang, Y.; Li, Y. Phytoextraction of metal (loid) s from contaminated soils by six plant species: A field study. Sci. Total Environ. 2022, 804, 150282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, X.; Ling, Q.; Li, S.; Wei, J.; Xin, M.; Xie, D.; Chen, X.; Liu, K.; Yu, F. Bacterial extracellular polymeric substances: Impact on soil microbial community composition and their potential role in heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 2022, 240, 113701. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Zhu, X.; Chai, J.; Ji, X. Ecological effects of heavy metal pollution on soil microbial community structure and diversity on both sides of a river around a mining area. Int. J. Environ. Res. Public Health 2020, 17, 5680. [Google Scholar] [CrossRef] [PubMed]
- Mundra, S.; Kjønaas, O.J.; Morgado, L.N.; Krabberød, A.K.; Ransedokken, Y.; Kauserud, H. Soil depth matters: Shift in composition and inter-kingdom co-occurrence patterns of microorganisms in forest soils. FEMS Microbiol. Ecol. 2021, 97, fiab022. [Google Scholar] [CrossRef]
- Ye, Z.; Li, J.; Wang, J.; Zhang, C.; Liu, G.; Dong, Q. Diversity and co-occurrence network modularization of bacterial communities determine soil fertility and crop yields in arid fertigation agroecosystems. Biol. Fertil. Soils 2021, 57, 809–824. [Google Scholar] [CrossRef]
- Siles, J.A.; García-Sánchez, M.; Gómez-Brandón, M. Studying microbial communities through co-occurrence network analyses during processes of waste treatment and in organically amended soils: A review. Microorganisms 2021, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; Heijden, M.G. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, J.; Huo, Y.; Zhao, X.; Xue, L. Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China. Sci. Total Environ. 2021, 752, 141827. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Si, Y.; Xing, Y.; Li, Y. Illumina MiSeq sequencing investigation on the contrasting soil bacterial community structures in different iron mining areas. Environ. Sci. Pollut. Res. 2015, 22, 10788–10799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, X.; Zhao, Y.; Zhou, J.; Sun, B.; Liang, Y. Patterns of microbial arsenic detoxification genes in low-arsenic continental paddy soils. Environ. Res. 2021, 201, 111584. [Google Scholar] [CrossRef]
- Sheik, C.S.; Mitchell, T.W.; Rizvi, F.Z.; Rehman, Y.; Faisal, M.; Hasnain, S.; McInerney, M.J.; Krumholz, L.R. Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS ONE 2012, 7, e40059. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Xiao, T.; Krumins, V.; Wang, Q.; Häggblom, M.; Dong, Y.; Tang, S.; Hu, M.; Li, B.; et al. Response of soil microbial communities to elevated antimony and arsenic contamination indicates the relationship between the innate microbiota and contaminant fractions. Environ. Sci. Technol. 2017, 51, 9165–9175. [Google Scholar] [CrossRef]



| Grades | Igeo |
|---|---|
| Uncontaminated | Igeo ≤ 0 |
| Uncontaminated to moderately contaminated | 0 < Igeo ≤ 1 |
| Moderately contaminated | 1 < Igeo ≤ 2 |
| Moderately to heavily contaminated | 2 < Igeo ≤ 3 |
| Heavily contaminated | 3 < Igeo ≤ 4 |
| Heavily to extremely contaminated | 4 < Igeo ≤ 5 |
| Extremely contaminated | Igeo > 5 |
| HMs | As | Cd | Cr | Cu | Ni | Mn |
|---|---|---|---|---|---|---|
| Si | 11 | 0.091 | 70 | 24 | 28 | 600 |
| Ti | 10 | 30 | 2 | 5 | 5 | 1 |
| Grades | ERI | Comprehensive ERI |
|---|---|---|
| Slight risk | <40 | <150 |
| Mild risk | 40–80 | 150–300 |
| Moderate risk | 80–160 | 300–600 |
| Severe risk | 160–320 | >600 |
| Extremely severe risk | >320 |
| Sampling Sites (HMs) | Min | Max | Mean ± SD | Background Values in Sanjiang Plain [35] (mg/kg) | Grade II Values of EQSS 1 | CCME 2 |
|---|---|---|---|---|---|---|
| AS (As) | 34.59 | 41.05 | 38.1 ± 3.3 b | 11 | 40 | 12 |
| AS (Cd) | 0.51 | 0.72 | 0.6 ± 0.1 b | 0.091 | 0.3 | 1.4 |
| AS (Cr) | 20.55 | 28.17 | 24.8 ± 3.9 b | 70 | 150 | 64 |
| AS (Cu) | 8.34 | 12.04 | 10.4 ± 1.9 a | 24 | 50 | 63 |
| AS (Ni) | 12.78 | 18.03 | 15.4 ± 2.6 b | 28 | 40 | 50 |
| AS (Mn) | 341.1 | 694 | 543.6 ± 182.1 a | 600 | - | - |
| NWS (As) | 18.54 | 22.19 | 20.5 ± 1.8 a | 11 | 40 | 12 |
| NWS (Cd) | 0.18 | 0.36 | 0.3 ± 0.1 a | 0.091 | 0.3 | 1.4 |
| NWS (Cr) | 12.02 | 15.5 | 13.6 ± 1.8 a | 70 | 150 | 64 |
| NWS (Cu) | 6.61 | 9.61 | 8.2 ± 1.5 a | 24 | 50 | 63 |
| NWS (Ni) | 8.28 | 11.17 | 9.6 ± 1.5 a | 28 | 40 | 50 |
| NWS (Mn) | 486.1 | 886.7 | 639.8 ± 215.9 a | 600 | - | - |
| RWS (As) | 30.7 | 37.15 | 33.1 ± 3.5 b | 11 | 40 | 12 |
| RWS (Cd) | 0.28 | 0.45 | 0.4 ± 0.1 a | 0.091 | 0.3 | 1.4 |
| RWS (Cr) | 17.22 | 22.84 | 19.5 ± 2.9 ab | 70 | 150 | 64 |
| RWS (Cu) | 7.21 | 11.18 | 8.7 ± 2.2 a | 24 | 50 | 63 |
| RWS (Ni) | 9.89 | 12.08 | 11.1 ± 1.2 a | 28 | 40 | 50 |
| RWS (Mn) | 547.3 | 985.2 | 737.6 ± 224.5 a | 600 | - | - |
| HMs | As | Cd | Cr | Cu | Ni | Mn |
|---|---|---|---|---|---|---|
| As | 1 | |||||
| Cd | 0.848 ** | 1 | ||||
| Cr | 0.714 * | 0.742 * | 1 | |||
| Cu | 0.29 | 0.624 | 0.745 * | 1 | ||
| Ni | 0.675 * | 0.628 | 0.652 | 0.284 | 1 | |
| Mn | −0.06 | 0.048 | −0.171 | 0.25 | −0.21 | 1 |
| Risk Index | Igeo (AS) | Igeo (NWS) | Igeo (RWS) | PLI (AS) | PLI (NWS) | PLI (RWS) | ERI (AS) | ERI (NWS) | ERI (RWS) |
|---|---|---|---|---|---|---|---|---|---|
| As | 1.8 | 0.9 | 1.59 | 3.46 | 1.9 | 3 | 34.66 | 18.66 | 30.08 |
| Cd | 2.22 | 1.22 | 1.63 | 6.7 | 2.64 | 4.07 | 202.2 | 82.42 | 123.08 |
| Cr | −1.84 | −2.74 | −2.18 | 0.35 | 0.19 | 0.28 | 0.71 | 0.39 | 0.56 |
| Cu | −1.51 | −1.89 | −1.79 | 0.43 | 0.34 | 0.36 | 2.16 | 1.71 | 1.81 |
| Ni | −1.15 | −1.84 | −1.64 | 0.54 | 0.34 | 0.4 | 2.74 | 1.71 | 1.99 |
| Mn | −1.56 | −1.32 | −1.12 | 0.87 | 1.03 | 1.19 | 0.91 | 1.07 | 1.23 |
| Comprehensive ERI | 243.38 | 105.96 | 158.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhu, B.; Guo, Y.; Tian, S.; Zhang, Z.; Hou, X. Assessment of the Pollution of Soil Heavy Metal(loid)s and Its Relation with Soil Microorganisms in Wetland Soils. Sustainability 2022, 14, 12164. https://doi.org/10.3390/su141912164
Wang C, Zhu B, Guo Y, Tian S, Zhang Z, Hou X. Assessment of the Pollution of Soil Heavy Metal(loid)s and Its Relation with Soil Microorganisms in Wetland Soils. Sustainability. 2022; 14(19):12164. https://doi.org/10.3390/su141912164
Chicago/Turabian StyleWang, Chunyong, Bo Zhu, Yitong Guo, Shasha Tian, Zhenbin Zhang, and Xintong Hou. 2022. "Assessment of the Pollution of Soil Heavy Metal(loid)s and Its Relation with Soil Microorganisms in Wetland Soils" Sustainability 14, no. 19: 12164. https://doi.org/10.3390/su141912164
APA StyleWang, C., Zhu, B., Guo, Y., Tian, S., Zhang, Z., & Hou, X. (2022). Assessment of the Pollution of Soil Heavy Metal(loid)s and Its Relation with Soil Microorganisms in Wetland Soils. Sustainability, 14(19), 12164. https://doi.org/10.3390/su141912164




