Abstract
The ability of grassland ecosystems to sequester carbon has a great potential to achieve carbon neutralization. Rhizosphere deposition is the most uncertain part of the soil carbon cycle. Since grazing is one of the main ways to utilize grasslands, we conducted experiments to clarify the role of rhizosphere on soil organic carbon (SOC) cycling under grazing in a typical steppe region of Inner Mongolia, China. The experiment was conducted in grasslands under light, moderate, and heavy grazing and in a control (no grazing) in Inner Mongolia, China. Here, we present our analysis of the total soil organic carbon (TOC) and fractions in both the rhizosphere and bulk soil. Light and moderate grazing increased SOC contents in rhizosphere soil, and we found more SOC in rhizosphere soil than in bulk soil. The rhizosphere showed SOC enrichment effect, and this effect increased with an increase in grazing intensity. As grazing intensity increased, microbial biomass carbon content and its percentage of TOC increased in rhizosphere soil and were more stable than those in bulk soil. Dissolved organic carbon content and its percentage of TOC also increased in rhizosphere soil with increasing grazing intensity. These changes were more than those observed in bulk soil. Changes in potentially mineralizable carbon and particulate organic carbon in the rhizosphere and bulk soil were not synchronized in plots under different grazing intensities. Grazing changed the quantity of bacteria, fungi, and actinomycetes, as well as the community structure of soil microbes, in rhizosphere soil and bulk soil. It also influenced the content and structure of SOC. Acidic components (e.g., organic acids) of root exudate reduced the pH in rhizosphere soil to less than that in bulk soil, which may have affected SOC cycling. The results can provide support to improve the ecosystem carbon sink function and help to achieve the goal of carbon peaking/carbon neutral target.
1. Introduction
On 1 December 2015, the United Nations Climate Change Conference (COP 21) in Paris launched an international initiative called the Four Per Thousands Initiative. The initiative aims to increase soil organic carbon (SOC) content in the top 30 to 40 cm of cropland, grassland, and forest soil by 0.4% per year [1,2]. A rough calculation by Kell [3] showed that sequestering 10% more CO2 in the soil could remove up to 20% more CO2 from the atmosphere. Therefore, increasing the SOC content is an important process for mitigating the climate change caused by CO2 emissions from natural and anthropogenic activities. SOC sequestration by plant root exudates plays an important role in the removal of CO2 from the atmosphere. Rhizodeposited C accounts for 5–20% of the photosynthetic products and is a highly bioavailable and readily metabolized substrate for soil microorganisms.
Carbon stocks in grassland ecosystems account for 28–30% of the total carbon stocks in global terrestrial ecosystems [4,5], and 80–95% of the carbon in grasslands is stored in soil [6]. However, the overgrazing of grasslands leads to degradation of the soil [7], which results in lost SOC [8]. Roots are the principal site of interaction between plants and soil [9,10]. Root biomass can account for 80% of total biomass in grassland ecosystems [6]. Roots absorb water and nutrients from the soil while excreting large amounts of low molecular organic compounds (e.g., sugars, organic acids, amino acids, and phenolic compounds) into the soil [11]. The organic matter exuded by roots can affect biogeochemical cycling in the soil [12] and regulate a large number of soil processes [11,13], e.g., carbon transformation and carbon stock maintenance [14,15], as well as soil microbe activity and community structure [16,17,18]. The rhizosphere plays an important role in ecosystem carbon cycling and is important for nutrient resource storage and ground stress tolerance [19].
Our understanding of the impact of grazing on soil organic carbon (SOC) and the mechanisms underlying these effects is incomplete [20,21]. Most relevant studies have been conducted on bulk soil. Fewer studies have focused on the interaction (energy flow and matter circulation) between vegetation and soil, especially in the rhizosphere, the most active site of plant and soil interaction [10]. Studies on the priming effect of root carbon exudation on rhizosphere processes have been mainly controlled experiments, with relatively few field experiments conducted [22]. Removal of the aboveground vegetation by grazing changes the underground food web [23]. Further, some studies have shown that defoliation can promote rhizodeposition [24,25]. Holland et al. [26] found that defoliation results in a compensatory action that drives plant carbon to the roots, and, in turn, carbon accumulation in roots may drive roots to exude more carbon. Studies have also shown that defoliation or grazing can inhibit rhizodeposition. Augustine et al. [27] found that carbon from the rhizosphere were reduced in C3 and C4 plants of a North American steppe after 8 days of defoliation. In addition, grazing was shown to reduce SOC content and rhizosphere enrichment effects in the rhizosphere of Stipa grandis in a typical steppe in Inner Mongolia, China [19], and grass clipping was shown to significantly reduce organic matter in the rhizosphere soil of Krascheninnikovia ceratoides in a steppe desert [28]. The rate of root exudation has been related to grazing intensity. The greater the grazing intensity, the lower the SOC content in bulk and rhizosphere soil associated with S. grandis [29]. Heavy grazing significantly reduced the rate of root exudation from Elymus nutans, but there was no significant effect of moderate grazing [22].
Grazing changed both the content of TOC and the contributions of the active SOC fractions to this total. Although the active SOC fractions account for only a small percentage of TOC, they directly participate in biochemical transformation and thus play a crucial role in the circulation of SOC. Such as, POC supplies the most stable C in soil [30]. PMC and POC may represent sensitive SOC fractions that could be useful as early predictors of the response of SOC to grazing in a particular location or environment [31]. There were significant and strong positive correlations between PMC and POC and between MBC and DOC [31,32].
The steppes of Inner Mongolia are important ecological barriers in northwestern China that maintain balance in global and regional ecosystems. However, typical steppe ecosystems are relatively fragile and are highly susceptible to human activity. Human activities, such as long-term overgrazing, have degraded nearly 90% of grasslands to various degrees [33,34]. S. grandis is a highly adaptable dominant temperate grassland species and one of the most stable plant species in this ecosystem [35]. S. grandis dominates approximately 2,789,081 ha of grasslands in Inner Mongolia [36] and plays a key role in defining the structure and function of ecosystems, as well as the circulation of material and flow of energy in these systems. We determined the total soil organic carbon (TOC) and SOC fractions contents in bulk and rhizosphere soils in experimental plots with S. grandis exposed to different grazing intensities in a typical steppe in Inner Mongolia, China. The objectives of the study were (1) to analyze the effects of different grazing intensities on SOC content in S. grandis rhizosphere soil, (2) to explore the role of the rhizosphere in determining SOC content in grazed grassland soil.
2. Materials and Methods
2.1. Study Site
This study was carried out at the experimental grazing plots of the Institute of Grassland Research at the Chinese Academy of Agricultural Sciences, as described by [37]. The plots are located in the Chaokewula Sumu region (ca. 44°15′ N and 116°32′ E, 1111 to 1121 m a.s.l.) in the Xilin Gol League of China’s Inner Mongolia Autonomous Region. The area is situated in a semiarid steppe ecosystem and has a typical continental monsoon climate with annual precipitation of 350 to 450 mm and an annual mean temperature of −0.1 °C, ranging from an average of −22.0 °C in January to 18.3 °C in July. The plant community is dominated by Leymus chinensis, Stipa krylovii, and S. grandis. The soils are classified as calcic chernozems [37], with physiochemical properties similar to those associated with chestnuts and other calcic chernozems [31,38].
The entire Chaokewula Sumu region is grazed by herds consisting of 70–90% sheep and 10–30% goats [39], and the carrying capacity is around 0.50–0.75 standard sheep units (SSU) ha−1 yr−1. From 2007 to 2014, grazing was prevented in the 1.33-ha experimental plots. During this time, the plots were mowed to provide fodder, and vegetation grew well. In 2014, at the start of the grazing experiment, plots were chosen so that vegetation cover, species composition, and soil properties were similar across plots. Each year, livestock were allowed to graze in the grazing areas for a period of 90 days beginning on 10 June. The livestock were 2-year-old Ujumqin sheep wethers (castrated males) with an average weight of 31.5 kg. We selected plots representing four grazing intensities: a control plot with no sheep grazing, and lightly, moderately, and heavily grazed plots. The grazing intensities were equivalent to 0, 0.47, 0.93, and 1.40 SSU ha−1 yr−1, with three spatial replicates for each grazing intensity, 12 in total.
2.2. Data Acquisition
2.2.1. Root Biomass
In late July 2017, we established three 1 × 1 m subplots in each plot for a total of 36 subplots. The upper 30–40 cm of soil has the highest concentration of soil organic matter and contains approximately 80% of root biomass [40]. Most studies (92%) considered in a 2010 review by Yang and colleagues reported SOC data within the top 30 cm, and SOC are mainly concentrated in the upper 30 cm of soil in China’s grassland [41,42]. We removed the aboveground biomass and then obtained soil cores to measure the root biomass. Samples were taken at depths of 0–10 cm, 10–20 cm, and 20–30 cm using a root drill (10 cm in diameter) at three locations in each subplot (i.e., a total of nine samples per plot). We separated the roots from the soil using a 1-mm sieve, washed the roots to remove any soil, and then oven-dried the samples at 65 °C for 24 h to a constant weight.
2.2.2. Soil Sampling and Measurements
We collected bulk soil samples at 0–10 cm, 10–20 cm, and 20–30 cm using a soil drill (8 cm in diameter) and obtained a mixed soil sample at each depth from three soil cores in each subplot. All means reported in this paper represent the means of measures determined at the three depths in each subplot. Three bulk rhizosphere soil samples were also collected in each plot. In grazing plots, we selected rhizosphere soil associated with S. grandis that had been grazed by sheep near the bulk soil sampling subplots. In the control plot, we sample soil associated with ungrazed S. grandis near the bulk soil sampling subplots. After removing the aboveground parts of the plant, we obtained the roots and soil with a root drill (10 cm in diameter) and then removed the soil by gentle shaking. The soil that stuck to the root was collected with a brush and considered rhizosphere soil. Three rhizosphere soil samples were obtained from each plot.
We removed roots and stones with a 2-mm sieve and immediately stored the samples in boxes with dry ice until they could be shipped to the laboratory for microbial analysis and measurement of potentially mineralizable carbon (PMC), microbial biomass carbon (MBC), and dissolved organic carbon (DOC). Subsamples of each soil sample were air-dried and passed through a 2-mm sieve, and then total soil organic carbon (TOC), particulate organic carbon (POC), and pH were measured.
TOC was measured using an Elementar Liqui TOCα analyzer (Elementar Co., Hanau, Germany). MBC was estimated using the chloroform-fumigation extraction method described by Brookes et al. [43], DOC and PMC were measured as described by Xu et al. [44], and POC was measured according to the method of Zhang et al. [37]. We analyzed the genetic and structural diversity of the soil microbial community, including bacteria, fungi, and actinomycetes, using primers and polymerase chain reaction conditions described by Aldezabal et al. [45] and denaturing-gradient gel electrophoresis. The primers were as follows, bacteria: Eub338/Eub518 (5′-ACTCCTACGGGAGGCAGCA-3′/5′-ATTACCGCGGCTGCTGG-3′), Fungi: 5.8s/ITSIf (5’-CGCTGCGTTCTTCATCG-3′/5′-TCCGTAGGTGAACCTGCCG-3′), Actinomycetes: Act920F/Act1200F (5′-TACGGCCGCAAGGCTA-3′/5′-TCRTCCCCACCTTCCTCCG-3′). Soil pH was measured using a Fisher Accumet probe (Thermo Fisher Scientific, Asheville, NC, USA) inserted into a 2:1 w/v mixture of soil and deionized water.
2.3. Data Analysis
We used the enrichment ratio (ER) of rhizosphere soil to bulk soil to reduce intersite variability. The ER was calculated as
where R and B are the TOC, MBC, DOC, PMC, and POC concentrations (mg/g) in rhizosphere and bulk soil, respectively; ER > 1 indicates a higher concentration of TOC, MBC, DOC, PMC, or POC in rhizosphere soil than in bulk soil [10,46].
ER = R/B
We used a one-way analysis of variance (ANOVA) and least significant difference (LSD) test to determine the significance of differences between the values for each variable in plots exposed to different grazing intensities, as well as between rhizosphere soil and bulk soil. Differences were considered significant at P < 0.05.
3. Results
3.1. Contents of the TOC and SOC Fractions
As the grazing intensity increased, TOC content increased and then decreased in the rhizosphere soil slightly (Figure 1a), but there was no significant difference in TOC content between plots exposed to different grazing intensities (P > 0.05). There were similar differences in bulk soils, but TOC content in plots under heavy grazing was significantly lower than that in control and light-grazing plots (P < 0.05). There was no significant difference between TOC content with different grazing treatments (Figure 1a). TOC content in rhizosphere soil was significantly higher than that in bulk soil (P < 0.05), and, as grazing intensity increased, changes in TOC content in rhizosphere soil were less than those in bulk soil. Rhizosphere enrichment was highest with moderate grazing (Table 1), indicating that the difference between rhizosphere and bulk soil was the greatest at this level of grazing. However, rhizosphere enrichment was not significantly different between the various grazing treatments (P > 0.05).
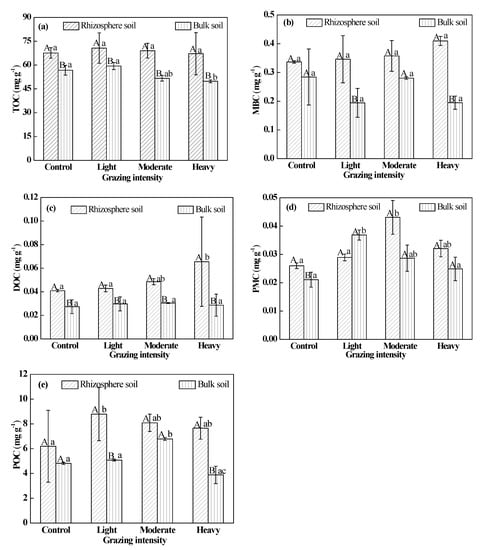
Figure 1.
The contents of TOC and SOC fractions. (a) TOC, total soil organic carbon; (b) MBC, microbial biomass carbon; (c) DOC, dissolved organic carbon; (d) PMC, potentially mineralizable carbon; (e) POC particulate organic carbon. Different uppercase letters indicate significant differences in rhizosphere and bulk soil under certain grazing intensity at 0.05 level. Different lowercase letters indicate significant differences under different grazing intensity at 0.05 level in rhizosphere and bulk soil, respectively.

Table 1.
The enrichment ratios of rhizosphere SOC. Bars indicate means ± SE (n = 6). Different letters on the bars indicate significant difference (P < 0.05).
MBC content increased in the rhizosphere soil as grazing intensity increased, but the differences between treatments were not significant (P > 0.05) (Figure 1b). MBC content in rhizosphere soil was higher than that in bulk soil, and there was a significant difference in plots under light and heavy grazing. Rhizosphere enrichment of MBC in plots under heavy grazing was significantly greater than that in the control plot (P < 0.05). However, there was no significant difference between enrichment associated with the other treatments and the control (Table 1).
DOC content increased in the rhizosphere soil as the grazing intensity increased. The mean value for plots under heavy grazing was significantly greater than those for the control and light-grazing plots (P < 0.05), but there were no significant differences between plots under the other treatments (P > 0.05) (Figure 1c). DOC content in rhizosphere soil was higher than that in bulk soil in plots under all treatments (P < 0.05). Rhizosphere enrichment of DOC in plots under heavy grazing was significantly greater than those in the control and light-grazing plots (P < 0.05), but there were no significant differences between enrichment associated with the other treatments and the control (Table 1).
PMC content increased and then decreased in rhizosphere soil as grazing intensity increased. The content in plots under moderate grazing was significantly higher than those in the control and light-grazing plots (P < 0.05), but there were no significant differences between plots under the other treatments (P > 0.05) (Figure 1d). PMC content in rhizosphere soil was significantly higher than that in bulk soil in the control (P < 0.05), but there were no significant differences between plots under the other treatments. However, in plots under light grazing, the PMC content in rhizosphere soil was lower than that in bulk soil. Rhizosphere enrichment of PMC in plots under moderate grazing was significantly greater than that in plots under light grazing (P < 0.05), and there were no significant differences between the other treatment plots and the control (Table 1).
POC content increased and then decreased in rhizosphere soil as grazing intensity increased. The content in plots under light grazing was significantly higher than that in the control (Figure 1e), and there were no significant differences between the other treatment plots and the control (P > 0.05). POC content in rhizosphere soil was higher than that in bulk soil, and there were significant differences in plots under light and heavy grazing (P < 0.05). Rhizosphere enrichment of POC in plots under light and heavy grazing was significantly higher than that in the control and moderate-grazing plots (P < 0.05) (Table 1).
3.2. Composition and Structure of SOC
As the intensity of grazing increased, the MBC/TOC in rhizosphere soil decreased, but there was no significant difference between the plots under each treatment and the control (P > 0.05) (Figure 2a). The MBC/TOC in bulk soil increased, and this difference was significant in plots under light grazing vs. the control and moderate grazing vs. the control (P < 0.05). The MBC/TOC under light and heavy grazing were significantly higher than those in bulk soil (P < 0.05). As the intensity of grazing increased, the DOC/TOC in rhizosphere soil changed more than that in the bulk soil (Figure 2b). The increase in grazing intensity resulted in trends of the PMC/TOC in the rhizosphere and bulk soil that were similar to trends in the PMC content in these soils (Figure 2a). Grazing increased the POC/TOC, where the maximum in rhizosphere soil or bulk soil was found in light grazing or moderate grazing, respectively (Figure 2d).
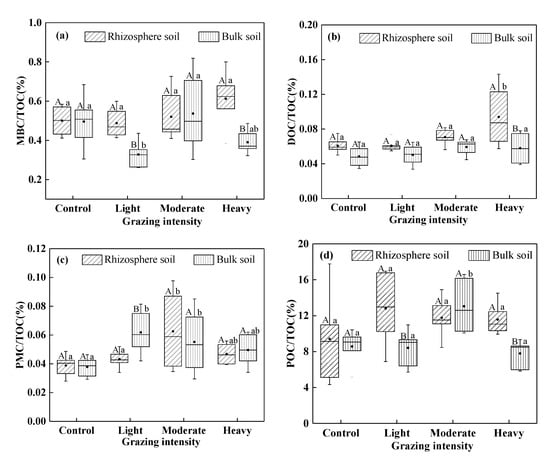
Figure 2.
The percentage of SOC fractions in TOC. (a) Percentage of MBC in TOC; (b) Percentage of DOC in TOC; (c) Percentage of PMC in TOC; (d) Percentage of POC in TOC. TOC, total soil organic carbon; MBC, microbial biomass carbon; DOC, dissolved organic carbon; PMC, potentially mineralizable carbon; POC particulate organic carbon. Different uppercase letters indicate significant differences in rhizosphere and bulk soil under certain grazing intensity at 0.05 level. Different lowercase letters indicate significant differences under different grazing intensity at 0.05 level in rhizosphere and bulk soil, respectively.
3.3. Soil Microbes
The abundance of soil bacteria in rhizosphere soil was significantly less than that in bulk soil in the control (P < 0.05). The quantity of soil bacteria in rhizosphere soil was greater than that in bulk soil in plots exposed to each grazing treatment, but these differences were not significant (P > 0.05) (Figure 3a). The quantity of fungi and actinomycetes in rhizosphere soil was greater than that in bulk soil under moderate and heavy grazing, and these differences were significant (P < 0.05) (Figure 3b,c). However, the quantity in rhizosphere soil was lower than that in bulk soil in the control and light-grazing plots.
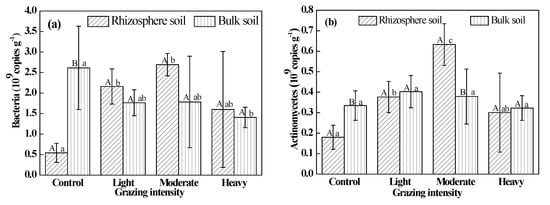
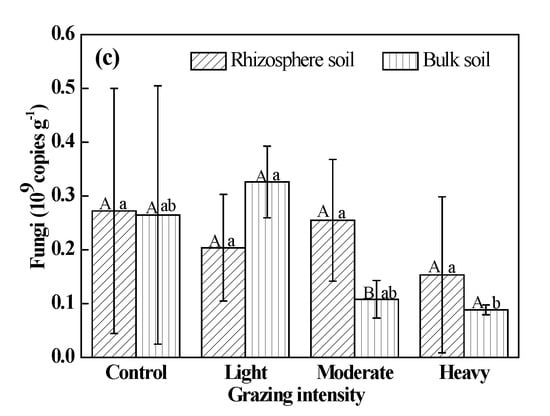
Figure 3.
Populations of soil microbes. (a) Bacteria; (b) Actinomycetes; (c) Fungi. Different uppercase letters indicate significant differences in rhizosphere and bulk soil under certain grazing intensity at 0.05 level. Different lowercase letters indicate significant differences under different grazing intensity at 0.05 level in rhizosphere and bulk soil, respectively.
Grazing decreased the ratio of actinomycetes to bacteria in rhizosphere soil, whereas it increased the ratios of actinomycetes to fungi and bacteria to fungi in the same soil (Table 2). In contrast, grazing increased the ratio of actinomycetes to bacteria in bulk soil. The ratios of actinomycetes to fungi and bacteria to fungi were lower in plots under light grazing than in the control plot, and these ratios increased with grazing intensity (Table 2).

Table 2.
Ratios between populations of soil microbial.
3.4. Root Biomass
As grazing intensity increased, root biomass decreased and then increased (Figure 4). However, there was no significant difference between the plots under different grazing intensities (P > 0.05).
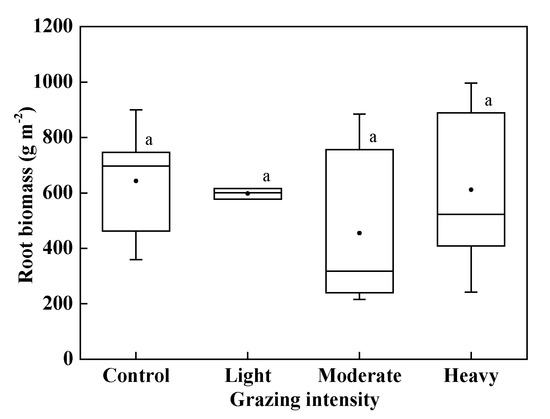
Figure 4.
Root biomass. Same lowercase letters indicate no significant differences under different grazing intensity at 0.05 level.
3.5. Soil pH
The pH in rhizosphere soil was lower than that in bulk soil in plots under all treatments (Figure 5). As grazing intensity increased, soil pH increased and then decreased, but this difference was not significant (P > 0.05).
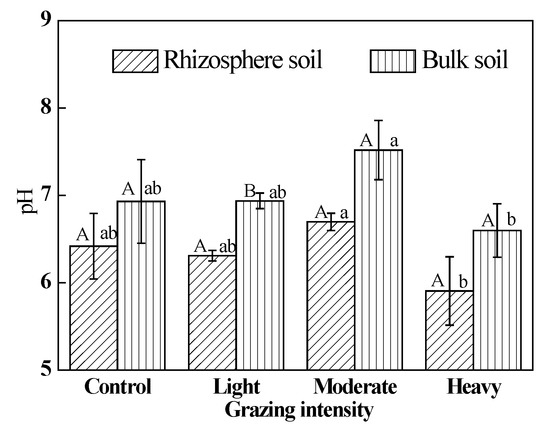
Figure 5.
Soil pH. Different uppercase letters indicate significant differences in rhizosphere and bulk soil under certain grazing intensity at 0.05 level. Different lowercase letters indicate significant differences under different grazing intensity at 0.05 level in rhizosphere and bulk soil respectively.
4. Discussion
4.1. Effects of Grazing on TOC and the Fractions
Grazing intensity affects the TOC, as well as the soil active organic carbon [20]. Some studies showed that the SOC and component contents decreased with increasing grazing intensity [40,47,48], but other results showed the complete opposite [49]. There are also results that showed that the SOC and component contents increased first and then decreased [37,50], which is the same as the results of this study. Soil active organic carbon components will be preferentially lost during the process of soil degradation [51]. Although active organic carbon components account for a small proportion of TOC. They play an important role in SOC cycling [52,53]. Some studies showed that grazing can reduce the MBC by 20% [21,54] or increase it first, which was then followed by a decrease [55,56]. There are also studies that showed that grazing has no significant effect on the MBC [57]. Grazing increases the POC content [58], decreases it [51], shows no significant difference [30], or first increases and then decreases it with only a slight difference [59]. POC is more stable than the other SOC fractions [30], and the rhizosphere is more conducive to maintaining the POC than the bulk soil in grazed grasslands. The formation of large aggregates contributes to carbon sequestration in the soil [60]. Root exudates can promote the formation of large aggregates [61] and take advantage of the soil properties to increase and stabilize the organic carbon content [62]. There are few related studies on PMC, DOC, and other components of the soil, but the existing results show that grazing has no significant difference in the PMC and DOC [59,63]. Chang et al. [64] found that the TOC content in Mongolian grasslands increased with the decrease in grazing intensity, and the cumulative increase of the soil TOC by reducing grazing intensity could reach 21.96–36.91 g C m−2 yr−1, indicating that an appropriate grazing intensity can solve the problem of the loss of soil carbon due to heavy grazing.
4.2. The Role of Rhizosphere in SOC Transformation
The rhizosphere is the most important site for nutrient cycling between plants and the environment. Organic matter can be released into the soil through root exudates [10,65]. Forage can transfer 30–50% of carbon assimilated through photosynthesis to underground parts of a plant [12]. In turn, the roots can release 3–5% of carbon fixed by photosynthesis into the soil [10,66]. There are more biogeochemical reactions in the plant and soil system of the rhizosphere than in bulk soil. Thus, the rhizosphere is more responsive to environmental changes [67]. Further, because the rhizosphere is the most active site for plant energy and material metabolism, physiological activities in the rhizosphere significantly affect soil properties, carbon cycling, and the distribution of carbon fractions in terrestrial ecosystems [68]. In the present study, SOC content in rhizosphere soil was higher than that in bulk soil (Figure 1). In addition, we found that the enrichment rates in plots under the various grazing treatments were greater than that in the control (Table 1). Grazing can stimulate the growth of root biomass (Figure 4) and root exudation rates, thereby affecting the amount of organic carbon released by roots into the soil [10,69,70]. The rhizosphere of S. grandis, therefore, is very important for grassland carbon enrichment in grazed grasslands.
In the present study, TOC, MBC, DOC, PMC, and POC contents in rhizosphere soil were higher than those in bulk soil (Figure 1). Because of interactions between the roots and soil, the rhizosphere soil environment is more stable than that of bulk soil, causing SOC to be maintained. Therefore, the impact of grazing on SOC in bulk soil was greater than that in rhizosphere soil. Because of rhizosphere exudation, rhizosphere soil is wet and dried repeatedly [71], which can stimulate the soil to continue exuding soluble nutrients. The activity of soil microbes in the rhizosphere may also stimulate soil organic matter mineralization, which causes the soil to exude soluble matter further [72]. In addition, root exudation can affect soil water action [71,73] and ultimately influence SOC content. The rhizosphere enrichment rates in plots under moderate and heavy grazing were higher than those in plots under control and light grazing. However, these differences were not significant (Table 1). Yang et al. [10] obtained similar results in a study of an alpine meadow. Roots release 3–5% of the carbon fixed by photosynthesis into the soil [66]. S. grandis has a developed root system and many fibrous roots. After the aboveground part is eaten, the production of root exudates is stimulated [10]. The total amount of organic matter secreted by the roots increased at last.
4.3. The Effects of Grazing on Soil Microbes in Rhizosphere Soil
Grazing is one of the main causes of changes in soil microbe community structure [22,74,75]. Changes in the abundance of soil microbes differed between rhizosphere and bulk soil as grazing intensity increased (Figure 3). Because of rhizodeposition, the responses of microbe community structure to different grazing intensities differed between rhizosphere and bulk soil. Defoliation stimulates root carbon exudation and increases soil microbe biomass in the rhizosphere [25], resulting in a greater abundance of soil microbes in rhizosphere soil than in bulk soil [19]. Root growth promotes rhizodeposition and soil microbial activity, and the repeated wetting and drying of soil at the root-soil interface results in a heterogeneous soil matrix with physical properties that are different from those of soil distant from the root [62].
Soil microbes play many roles in soil-plant interactions and the degradation of soil organic matter and plant litter, including aiding the circulation of soil elements and especially carbon. Therefore, small changes in soil microbial processes can have major impacts on greenhouse gas flux [76,77]. Because different soil microbes obtain different nutrients, changes in soil microbe community structure can affect the turnover of soil organic matter and nutrients [22,71,78]. For example, bacteria can produce extracellular metabolites, such as polysaccharides, lipids, and proteins that stabilize aggregates [79]. Actinomycetes mainly decompose components of plant tissues that are difficult to break down, converting these materials to soil organic components and forming the most stable organic compounds in the soil [79]. Fungi act to decompose cellulose, lignin, and pectin from plant residues in the soil. They can also decompose protein compounds to release nitrogen and participate during soil organic matter decomposition and humus synthesis [79].
4.4. The Effects of pH on SOC in Rhizosphere Soil
On the one hand, carbon dioxide is released through root respiration during the process of plant growth. Alternatively, protons and organic acids are secreted during the active absorption of ions and elongation of root tip cells, which thus, change the pH of rhizosphere. Once under stress, the intensity of protons and organic acids secreted by roots could increase, and the pH in rhizosphere could also change more. No correlation between the root biomass and SOC content in rhizosphere soil (r = −0.196, P = 0.407) was identified in this study. By elimination, the rhizosphere soil is acidified by carbon dioxide and organic acids excreted by the roots. Therefore, the pH in rhizosphere soil is lower than that in the bulk soil [25,29,71]. We confirmed this association in our study (Figure 5).
SOC transformation occurs within a certain pH range, and a change in pH can affect this transformation. For example, when pH is reduced, the abundance of fungi adapted to acidic environments increases [80]. Some studies have shown that a reduction in the pH of rhizosphere soil can increase the availability and activity of various mineral nutrients, allowing the use of additional soil nutrients in fragile environments [81]. This is mainly because the rhizosphere pH affects the rhizosphere environment in many ways, such as increasing the availability of nutrients, nutrient absorption by the roots, permeability of the root cell membranes, status of the roots, type and quantity of root exudates, and the species and quantity of rhizosphere microbial and enzyme activity.
5. Conclusions
Appropriate grazing, such as light and moderate grazing, can increase the SOC content. Controlled grazing can not only provide resources for grassland production but also improve the ecosystem carbon sink function. The rhizosphere showed an SOC enrichment effect, and this effect tended to increase as the intensity of grazing increased. As the grazing intensity increased, the MBC content and MBC/TOC increased in both the rhizosphere and the bulk soil, but the data were more stable in the rhizosphere soil. The DOC content and DOC/TOC only increased in the rhizosphere soil. Light and moderate grazing can increase the contents of PMC and POC, as well as those of the PMC/TOC and POC/TOC. The maximum PMC and PMC/TOC in the rhizosphere soil was found in moderate grazing, while it was found in light grazing in bulk soil. POC acts in an opposite manner to PMC. Grazing and the rhizosphere affected the conversion and mineralization of SOC mainly by changing the number and structure of soil microorganisms and soil pH. It is also important to study the technical aspects of root exudates in soil carbon sequestration in natural ecosystems. We can increase organic carbon in these ecosystems through the deposition of root exudates. Therefore, in the adaptive management of typical grasslands in Inner Mongolia, we can explore ways to increase the carbon stock of the ecosystem through fine-grained measures, such as optimizing grazing intensity and re-seeding local species with advanced root systems. It is of great significance to achieve efficient synergy between ecological protection and economic development, increase the function of carbon sink, and help to achieve the goal of carbon peaking/carbon neutral target.
Author Contributions
Conceptualization, M.Z. and X.L.; methodology, X.L.; investigation, M.Z. and M.L.; resources, X.L. and P.Y.; data curation, M.Z.; writing—original draft preparation, M.Z. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Basic Research Program of China (2016YFC0500502) and the National Natural Science Foundation of China (31570451).
Acknowledgments
We thank the Institute of Grassland Research of Chinese Academy of Agricultural Sciences for offering experimental grazing plots.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arun, J.N.; Rattan, L.; Gudeta, W.S.; Ashesh, K.D. Managing India’s small landholder farms for food security and achieving the “4 per Thousand” target. Sci. Total Environ. 2018, 634, 1024–1033. [Google Scholar]
- Rumpel, C.; Lehmann, J.; Chabbi, A. Boost soil carbon for food and climate. Nature 2018, 553, 27. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: Why and how. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Summary for policymakers. In Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Monkany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Jeddi, K.; Chaieb, M. Changes in soil properties and vegetation following livestock grazing exclusion in degraded arid environments of South Tunisia. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 184–189. [Google Scholar] [CrossRef]
- Dlamini, P.; Chivenge, P.; Chaplot, V. Overgrazing decreases soil organic carbon stocks the most under dry climates and low soil pH: A meta-analysis shows. Agric. Ecosyst Environ. 2016, 221, 258–269. [Google Scholar] [CrossRef]
- Hartmann, A.; Lemanceau, P.; Prosser, J.I. Multitrophic interactions in the rhizosphere—Rhizosphere microbiology: At the interface of many disciplines and expertises. FEMS Microbiol. Ecol. 2008, 65, 179. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.N.; Zhu, Q.A.; Zhan, W.; Xu, Y.Y.; Zhu, E.X.; Gao, Y.H.; Li, S.Q.; Zheng, Q.Y.; Zhu, D.; He, Y.X.; et al. The linkage between vegetation and soil nutrients and their variation under different grazing intensities in an alpine meadow on the eastern Qinghai-Tibetan Plateau. Ecol. Eng. 2018, 110, 128–136. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Rev. J. Plant Nutr. Soil. Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Zhao, X.F.; Tian, P.; Sun, Z.L.; Liu, S.G.; Wang, Q.K.; Zeng, Z.Q. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil. Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Li, W.J.; Li, Y.; Lv, J.; He, A.M.; Wang, J.L.; Teng, D.X.; Jiang, L.M.; Wang, H.F.; Lv, G.H. Rhizosphere effect alters the soil microbiome composition and C, N transformation in an arid ecosystem. Appl. Soil. Ecol. 2022, 170, 104296. [Google Scholar] [CrossRef]
- Paterson, E.; Midwood, A.J.; Millard, P. Through the eye of the needle: A review of isotope approaches to quantify microbial processes mediating soil carbon balance. New Phytol. 2009, 184, 19–33. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil. Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Noah, W.S.; Eric, S.; Gianna, L.M.; Alexa, N.; Steven, J.B.; Eoin, L.B.; Mary, K.F.; Megan, M.F.; Rachel, H.; Bruce, A.H.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar]
- Hu, J. The Effects of Grazing on Soil Nutrient and Soil Microbes in Rhizosphere of Stipa Grandis in the Typical Steppe. Ph.D. Thesis, Institute of Grassland Research of Chinese Academy of Agricultural Science, Hohhot, China, 2015. (In Chinese with English abstract). [Google Scholar]
- Mcsherry, M.; Ritchie, M.E. Effects of grazing on grassland soil carbon: A global review. Glob. Chang. Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhou, X.H.; He, Y.H.; Shao, J.J.; Hu, Z.H.; Liu, R.Q.; Zhou, H.M. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Chang. Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef]
- Sun, G.; Zhu-Barker, X.; Chen, D.M.; Liu, L.; Zhang, N.N.; Shi, C.G.; He, L.P.; Lei, Y.B. Responses of root exudation and nutrient cycling to grazing intensities and recovery practices in an alpine meadow: An implication for pasture management. Plant Soil. 2017, 416, 515–525. [Google Scholar] [CrossRef]
- Rajaniemi, T.K.; Goldberg, D.E.; Turkington, R.; Dyer, A.R. Local filters limit species diversity, but species pools determine composition. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 373–380. [Google Scholar] [CrossRef]
- Paterson, E.; Thornton, B.; Midwood, A.J.; Sim, A. Defoliation alters the relative contributions of recent and non-recent assimilate to root exudation from Festuca rubra. Plant Cell Environ. 2005, 28, 1525–1533. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Frank, D.A.; Hinchey, P.M.; Murray, T.R. Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil. Biol. Biochem. 2008, 40, 2865–2873. [Google Scholar] [CrossRef]
- Holland, J.N.; Cheng, W.X.; Crossley, D.A. Herbivore-induced changes in plant carbon allocation: Assessment of below-ground C fluxes using carbon-14. Oecologia 1996, 107, 87–94. [Google Scholar] [CrossRef]
- Augustine, D.J.; Dijkstra, F.A.; Hamilton, E.W.; Morgan, J.A. Rhizosphere interactions, carbon allocation, and nitrogen acquisition of two perennial North American grasses in response to defoliation and elevated atmospheric CO2. Oecologia 2011, 165, 755–770. [Google Scholar] [CrossRef]
- Zhang, W.W.; Yang, J.; Song, B.Y.; Qing, H. Impacts of moving on the rhizosphere soil properties of Krascheninnikovia ceratoides in the steppe desert. Acta Ecol. Sin. 2016, 36, 6842–6849, (In Chinese with English abstract). [Google Scholar]
- Wei, X.J. The Effect of Grazing Intensity to Nutrition of Stipa Grandis Rhizosphere which is the Mainly Built Plants of Typical Steppe. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2011. (In Chinese with English abstract). [Google Scholar]
- Herfurth, D.; Vassal, N.; Louault, F.; Alvarez, G.; Pottier, J.; Picon-Cochard, C.; Bosio, I.; Carrère, P. How does soil particulate organic carbon respond to grazing intensity in permanent grasslands? Plant Soil 2015, 394, 239–255. [Google Scholar]
- Zhang, M.; Li, X.B.; Wang, H.; Huang, Q. Comprehensive analysis of grazing intensity impacts soil organic carbon: A case study in typical steppe of Inner Mongolia, China. Appl. Soil Ecol. 2018, 129, 1–12. [Google Scholar] [CrossRef]
- Qiu, Q.Y.; Wu, L.F.; Ouyang, Z.; Li, B.B.; Xu, Y.Y.; Wu, S.S.; Gregorich, E.G. Effects of plant-derived dissolved organic matter (DOM) on soil CO2 and N2O emissions and soil carbon and nitrogen sequestrations. Appl. Soil Ecol. 2015, 96, 122–130. [Google Scholar] [CrossRef]
- Jiang, G.; Han, X.; Wu, J. Restoration and management of the Inner Mongolia grassland require a sustainable strategy. Ambio 2009, 35, 269–270. [Google Scholar]
- Pan, W.J.; Song, Z.L.; Liu, H.Y.; Müellerd, K.; Yang, X.M.; Zhang, X.D.; Lie, Z.M.; Liu, X.; Qiu, S.; Hao, Q.; et al. Impact of grassland degradation on soil phytolith carbon sequestration in Inner Mongolian steppe of China. Geoderma 2017, 308, 86–92. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, Z.L.; Chang, Y.; Ren, L.J.; Wang, R.; Wu, X.X.; Fan, Q. The stability and compensation of the primary productivity of the typical steppe in Inner Mongolia. J. Arid Land Res. Environ. 2014, 28, 1–8, (In Chinese with English abstract). [Google Scholar]
- Shan, D.; Zhao, M.L.; Han, B.; Han, G.D. Genetic diversity of Stipa grandis under different grazing pressures. Acta Ecol. Sin. 2006, 10, 3175–3181, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, 2nd ed.; World Soil Resources Reports; FAO: Rome, Italy, 2006. [Google Scholar]
- Hoffmann, C.; Funk, R.; Wieland, R.; Li, Y.; Sommer, M. Effects of grazing and topography on dust flux and deposition in the Xilingele grassland, Inner Mongolia. J. Arid Environ. 2008, 72, 792–807. [Google Scholar] [CrossRef]
- Steffens, M.; Koelbl, A.; Totsch, E.K.U.; Kögel-Knabner, I. Grazing effects on soilchemical and physical properties in a semiarid steppe of Inner Mongolia (PR China). Geoderma 2008, 143, 63–72. [Google Scholar] [CrossRef]
- Shrestha, G.; Stahl, P.D. Carbon accumulation and storage in semi-arid sagebrush steppe: Effects of long-term grazing exclusion. Agric. Ecosyst. Environ. 2008, 125, 173–181. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ma, W.H.; Smith, P.; Mohammat, A.; Wang, S.P.; Wang, W. Soil carbon stock and its changes in northern China’s grassland from 1980s to 2000s. Glob. Chang. Biol. 2010, 16, 3036–3047. [Google Scholar] [CrossRef]
- Xiong, D.P.; Shia, P.L.; Zhang, X.Z.; Zou, C.B. Effects of grazing exclusion on carbon sequestration and plant diversity in grasslands of China-a meta-analysis. Ecol. Eng. 2016, 94, 647–655. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil. Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Xu, M.G.; Lou, Y.L.; Sun, X.L.; Wan, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fert. Soils 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Aldezabal, A.; Moragues, L.; Odriozola, I.; Mijangos, I. Impact of grazing abandonment on plant and soil microbial communities in an Atlantic mountain grassland. Appl. Soil Ecol. 2015, 96, 251–260. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, H.L.; Li, Y.L.; Cui, J.Y. Influencing mechanisms of several shrubs on soil chemical properties in semiarid Horqin Sandy Land, China. Arid Land Res. Manag. 2010, 18, 251–263. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Barthold, F.K.; Blank, F.B.; Kögel-Knabner, I. Digital mapping of soil organic matter stocks using Random Forest modeling in a semi-arid steppe ecosystem. Plant Soil 2011, 340, 7–24. [Google Scholar] [CrossRef]
- Li, X.D.; Zhang, C.P.; Fu, H.; Guo, D.; Song, X.R.; Wan, C.G.; Ren, J.Z. Grazing exclusion alters soil microbial respiration, root respirationand the soil carbon balance in grasslands of the Loess Plateau, northern China. Soil Sci. Plant Nutr. 2013, 59, 877–887. [Google Scholar] [CrossRef]
- Chen, J.B.; Hou, F.J.; Chen, X.J.; Wan, X.L.; Millner, J. Stocking rate and grazing season modify soil respiration on the Loess Plateau, China. Rangel. Ecol. Manag. 2015, 68, 48–53. [Google Scholar] [CrossRef]
- Holt, J.A. Grazing pressure and soil carbon, microbial biomass and enzyme activities in semi-arid northeastern Australia. Appl. Soil Ecol. 1997, 5, 143–149. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Munro, S.; Barthold, F.; Steffens, M.; Schad, P.; Kogel-knabner, I. Carbon storage capacity of semi-arid grassland soils and sequestration potentials in northern China. Glob. Chang. Biol. 2015, 21, 3836–3845. [Google Scholar] [CrossRef]
- Wander, M.M.; Traina, S.J.; Stinner, B.R.; Peters, S.E. Organic and conventional management effects on biologically active soil organic matter pools. Soil. Sci. Soc. Am. J. 1994, 58, 1130–1139. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Ma, X.Z. Effects of cultivation and grazing on soil carbon pool and greenhouse gases fluxes in the Inner Mongolia Steppes. Ph.D. Thesis, Institute of Botany, The Chinese Academy of Science, Beijing, China, 2006. (In Chinese with English abstract). [Google Scholar]
- Li, S.Q.; Wang, X.Z.; Guo, Z.G.; Zhou, J.; Xue, R.; Shen, Y.Y. Effects of short-term grazing on C and N content in soil and microbe in Alpine Meadow in the north-eastern edge of the Qinghai-Tibetan Platesu. Chin. J. Grassl. 2013, 31, 367–374, (In Chinese with English abstract). [Google Scholar]
- Zhao, N.; Zhuang, Y.; Zhao, J. Effects of grassland managements on soil organic carbon and microbial biomass carbon. Pratacultural Sci. 2014, 31, 367–374, (In Chinese with English abstract). [Google Scholar]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; Conover, D.M. Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agric. Ecosyst. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- Leifeld, J.; Fuhrer, J. Long-term management effects on soil organic matter in two cold, high-elevation grasslands: Clues from fractionation and radiocarbon dating. Eur. J. Soil Sci. 2009, 60, 230–239. [Google Scholar] [CrossRef]
- Martinsen, V.; Mulder, J.; Austrheim, G.; Mysterud, A. Carbon storage in low-alpine grassland soils: Effects of different grazing intensities of sheep. Eur. J. Soil Sci. 2011, 62, 822–833. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Valadimirov, A.A.; Meier, I.C.; Mueller, C.W. Root exudates induce soil macroaggregation facilitated by fungi in subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Poonam, P.; Catherine, P.; Josep, P.; Jlitender, G. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar]
- Fu, G.; Zhang, X.Z.; Yu, C.Q.; Shi, P.L.; Zhou, Y.T.; Li, Y.L.; Yang, P.W.; Shen, Z.X. Response of soil respiration to grazing in an alpine meadow at three elevations in Tibet. Sci. World J. 2014, 2014, 265142. [Google Scholar] [CrossRef]
- Chang, X.F.; Bao, X.Y.; Wang, S.P.; Wilkes, A.; Erdenetsetseg, B.; Baival, B.; Avaadorj, D.; Maisaikhan, T.; Damdinsuren, B. Simulating effects of grazing on soil organic carbon stocks in Mongolian grasslands. Agric. Ecosyst. Environ. 2015, 212, 278–284. [Google Scholar] [CrossRef]
- Zhang, F.S.; Shen, J.B.; Zhang, J.L.; Zuo, Y.M.; Li, L.; Chen, X.P. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107, 1–32. [Google Scholar]
- Dijkstra, F.A.; Cheng, W.X.; Johnson, D.W. Plant biomass influences rhizosphere priming effects on soil organic matter decomposition in two differently managed soils. Soil Biol. Biochem. 2006, 38, 2519–2526. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.L.; Bowatte, S.; Newton, P.C.D.; Zhang, Q.F. Rhizospheric carbon-nitrogen interactions in a mixed-species pasture after 13 years of elevated CO2. Agric. Ecosyst. Environ. 2016, 235, 134–141. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, L.; Li, X.B.; Li, Y.F.; Yang, X.G. Soil nutrients and carbon management indexes in the rhizosphere versus non rhizosphere area of different plant species in desert grassland. Acta Prataculturae Sci. 2017, 26, 24–34, (In Chinese with English abstract). [Google Scholar]
- Jiang, L.L.; Wang, S.P.; Pang, Z.; Wang, C.S.; Kardol, P.; Zhou, X.Q.C.; Wang, Y.F.; Xu, X.L. Grazing modifies inorganic and organic nitrogen uptake by coexisting plant species in alpine grassland. Biol. Fertil. Soils 2016, 52, 211–221. [Google Scholar] [CrossRef]
- Wilson, C.H.; Strickland, M.S.; Hutchings, J.A.; Bianchi, T.S.; Flory, S.L. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Glob. Chang. Biol. 2018, 24, 2997–3009. [Google Scholar] [CrossRef]
- Gregory, P.J. Roots, rhizosphere and soil: The route to a better understanding of soil science? Eur. J. Soil Sci. 2006, 57, 2–12. [Google Scholar] [CrossRef]
- Khalid, M.; Soleman, N.; Jones, D.L. Grassland plants affects dissolved organic carbon and nitrogen dynamic in soil. Soil Biol. Biochem. 2007, 39, 378–381. [Google Scholar] [CrossRef]
- Passioura, J.B. Water transport in and to roots. Annu. Rev. Plant Physiol. Mol. Biol. 1988, 39, 245–265. [Google Scholar] [CrossRef]
- Patra, A.; Abbadie, L.; Clays-Josserand, A.; Degrange, V.; Grayston, S.J.; Loiseau, P.; Louault, F.; Mahmood, S.; Nazaret, S.; Philippot, L.; et al. Effects of grazing on microbial functional groups involved in soil N dynamics. Ecol. Monogr. 2005, 74, 65–80. [Google Scholar] [CrossRef]
- Eldridge, D.; Delgado-Baquerizo, M.; Travers, S.K.; Val, J.; Oliver, I.; Hamonts, K.; Singh, B.K. Competition drives the response of soil microbial diversity to increased grazing by vertebrate herbivores. Ecology 2017, 98, 1922–1931. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.C.; An, S.H.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lakshmaiian, V.; Kitto, S.L.; Caplan, J.L.; Hsueh, Y.H.; Kearns, D.B.; Wu, Y.S.; Bais, H.P. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 2012, 160, 1642–1661. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Q.; Yang, G.X.; Cui, Y.X.; Xin, X.P.; Liu, Z.L.; Dai, Y.T.; Zhao, J. Distribution characteristics of the number of soil microorganisms under different grazing intensity in Xilingol typical steppe. Chin. J. Grassl. 2011, 33, 63–68, (In Chinese with English abstract). [Google Scholar]
- Liu, T.; Diao, Z.M.; Qi, Y.Q. The primary advances rhizosphere microbiology. Qinghai Pr. 2008, 17, 41–47, (In Chinese with English abstract). [Google Scholar]
- Yang, Y.; Liu, B.R. Distribution of soil nutrient and microbial biomass in rhizosphere versus non-rhizosphere area of different plant species in desertified steppe. Acta Ecol. Sin. 2015, 35, 7562–7570, (In Chinese with English Abstract). [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).