The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation
Abstract
:1. Introduction
2. Overview of Hydrogen and Ammonia Energy Development
2.1. Overview of Hydrogen Energy Development
2.2. Overview of Ammonia Energy Development
3. Production, Storage and Transportation of Hydrogen and Ammonia Energy
3.1. Production, Storage and Transportation of Hydrogen Energy
3.1.1. Production of Hydrogen
3.1.2. Storage of Hydrogen
3.1.3. Transportation of Hydrogen
3.2. Production, Storage and Transportation of Ammonia Energy
3.2.1. Production of Ammonia
- (1)
- H–B process is the synthesis of ammonia by hydrogen and nitrogen under the action of a catalyst at high temperature and high pressure. The H–B process is employed as the mainstream ammonia production method today. Many carbon emissions will be generated in the synthesis process due to the generation mode of raw material hydrogen (gray hydrogen), so it is also called gray ammonia. Ammonia production currently accounts for 1.0% of global greenhouse gas emissions [50]. Similar to the classification of hydrogen energy, when blue hydrogen introduced into carbon capture technology (CCUS) is used to produce ammonia, the product is called blue ammonia;
- (2)
- In essence, hydrogen production from renewable energy still adopts the H–B process. Because the hydrogen at source is derived from renewable production (green hydrogen), the ammonia produced in this way is also called green ammonia. It is worth mentioning that green ammonia provides more clean options on the supply side as a fuel and has great potential as a fixed carrier and hydrogen transport carrier of renewable energy (wind energy, solar energy, etc.);
- (3)
- Electrochemical ammonia synthesis uses electrochemical means to synthesize ammonia from nitrogen molecules and hydrogen in H2O through a redox medium. This method has lower requirements for the purity of raw materials than other methods, which has a high intermittence tolerance to the reaction. Therefore, the electrochemical synthesis method is restricted when taking the total carbon and nitrogen oxide emissions cycle into consideration.
| Mode of Production | Basic Principles | Characteristic |
|---|---|---|
| Haber–Bosch process | Ammonia is synthesized by hydrogen and nitrogen under catalyst (iron), high temperature (500 °C) and high pressure (20–50 mpa). The hydrogen source is a fossil fuel. | The mainstream ammonia production method has large output and is mainly used for fertilizers and drugs; High purity requirements for hydrogen and nitrogen materials, significant factory investment and high carbon emissions in the production process. |
| Ammonia production from renewable energy | Greener Haber–Bosch process: synthesis of ammonia from hydrogen and nitrogen generated from renewable energy. | The investment cost can be reduced by transforming the equipment for the H–B process; Low carbon emissions; The cost of electrolysis is high, and the form of energy storage needs to be considered. |
| Electrochemical ammonia synthesis | Ammonia is electrochemically generated from nitrogen and water through a REDOX medium. | The purity of raw materials is not high; Theoretically, energy efficiency is expected to be high; The response can be interrupted/intermittent. In the experimental stage, easy to generate nitrogen oxides, resulting in environmental severe pollution. |
3.2.2. Storage and Transportation of Ammonia
4. Road Transportation Application form of Hydrogen Energy
4.1. Hydrogen Fuel Engine
4.2. Hydrogen Fuel Cell
5. Road Transportation Application Form of Ammonia Energy
5.1. Ammonia Fuel Engine
- (1)
- SI Engine
- (2)
- CI engine
5.2. Ammonia Fuel Cell
- (1)
- Indirect ammonia fuel cell: The indirect ammonia fuel cell is to decompose ammonia into nitrogen and hydrogen through the electrochemical method or catalytic pyrolysis with catalyst, and then uses the hydrogen.
- (2)
- Direct ammonia fuel cell: At present, direct ammonia fuel cells can be classified into three types according to different electrolytes: alkaline fuel cell (AFC), alkaline membrane fuel cell (AMFC) and solid oxide fuel cell (SOFC).
6. Road Transportation Application Mode of Hydrogen and Ammonia
6.1. Application Mode of Hydrogen Energy Highway
6.1.1. Application Mode of Hydrogen Fuel Engine
6.1.2. Application Mode of Hydrogen Fuel Cell
6.2. Ammonia Energy Highway Application Mode
6.2.1. Application Mode of Ammonia Fuel Engine
- (1)
- Using ammonia as auxiliary fuel can improve the working efficiency of traditional engines and effectively reduce NOx emission. Ammonia can also be used as the primary fuel. Through the hydrogen produced by the early decomposition of ammonia, the combustion speed can be accelerated, and the availability of an ammonia fuel engine can be improved.
- (2)
- Aiming at the incomplete combustion of ammonia that cannot be ignored, how to deal with the tail gas has also become a research hotspot. The unified way is to install post-treatment devices for engines [86].
6.2.2. Application Mode of Ammonia Fuel Cell
- (1)
- Solid Oxide Cells (SOFC)
- (2)
- Direct ammonia fuel cells (DAFC)
7. Challenges and Suggestions
7.1. Opportunities and Challenges
7.1.1. Opportunity—Joint Development Trend of Hydrogen and Ammonia
7.1.2. Challenges
- (1)
- Security. Security is an inevitable problem for emerging energy sources. The flammable and explosive nature of hydrogen energy poses an obstacle to its widespread utilization. Although the liquid ammonia transportation technology is mature, problems such as fluid ammonia leakage also need to be solved. The volatility and toxicity of ammonia also bring certain risks to energy utilization;
- (2)
- High cost. Due to the limitations of transportation and storage technology and the high price of fuel cells, promoting the commercialization of hydrogen energy is still a challenge. Although ammonia energy has formed a particular energy economy system, its application scale as road traffic energy is small and is not as economical as gasoline or diesel. It is also a challenging issue to promote utilization commercially.
7.2. Policy Recommendations
- (1)
- Formulate supporting policies to promote the development of hydrogen and ammonia energy technology
- (2)
- Developing hydrogen literacy to build acceptance
- (3)
- Emphasizing personnel training and deepening international cooperation
8. Summary and Conclusions
- (1)
- Hydrogen can provide powerful traction energy and has excellent performance in long-distance road transportation. The application research of hydrogen fuel cells is relatively mature, and the road vehicle carrying hydrogen fuel cells is gradually popularized and has certain commercial advantages;
- (2)
- As an auxiliary fuel in road transportation, ammonia has significant benefits in ammonia fuel engines. The participation of ammonia in combustion reactions can improve engine efficiency and reduce NOx emission to a certain extent;
- (3)
- The technology development of hydrogen and ammonia also has limitations in storage and transferring because of their performance and characteristics. Hydrogen and ammonia have different advantages in their own development pathways. Joint action can support each other’s industries, make up for insufficient expenditure, broaden application scenarios and improve the penetration of clean energy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IEA | International Energy Agency |
| CNG | Compressed natural gas |
| LPG | Liquefied petroleum gas |
| EVs | Electric vehicles |
| R&D | Research and Development |
| DOE | Department of Energy |
| CCS | Carbon capture and storage |
| CCUS | Carbon capture, utilization, and storage |
| H–B process | Haber–Bosch process |
| DI | Direct injection |
| PFI | Port fuel injection |
| PEMFC | Proton exchange membrane fuel cell |
| SOFC | Solid oxide fuel cell |
| PAFC | Phosphoric acid fuel cell |
| AFC | Alkaline fuel cell |
| SI | Spark-ignition |
| CI | Compression ignition |
| AMFC | Alkaline membrane fuel cell |
| MFC | Microbial fuel cell |
| EGR | Exhaust gas recirculation |
| TCR | Thermochemical recovery |
| RES | Renewable energy sources |
| DAFC | Direct ammonia fuel cells |
| DSC | Dissociation and separation device |
References
- Marrero, Á.S.; Marrero, G.A.; González, R.M.; Rodríguez-López, J. Convergence in road transport CO2 emissions in Europe. Energy Econ. 2021, 99, 105322. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Abdalla, A.M.; Radenahmad, N.; Zakaria, A.K.M.; Zaini, J.H.; Rahman, S.M.H.; Eriksson, S.G.; Irvine, J.T.S.; Azad, A.K. Highly dense and chemically stable proton conducting electrolyte sintered at 1200 °C. Int. J. Hydrog. Energy 2018, 43, 894–907. [Google Scholar] [CrossRef]
- Candelaresi, D.; Valente, A.; Iribarren, D.; Dufour, J.; Spazzafumo, G. Comparative life cycle assessment of hydrogen-fuelled passenger cars. Int. J. Hydrog. Energy 2021, 46, 35961–35973. [Google Scholar] [CrossRef]
- Chapman, A.; Nguyen, D.H.; Farabi-As, H.; Itaoka, K.; Hirose, K.; Fujii, Y. Hydrogen penetration and fuel cell vehicle deployment in the carbon constrained future energy system. arXiv 2008, arXiv:2008.13414. [Google Scholar] [CrossRef]
- Cuevas, F.; Zhang, J.X.; Latroche, M. The Vision of France, Germany, and the European Union on Future Hydrogen Energy Research and Innovation. Engineering 2021, 7, 715–718. [Google Scholar] [CrossRef]
- Ritchie, H. Sector by Sector: Where Do Global Greenhouse Gas Emissions Come From? Our World Data. Available online: https://ourworldindata.org/ghg-emissions-by-sector (accessed on 18 September 2020).
- Plötz, P. Hydrogen technology is unlikely to play a major role in sustainable road transport. Nat. Electron. 2022, 5, 8–10. [Google Scholar] [CrossRef]
- Kim, G.; Ghorpade, R.; Vasu, S.S. Laminar Flame Speed Measurements of Hydrogen/Natural Gas Mixtures for Gas Turbine Applications. Turbo Expo: Power for Land, Sea and Air. Am. Soc. Mech. Eng. 2022, 6, 84997. [Google Scholar]
- Maria, R.L.; Johannes, S.; Rolf, A.; Görner, K. Current Legislative Framework for Green Hydrogen Production by Electrolysis Plants in Germany. Energies 2022, 15, 1786. [Google Scholar]
- Cheng, W.; Lee, S. How Green Are the National Hydrogen Strategies? Sustainability 2022, 14, 1930. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, S.; Kim, H.M. A Study on the Hydrogen Economic Law for the Realization of Hydrogen Society in Korea. Leg. Essays 2020, 46, 1–30. [Google Scholar]
- Li, Y.; Taghizadeh-Hesary, F. The economic feasibility of green hydrogen and fuel cell electric vehicles for road transport in China. Energy Policy 2022, 160, 112703. [Google Scholar] [CrossRef]
- China Hydrogen Energy Alliance. China Hydrogen Energy and Fuel Cell Industry Manual (2020). 2021. Available online: https://www.dx2025.com/archives/137687.html (accessed on 25 July 2022).
- International Energy Network, International Energy Network. Available online: https://h2.in-en.com/html/h2-2415221.shtml (accessed on 10 August 2022).
- Energy Bureau of Shandong Province. The 14th Five-Year Plan of Energy Science and Technology Innovation in Shandong Province. Available online: http://nyj.shandong.gov.cn/art/2022/1/5/art_100399_10291073.html (accessed on 5 January 2022).
- People’s Government of Gansu Province. The 14th Five-Year Energy Development Plan of Gansu Province. 2022. Available online: http://gsnea.cn/page211?article_id=325 (accessed on 20 February 2022).
- Development and Reform Commission of Ningxia Hui Autonomous Region. Implementation Opinions of The People’s Government of the Autonomous Region on Accelerating the Establishment and Improvement of Green, Low-Carbon and Circular Development Economic System. Available online: http://fzggw.nx.gov.cn/zcgh/zzqzc/202201/t20220120_3290351.html (accessed on 20 January 2022).
- Liaoning Provincial People’s Government. Liaoning Provincial “14th Five-Year” Ecological and Economic Development Plan. Available online: http://www.ln.gov.cn/zwgkx/zfwj/szfbgtwj/zfwj2011_153687/202201/t20220114_4491486.html (accessed on 3 January 2022).
- Tianjin Municipal People’s Government. Tianjin Eco-Environmental Protection “14th Five-Year Plan”. Available online: http://www.tj.gov.cn/zwgk/zcjd/tjzc/202202/t20220210_5801253.html (accessed on 7 February 2022).
- People’s Government of Guangxi Zhuang Autonomous Region. The 14th Five-Year Plan for the Development of Guangxi New Energy Automobile Industry. Available online: http://www.gxzf.gov.cn/zcjd/t11899590.shtml (accessed on 14 February 2022).
- National Development and Reform Commission, National Energy Administration. Opinions on Improving the Energy, Green and Low-Carbon Transition Institutions and Reform Measures. Available online: http://zfxxgk.nea.gov.cn/2022-01/30/c_1310464313.htm (accessed on 30 January 2022).
- Inner Mongolia Autonomous Region Energy Bureau. Inner Mongolia Autonomous Region “14th Five-Year” Hydrogen Energy Development Plan. Available online: http://nyj.nmg.gov.cn/zwgk/zfxxgkzl/fdzdgknr/tzgg_16482/tz_16483/202202/t20220228_2010712.html (accessed on 28 February 2022).
- Innovation Promotion Program Energy Carriers. SIP 2016. Available online: https://www.jst.go.jp/sip/pdf/SIP_energycarriers2016_en.pdf (accessed on 25 July 2022).
- Opportunities for Australia from Hydrogen Exports. 2018. Available online: https://arena.gov.au/assets/2018/08/opportunities-for-australia-from-hydrogen-exports.pdf (accessed on 25 July 2022).
- Atchion, J. Blue Ammonia in the Northern Territory & Wyoming, 2022 Ammonia Energy Association. Available online: https://www.ammoniaenergy.org/articles/blue-ammonia-in-the-northern-territory-wyoming/ (accessed on 25 July 2022).
- Ammonia—A Potential Key Player in Energy Storage. COUDIS EU Research Results. 2020. Available online: https://cordis.europa.eu/project/id/862482 (accessed on 25 July 2022).
- Radwan, R. New Agreement for Green Hydrogen Production Plant in NEOM, Arab News. 2020. Available online: https://www.arabnews.com/node/1701311/saudi-arabia (accessed on 25 July 2022).
- Saudi Aramco to Explore Carbon-Free Ammonia Production in the Kingdom. Aramco. Available online: https://japan.aramco.com/en/news-media/news/2019/20190710_ammonia# (accessed on 10 July 2019).
- Lee, S. Hyundai Heavy Industry and Saudi Aramco conclude “Hydrogen Ammonia Alliance” The Dong-a Ilbo. Available online: https://www.donga.com/cn/article/all/20210304/2476453/1 (accessed on 4 March 2021).
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrog. Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Veysey, J. Seasonal hydrogen storage for sustainable renewable energy integration in the electricity sector: A case study of Finland. J. Energy Storage 2021, 44, 103474. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Research and Innovation. Strategic Research and Innovation Agenda (SRIA) of the European Open Science Cloud (EOSC). 2022. Available online: https://data.europa.eu/doi/10.2777/935288 (accessed on 25 July 2022).
- Alvarez, R.A.; Zavala-Araiza, D.; Lyon, D.R.; Allen, D.T.; Barkley, Z.R.; Brandt, A.R.; Davis, K.J.; Herndon, S.C.; Jacob, D.J.; Karion, A.; et al. Assessment of methane emissions from the U.S. oil and gas supply chain. Science 2018, 361, 186e8. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- O’Neil, S. Unlocking the Potential of Hydrogen Energy Storage, Fuel Cell & Hydrogen Energy Association. Available online: https://www.fchea.org/in-transition/2019/7/22/unlocking-the-potential-of-hydrogen-energy-storage (accessed on 22 July 2019).
- World Energy Council, Hydrogen on the Horizon: Hydrogen Demand and Cost Dynamics, World Energy Council Working Paper. 2021. Available online: https://www.worldenergy.org/publications/entry/working-paper-hydrogen-demand-and-cost-dynamics (accessed on 25 July 2022).
- Muhammad, R.U. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy 2022, 167, 112743. [Google Scholar]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Etienne, R.; Michel, T.; Karim, Z. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.-W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chin. J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrog. Energy 2016, 42, 7254–7262. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.; Malinowski, M.; van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrog. Energy 2019, 44, 29263–29272. [Google Scholar] [CrossRef]
- Cao, T.Y.; Lee, W.J.; Huang, R.J.; Raymond, J. Liquid-Organic hydrogen carriers as endothermic fuels. Fuel 2022, 313, 123063. [Google Scholar] [CrossRef]
- Blokland, H.; Sweelssen, J.; Isaac, T.; Boersma, A. Detecting hydrogen concentrations during admixing hydrogen in natural gas grids. Int. J. Hydrog. Energy 2021, 46, 32318–32330. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Boerner, L.K. Industrial ammonia production emits more CO2 than any other chemical-making reaction. Chemists want to change that. Chem. Eng. News 2019, 97, 1–9. [Google Scholar]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Yan, C.; Zhao, P.; Hu, Y.; Zhang, W.; Jin, Z. Review on photocatalytic and electrocatalytic artificial nitrogen fixation for ammonia synthesis at mild conditions: Advances, challenges and perspectives. Nano Res. 2019, 12, 1229–1249. [Google Scholar] [CrossRef]
- Chen, H.; Dong, F.; Minteer, S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals. Fuels Mater. Nat. Catal. 2020, 3, 225–244. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrog. Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- BMW iX5 Hydrogen in action at IAA show. Fuel Cells Bull. 2021, 2021, 2.
- Verhelst, S. Recent progress in the use of hydrogen as a fuel for internal combustion engines. Int. J. Hydrog. Energy 2014, 39, 1071–1085. [Google Scholar] [CrossRef]
- Costa, R.; Hernández, J.; Teixeira, R.; Netto, D.A.D. Combustion, performance and emission analysis of a natural gas-hydrous ethanol dual-fuel spark ignition engine with internal exhaust gas recirculation. Energy Convers. Manag. 2019, 195, 1187–1198. [Google Scholar] [CrossRef]
- Lai, X.; Chen, Q.W.; Tang, X.P.; Zhou, Y.; Gao, F.; Guo, Y.; Bhagat, R.; Zheng, Y. Critical review of life cycle assessment of lithium-ion batteries for electric vehicles: A lifespan perspective. eTransportation 2022, 12, 100169. [Google Scholar] [CrossRef]
- Bououchma, Z.; Sabor, J.; Aitbouh, H. New electrical model of supercapacitors for electric hybrid vehicle applications. Mater. Today Proc. 2019, 13, 688–697. [Google Scholar] [CrossRef]
- Wilailak, S.; Yang, J.; Heo, C.; Kim, K.-S.; Bang, S.-K.; Seo, I.-H.; Zahid, U.; Lee, C.-J. Thermo-economic analysis of Phosphoric Acid Fuel-Cell (PAFC) integrated with Organic Ranking Cycle (ORC). Energy 2021, 220, 119744. [Google Scholar] [CrossRef]
- Hu, D.H.; Wang, Y.T.; Li, J.W.; Yang, Q.; Wang, J. Investigation of optimal operating temperature for the PEMFC and its tracking control for energy saving in vehicle applications. Energy Convers. Manag. 2021, 249, 114842. [Google Scholar] [CrossRef]
- Abdalla, M.; Shahzad, H.; Atia, T.A.; Petra, M.L.; Begum, F.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Hj, Z.J.; Abul, K.A. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Kargupta, K.; Saha, S.; Banerjee, D.; Seal, M.; Ganguly, S. Performance enhancement of phosphoric acid fuel cell by using phosphosilicate gel based electrolyte. J. Fuel Chem. Technol. 2012, 40, 707–713. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Combustion characteristics of ammonia in a modern spark-ignition engine. In Proceedings of the Conference on Sustainable Mobility, Catania, Italy, 14 October 2019. [Google Scholar]
- Mounaïm-Rousselle, C.; Brequigny, P. Ammonia as fuel for low-carbon spark-ignition engines of tomorrow’s passenger cars. Front. Mech. Eng. 2020, 70. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Bréquigny, P.; Dumand, C.; Houillé, S. Operating limits for ammonia fuel spark-ignition engine. Energies 2021, 14, 4141. [Google Scholar] [CrossRef]
- Boretti, A. Novel dual fuel diesel-ammonia combustion system in advanced TDI engines. Int. J. Hydrog. Energy 2017, 42, 7071–7076. [Google Scholar] [CrossRef]
- Wallner, T.; Lohse-Busch, H.; Gurski, S.; Duoba, M.; Thiel, W.; Martin, D.; Korn, T. Fuel economy and emissions evaluation of BMW Hydrogen 7 Mono-Fuel demonstration vehicles. Int. J. Hydrog. Energy 2008, 33, 7607–7618. [Google Scholar] [CrossRef]
- Szwabowski, S.J.; Hashemi, S.; Stockhausen, W.F.; Natkin, R.J.; Reams, L.; Kabat, D.M.; Potts, C. Ford hydrogen engine powered P2000 vehicle. SAE Tech. Pap. 2002, 43. [Google Scholar] [CrossRef]
- Park, C.; Kim, Y.; Oh, S. Effect of fuel injection timing and injection pressure on performance in a hydrogen direct injection engine. Int. J. Hydrog. Energy 2022, 47, 21552–21564. [Google Scholar] [CrossRef]
- Dhyani, V.; Subramanian, K.A. Control of backfire and NOx emission reduction in a hydrogen fueled multi-cylinder spark ignition engine using cooled EGR and water injection strategies. Int. J. Hydrog. Energy 2019, 44, 6287–6298. [Google Scholar] [CrossRef]
- The Future of Hydrogen, IEA. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 25 July 2022).
- Sustainable and Smart Mobility Strategy—European Transport on Track for the Future, 2zeroemission. Available online: https://www.2zeroemission.eu/mediaroom/sustainable-and-smart-mobility-strategy-european-transport-on-track-for-the-future/ (accessed on 25 July 2022).
- Toyota Official Website. Available online: https://www.toyota.com.au/mirai (accessed on 25 July 2022).
- Honda Official Website. Available online: https://www.fuel-cell.info/fuel-cell-cars/honda-clarity-fuel-cell/ (accessed on 25 July 2022).
- Saicmaxus Official Website. Available online: https://www.saicmaxus.com/fcv80.shtml (accessed on 25 July 2022).
- Hyundai Official Website. Available online: https://www.hyundai.com.cn/aboutNexo.html (accessed on 25 July 2022).
- Quantron implements hydrogen drivetrain in vans and heavy trucks. Fuel Cells Bull. 2020. [CrossRef]
- Yanshan Petrochemical Started to Operate Two 49 Ton Hydrogen Energy Heavy Trucks, Qingdao Institute Bioenergy and Bioprocess Technology, Chinese Academy of Science. Available online: http://www.qibebt.cas.cn/xwzx/kydt/202107/t20210726_6148326.html (accessed on 26 July 2021).
- Yutong delivers fuel cell buses for fleets in two cities in China. Fuel Cells Bull. 2018, 1464–2859.
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies 2022, 15, 1023. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G. NOx reduction in diesel-hydrogen engines using different strategies of ammonia injection. Energies 2019, 12, 1255. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrog. Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Kane, S.P.; Northrop, W.F. Thermochemical Recuperation to Enable Efficient Ammonia-Diesel Dual-Fuel Combustion in a Compression Ignition Engine. Energies 2021, 14, 7540. [Google Scholar] [CrossRef]
- Angeles, D.A.; Tan, R.R.; Aviso, K.B.; Are, K.R.A.G.; Razon, L.F. Fuzzy optimization of the automotive ammonia fuel cycle. Clean 2018, 186, 877–882. [Google Scholar] [CrossRef]
- Fournier, G.G.M.; Cumming, I.W.; Hellgardt, K. High performance directammonia solid oxide fuel cell. J. Power Sources 2006, 162, 198–206. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A.; Di Trolio, P.; Di Micco, S.; Jannelli, E. Techno-economics of novel refueling stations based on ammonia-to-hydrogen route and SOFC technology. Int. J. Hydrog. Energy 2021, 46, 10059–10071. [Google Scholar] [CrossRef]
- Zhao, Y.; Setzler, B.P.; Wang, J.; Nash, J.; Wang, T.; Xu, B.; Yan, Y. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule 2019, 3, 2472–2484. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, S.; Yao, Y.; Lin, Z.; Ouyang, W.; Zhuo, L.; Wang, Z. Performance of direct ammonia fuel cell with PtIr/C, PtRu/C, and Pt/C as anode electrocatalysts under mild conditions. Int. J. Hydrog. Energy 2021, 46, 27749–27757. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Comparative assessments of two integrated systems with/without fuel cells utilizing liquefied ammonia as a fuel for vehicular applications. Int. J. Hydrog. Energy 2018, 43, 4597–4608. [Google Scholar] [CrossRef]
- Koike, M.; Miyagawa, H.; Suzuoki, T.; Ogasawara, K. Ammonia as a hydrogen energycarrier and its application to internal combustion engines. In Sustainable Vehicle Technologies; Warwickshire, G., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 61–70. [Google Scholar]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Performance and emissions of an ammonia-fueled SI engine with hydrogen enrichment. In Proceedings of the 14th International Conference on Engines & Vehicles, Napoli, Italy, 15 September 2019. [Google Scholar]
- Dinesh, M.H.; Pandey, J.K.; Kumar, G.N. Study of performance, combustion, and NOx emission behavior of an SI engine fuelled with ammonia/hydrogen blends at various compression ratio. Int. J. Hydrog. Energy 2022, 47, 25391–25403. [Google Scholar] [CrossRef]
- Siddiqui, O.; Ishaq, H.; Dincer, I. Experimental investigation of improvement capability of ammonia fuel cell performance with addition of hydrogen. Int. J. Hydrog. Energy 2020, 205, 112372. [Google Scholar] [CrossRef]
- Hydrogen on the Horizon: Inputs from Senior Leaders on Hydrogen Developments, World Energy Council. 2021. Available online: https://www.worldenergy.org/publications/entry/working-paper-inputs-from-senior-leaders-on-hydrogen-developments (accessed on 25 July 2022).
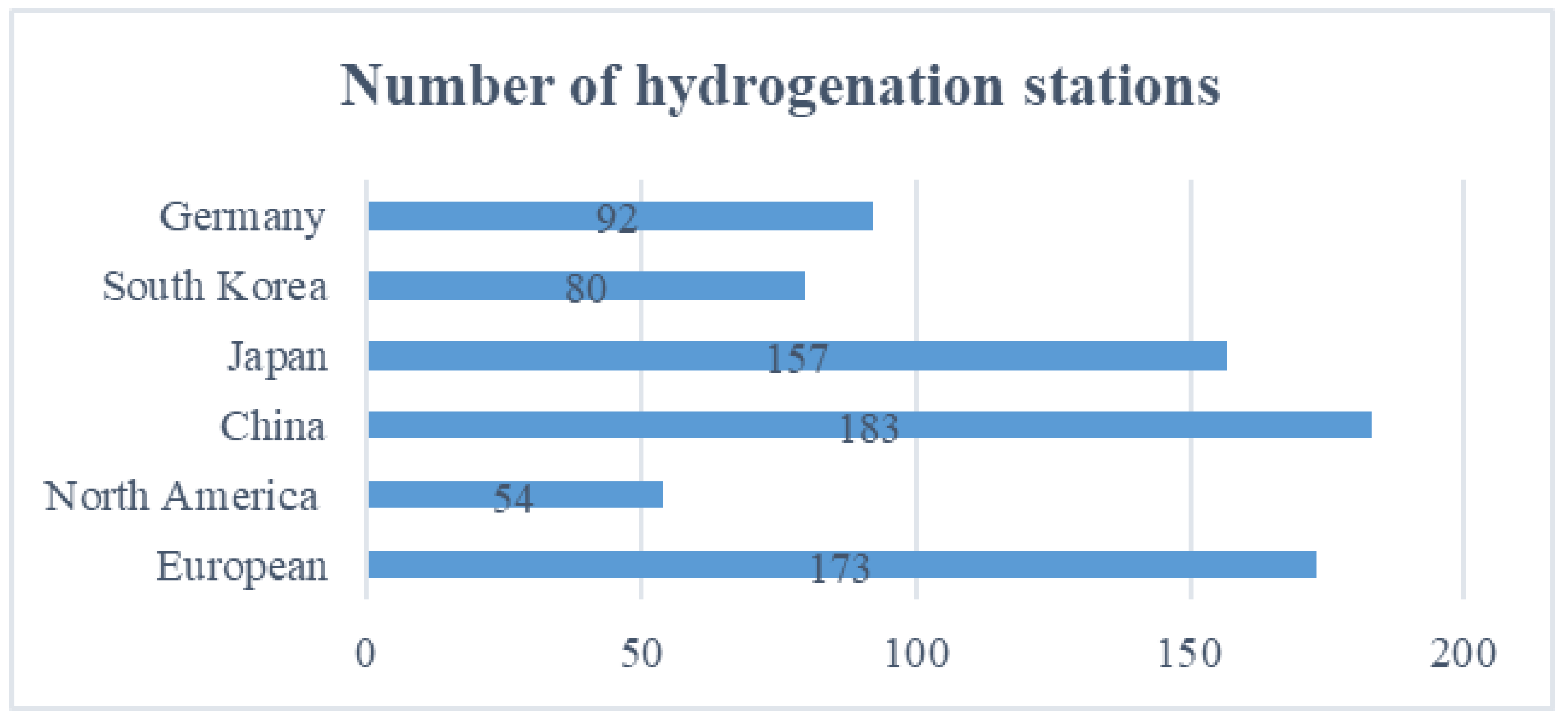
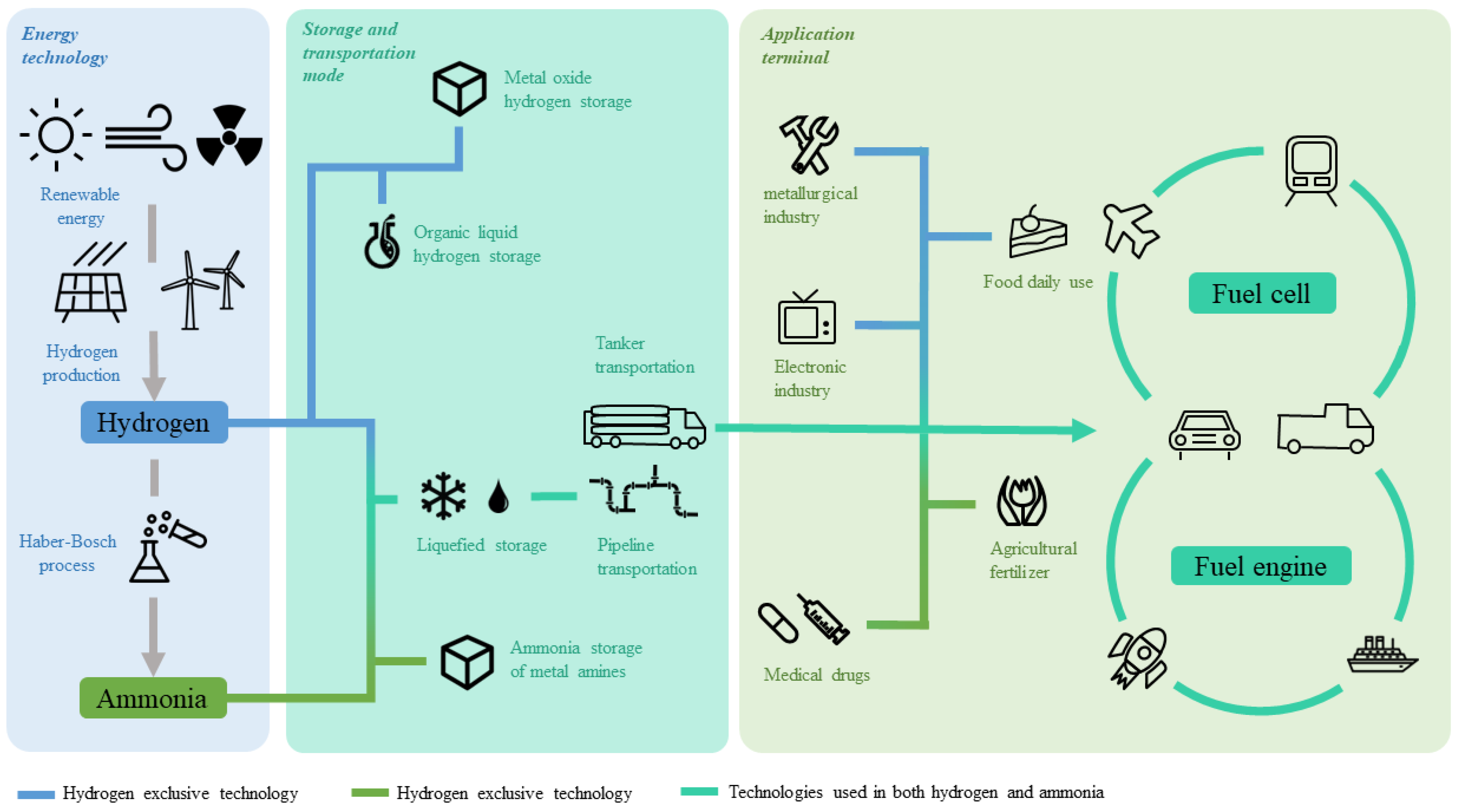
| Region | Time | Policy Name | Content of the Policy | Department | Reference |
|---|---|---|---|---|---|
| Shandong | January | “14th five-year plan” for energy science and technology innovation in Shandong Province | As for the key layout in hydrogen energy, solar hydrogen production and offshore wind power hydrogen production are put on the agenda. | Shandong Provincial Development and Reform Commission | [16] |
| Gansu | January | “14th five-year energy development plan” of Gansu Province | Promote the development of the hydrogen energy industry. Cultivate the hydrogen energy industry and plan a “five in one” hydrogen energy industrial park for hydrogen production, hydrogen storage, hydrogen transportation, hydrogen refueling station, and hydrogen fuel cell | General Office of Gansu Provincial People’s Government | [17] |
| Ningxia | January | Ningxia Hui Autonomous Region’s 14th five-year plan to deal with climate change | Accelerate the development of hydrogen energy, reasonably optimize the layout of hydrogen refueling stations, and accelerate the demonstration operation of hydrogen fuel cell city buses, logistics vehicles, municipal sanitation vehicles, forklifts, and hydrogen refueling stations | Department of ecological environment of Ningxia Hui Autonomous Region | [18] |
| Liaoning | January | “14th five-year plan” for eco-economic development | Support the construction of low-cost hydrogen production, storage and transportation demonstration projects, and plan to build a hydrogen energy wharf and hydrogen energy trade center | People’s Government of Liaoning Province | [19] |
| Tianjin | January | Tianjin’s 14th-five year plan for ecological and environmental protection | Promote the demonstration application of hydrogen fuel cell vehicles and accelerate the construction of corresponding hydrogenation supporting facilities | General Office of Tianjin Municipal People’s Government | [20] |
| Guangxi | January | Guangxi new energy vehicle industry development “14th five-year plan” | Layout and build a fuel cell vehicle application demonstration city cluster and accelerate the commercial application of fuel cell vehicles | General Office of the People’s Government of Guangxi Zhuang Autonomous Region | [21] |
| China | February | Opinions on improving the institutions, mechanisms, policies and measures for energy, green and low-carbon transformation | Improve the charging and exchanging, hydrogenation, gas station layout and service facilities, and explore efficient ways of hydrogen transportation, such as hydrogen transportation, pure hydrogen pipeline transportation and liquid hydrogen transportation. | National Development and Reform Commission, National Energy Administration | [22] |
| Inner Mongolia | February | The 14th Five-year Plan of Hydrogen Energy development in Inner Mongolia Autonomous Region | The plan puts forward the development goals of hydrogen energy in Inner Mongolia, speeds up the construction of hydrogen stations, promotes fuel cell vehicles, encourages the development of the hydrogen energy industry and creates hydrogen energy application demonstration projects. | Energy Bureau of Inner Mongolia Autonomous Region | [23] |
| Associated Color | Grey Hydrogen | Blue Hydrogen | Green Hydrogen | Purple Hydrogen | Turquoise Hydrogen | Yellow Hydrogen |
|---|---|---|---|---|---|---|
| Energy utilization type | natural gas, coal | natural gas | renewable electricity | nuclear electricity | natural gas | grid electric |
| Conversion route of hydrogen production | coal gasification natural gas reforming | methanol cracking or natural gas reforming + CCUS | Electrolysis of water using electricity generated from RES | Electrolysis of water using electricity generated from nuclear power plants | produced by pyrolysis of methane | Electrolysis of water using grid electricity |
| Maturity | Large scale application | Commerc-ialization | Commercia-lization | Experimental stage | Not yet Commercia-lly available | Commerc-ialization |
| Process-related CO2 emissions | High-CO2 | Low-CO2 | CO2-free | CO2-free | solid carbon | CO2 (from fossil fuel power plants) |
| Hydrogen Storage Type | Working Principle | Volumetric Densit (g/L) | Volumetric Energy Density (MJ/L) |
|---|---|---|---|
| High-pressure gaseous hydrogen storage | The hydrogen is pressurized and compressed into a high-pressure hydrogen storage tank | 24.5 (350 bar, RT) 41.4 (700 bar, RT) | 2.94 (350 bar, RT) 4.97 (700 bar, RT) |
| Low-temperature liquid hydrogen storage | Cooling hydrogen from gaseous state to liquid state; | 70.8 (1 bar, −253 °C) | 8.5 |
| Organic liquid hydrogen storage | Hydrogen is stored chemically by reacting with a hydrogen defi- cient organic molecule | 47.3 (Methylcyclohexane/ Toluene) 56.0 (perhydro-benzyltoluene/benzyltoluene) | 5.68 (Methylcyclohexane/ Toluene) 6.72 (perhydro-benzyltoluene/benzyltoluene) |
| Complex hydrides | hydrogen molecules are dissociated into hydrogen atoms and integrated in the lattice of the materials. | 80 (NaAlH4) | 9.6 (NaAlH4) |
| physical adsorption hydrogen | In the form of physical adsorption, hydrogen is adsorbed on solid surfaces | 20 (Zeolite,7 K and 40 bar) | 2.4 (Zeolite,7 K and 40 bar) |
| Parameter | Unit | Hydrogen | Ammonia | Gasoline | Diesel | Natural Gas | Ethanol | Methanol |
|---|---|---|---|---|---|---|---|---|
| density | kg·m−3 | 39.1 | 600 | 737 | 820–950 | 0.7–0.9 | 789 | 791 |
| Low calorific value | MJ/kg | 120 | 18.8 | 46.4 | 48 | 53.6 | --- | 19.92 |
| Spontaneous combustion temperature | K | 858 | 930 | 520–583 | 503 | 813 | 690 | 742 |
| laminar burning velocity | cm/s | 351 | 7 | 58 | 128 | 37.3 | --- | 36 |
| Combustion limit | % | 4–75 | 15–28 | 1.4–7.6 | 0.6–7.5 | 5–15 | 4.3–19 | 6–36.5 |
| Minimum ignition energy | MJ | 0.017 | 8 | 0.2 | --- | 0.29 | --- | 0.215 |
| Electrolyte Type | PEMFC [62] | SOFC [61] | PAFC [63] | AFC [65] |
|---|---|---|---|---|
| electrolyte | PEM | --- | phosphate | Alkaline electrolyte |
| Working temperature/°C | <80 | >700 | 150~200 | >70 |
| electrolytic efficiency/% | 70~90 | 85~100 | --- | 60~75 |
| maturity | Not in mass use | --- | Not commercialized | Industrial production |
| environmental protection | pollution-free | --- | pollution-free | Asbestos is poisonous |
| Advantage | Disadvantage |
|---|---|
| Complete infrastructure and large-scale production facilities. As ammonia has a high industrial manufacturing level as a common chemical, it has sufficient raw materials and low cost, which provides a good foundation for developing ammonia fuel. | As a fuel for road vehicles, safety needs to be considered. Once liquid ammonia leakage, volatilization and other accidents occur, it will cause significant harm to air pollution and the human respiratory tract. Therefore, it is necessary to consider the safety of transportation and use. |
| Convenient transportation and storage. This has a significant advantage over hydrogen energy and is also the key reason why ammonia energy may achieve a remarkable breakthrough after the bottleneck of hydrogen energy development. | Although it has a vast source supply, the infrastructure for adding ammonia fuel, such as gas stations, is still incomplete. |
| With high energy density, it has unique advantages as a vehicle fuel. Liquid ammonia has similar specific gravity to gasoline. The engine compression ratio can be significantly increased to improve the output power. The thermal efficiency of an ammonia engine can reach 50% or even nearly 60%, which is more than twice that of an ordinary gasoline engine. |
| Toyota Mirai (2021) [76] | Honda Clarity [77] | SAIV FCV 80 [78] | Hyundai NEXO [79] | |
|---|---|---|---|---|
| Body size (mm × mm × mm) | 4890 × 1815 × 1535 | 4915 × 1875 × 1480 | 6120 × 1998 × 2612 | 4670 × 1860 × 1630 |
| Energy type | hydrogen | hydrogen | hydrogen | hydrogen |
| Maximum output power/kW | 136 | 130 | 100 | 120 |
| Maximum torque/N·m | 300 | 256 | 350 | 395 |
| the highest speed/km·h−1 | 175 | 165 | 100 | 179 |
| Low-temperature performance of fuel cell/°C | −30 | −30 | −10 | −30 |
| Hydrogen consumption per 100 km/kg | 0.55 | 0.97 | 1.7 | 0.706 |
| Hydrogen storage quality/kg | 5.6 | 5.46 | 6.2 | 6.3 |
| Range/km | 650 | 750 | 500 | 800 |
| 100 km acceleration time/s | 9.2 | 8.8 | - | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Chen, X.; Qin, J.; Wu, P.; Jia, L. The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation. Sustainability 2022, 14, 11904. https://doi.org/10.3390/su141911904
Shi R, Chen X, Qin J, Wu P, Jia L. The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation. Sustainability. 2022; 14(19):11904. https://doi.org/10.3390/su141911904
Chicago/Turabian StyleShi, Ruifeng, Xiaoxi Chen, Jiajun Qin, Ping Wu, and Limin Jia. 2022. "The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation" Sustainability 14, no. 19: 11904. https://doi.org/10.3390/su141911904
APA StyleShi, R., Chen, X., Qin, J., Wu, P., & Jia, L. (2022). The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation. Sustainability, 14(19), 11904. https://doi.org/10.3390/su141911904








