Chlorination Treatment for Gold Extraction from Refractory Gold-Copper-Arsenic-Bearing Concentrates
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Sample Preparation
2.3. Thermodynamic Calculations
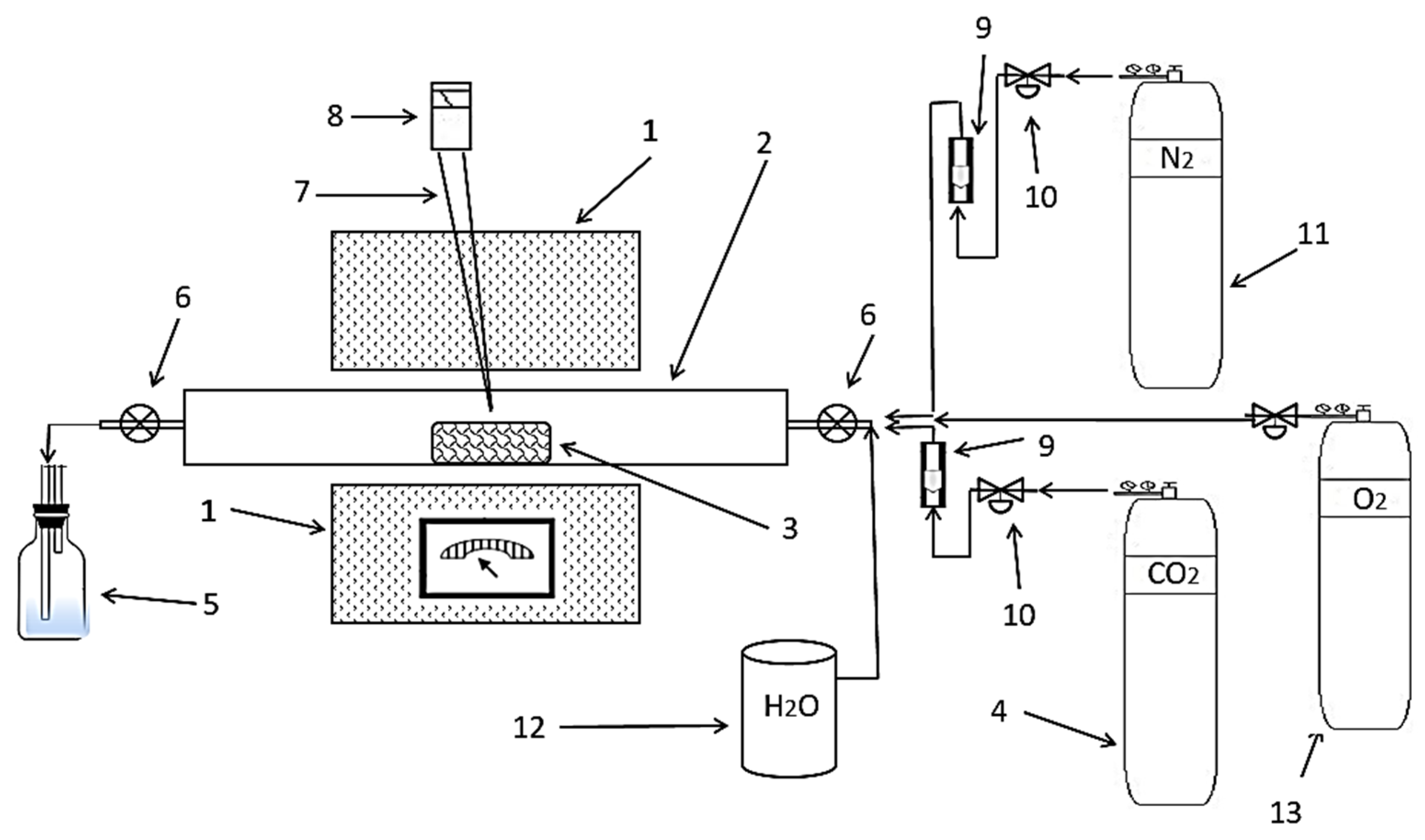
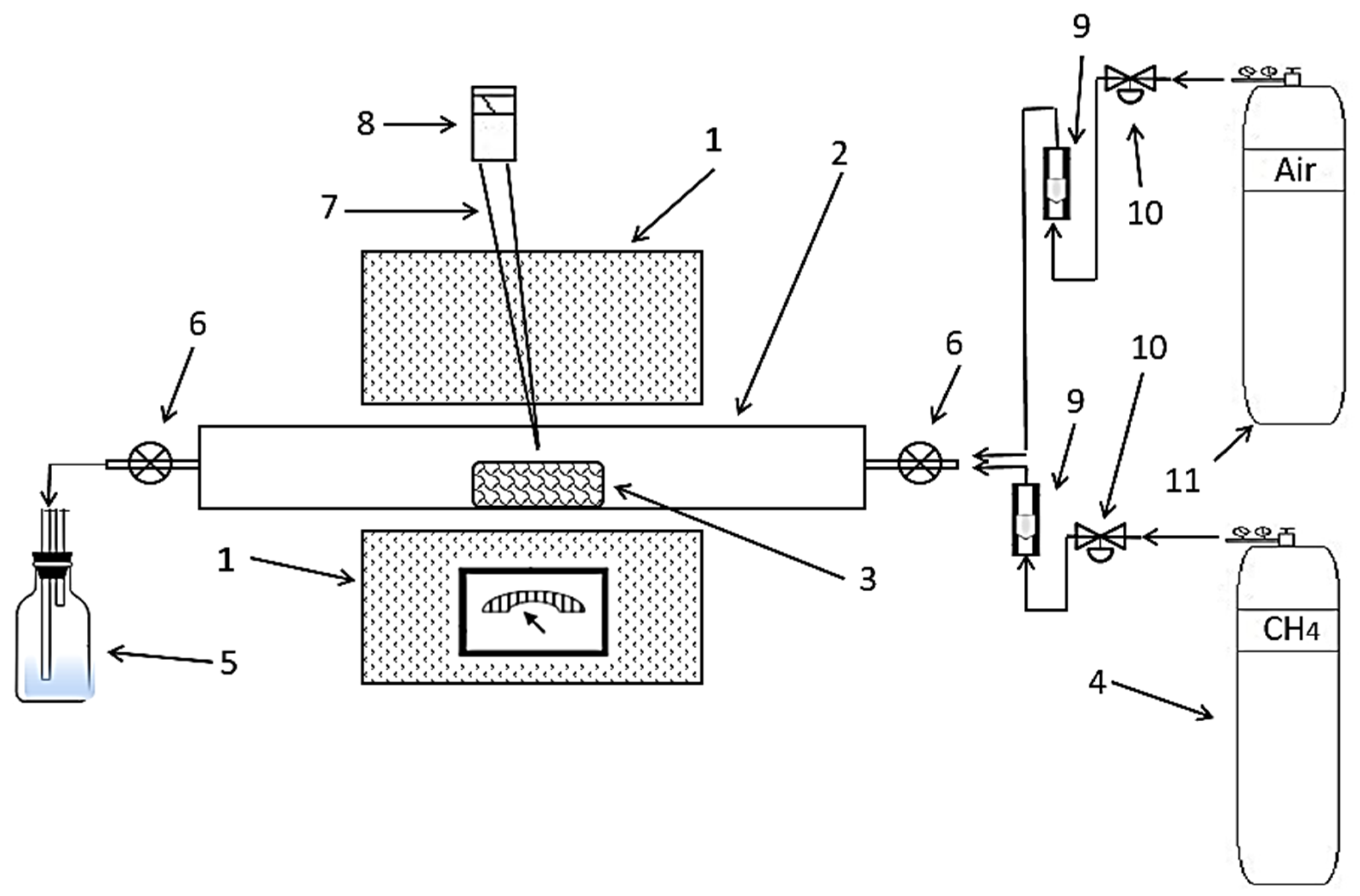
2.4. Oxidation Sintering
2.5. Reduction Sintering
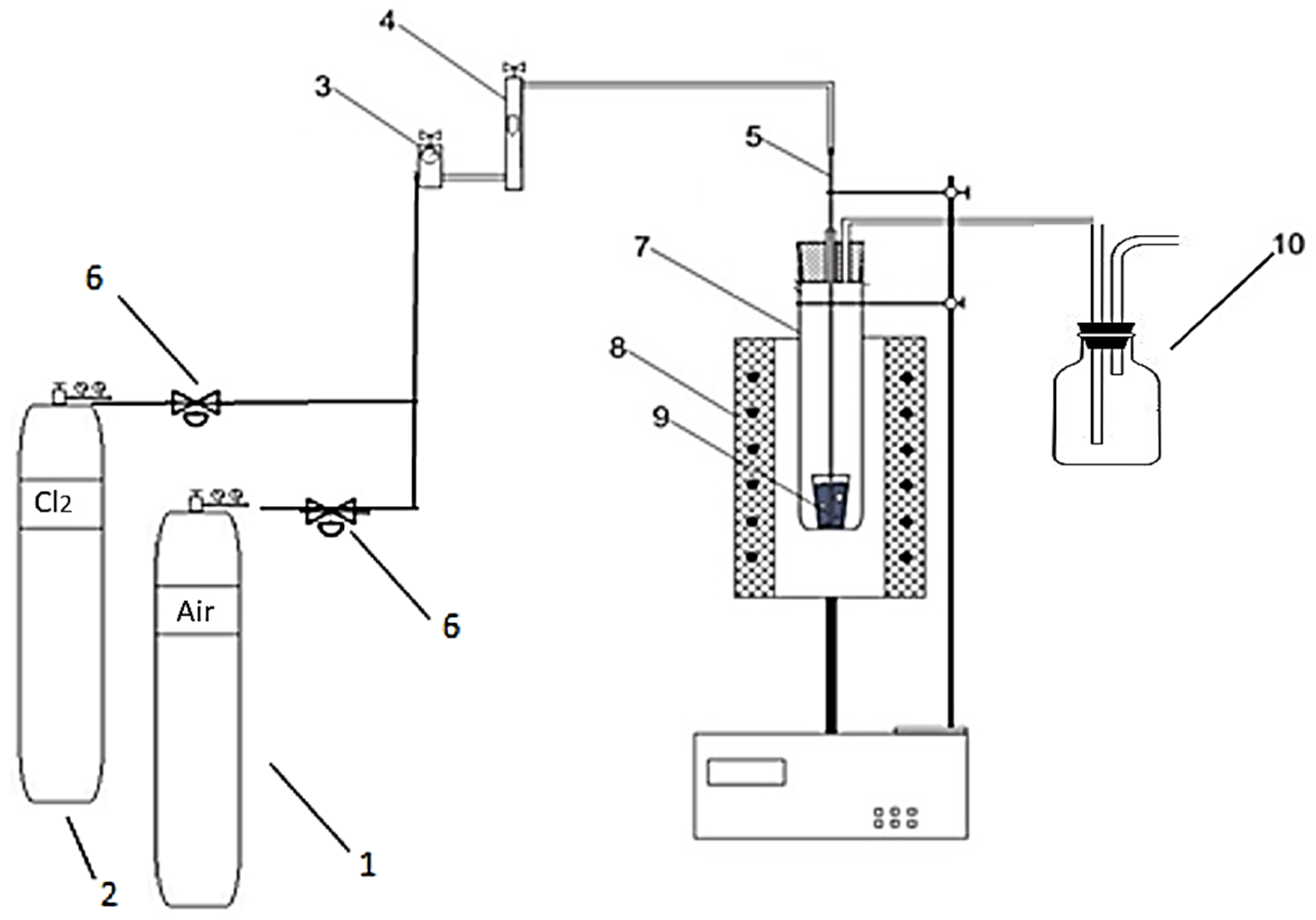
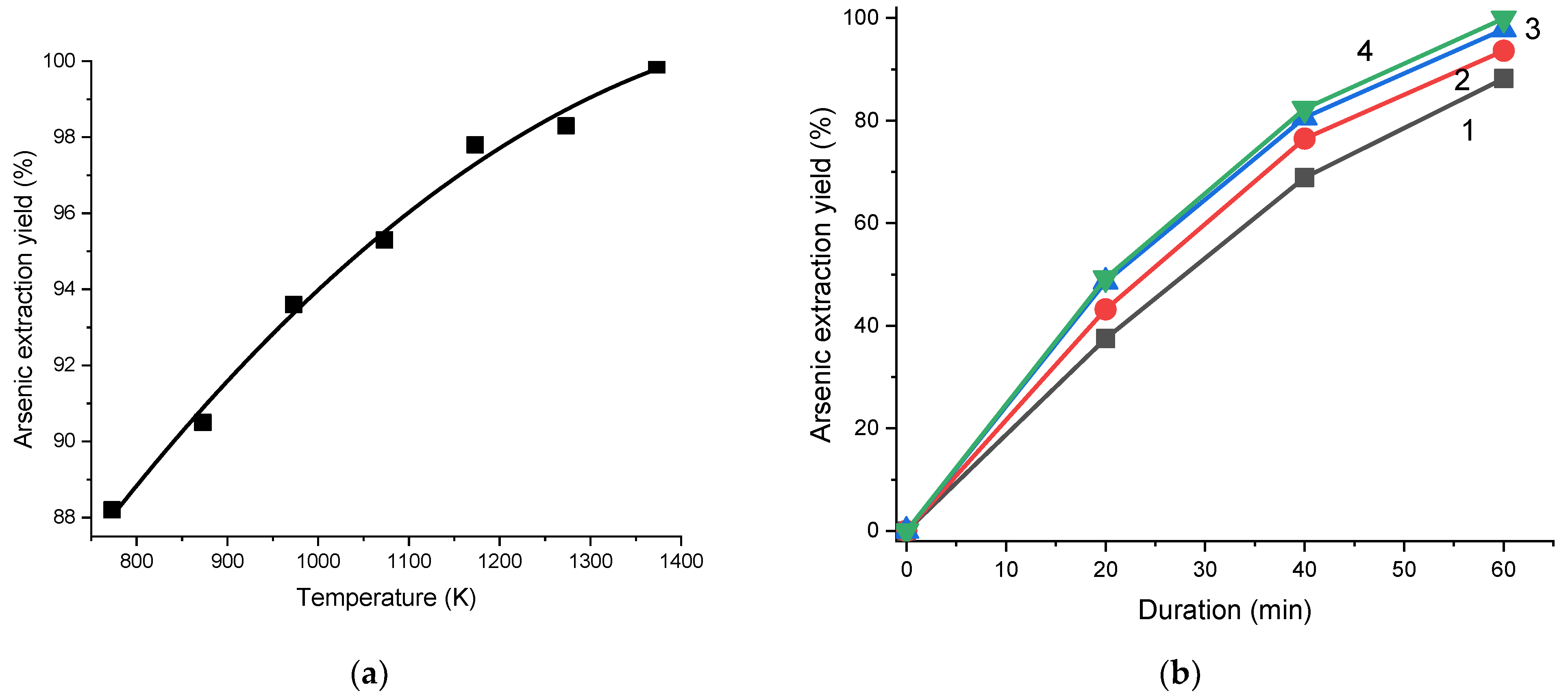
2.6. Chlorination
3. Results and Discussion
3.1. Thermodynamic Calculations
3.2. Oxidation Sintering
3.3. Reduction Sintering
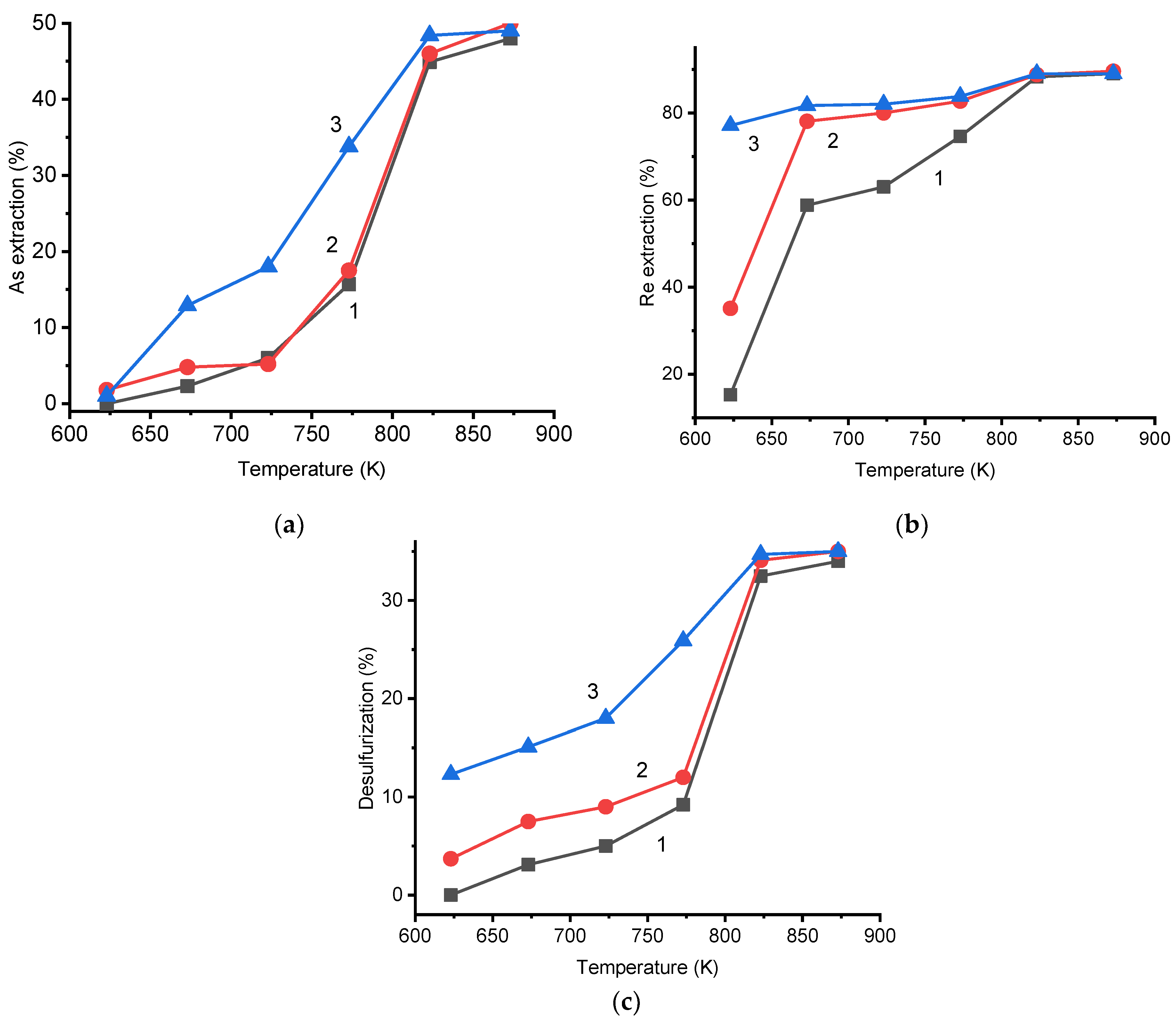
3.4. Chlorination Processing of the Cu-Au-As Concentrate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.Y.; Xin, Y.T.; Wang, H.; Tian, Q.H. Mineralogical characterization and pretreatment for antimony extraction by ozone of antimony-bearing refractory gold concentrates. Trans. Nonferrous Met. Soc. China 2017, 27, 1888–1895. [Google Scholar] [CrossRef]
- Khudoyarov, S.R.; Ilkhomov, D.A.; Bozorov, A.B. Technology of Extraction of Gold from Persistent Carbonaceous Gold-Bearing Ores. In Proceedings of the Results of Modern Scientific Research and Development: International Scientific and Practical Conference, Chelyabinsk, Russia, 7–8 November 2019; Science and Education: Penza, Russia, 2019. [Google Scholar]
- Panias, D.; Neou-Syngouna, P. Gold extraction from pyrite cinders by high temperature chlorination. Erzmetall 1990, 43, 41–44. [Google Scholar]
- Tukibaev, B.; Zholdasov, A.; Zholdasbay, E.; Dosmukhamedov, N.K. Investigation of gold extraction from resistant gold-bearing ores. Poisk 2015, 1, 300–305. [Google Scholar]
- Volkov, A.V. Prospects for the development of gold mining in Kazakhstan. Zel. Technol. 2011, 3, 13–16. [Google Scholar]
- Wang, H.J.; Feng, Y.L.; Li, H.R.; Kang, J.X. Simultaneous extraction of gold and zinc from refractory carbonaceous gold ore by chlorination roasting process. Trans. Nonferrous Met. Soc. China 2020, 30, 1111–1123. [Google Scholar] [CrossRef]
- Magyar, M.J. Rhenium. In US Geological Survey Minerals Yearbook; USGS: Washington, DC, USA, 2004; pp. 62.1–62.4. [Google Scholar]
- ADB. Mineral Resources Geologists’ Paradise. In Central Asia Atlas of Natural Resources; Asian Development Bank: Manila, Philippines, 2006; pp. 62–67. [Google Scholar]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Bukurov, T.N. Distribution of rhenium and osmium by products of processing of sulfide copper raw materials. Tsvetnye Met. 1997, 9, 47–50. [Google Scholar]
- Smirnov, M.P.; Khvan, V.T.; Shmakova, R.P. Study of the interaction of rhenium vapors with oxides, sulfates of heavy non-ferrous metals and industrial dusts. Tsvetnye Met. 1979, 9, 30–32. [Google Scholar]
- Malykhin, V.F.; Smirnov, M.P.; Besser, A.D. Influence of temperature and chemical composition of lead-containing dusts on the transition of rhenium in them. Tsvetnye Met. 1986, 2, 31–34. [Google Scholar]
- Ministry of Agriculture of the Republic of Kazakhstan. Raw Material Base of Lead and Zinc, COPPER, gold of Kazakhstan: Tez. Dokl. Confer. in the Committee of Geology and Subsoil Use of the Ministry of Agriculture of the Republic of Kazakhstan; Ministry of Agriculture of the Republic of Kazakhstan: Almaty, Kazakhstan, 2002; 102p.
- Kulkashev, N.T.; Baibatsha, A.B. On the Genetic Classification of Mineral Deposits. Satpayev Readings: Problems of Geology and Mineralogy of the Development of Mineral Resources; Satpayev University: Almaty, Kazakhstan, 2010; pp. 192–198. [Google Scholar]
- Dosmukhamedov, N.K.; Egizekov, M.G. Conditions for the Sustainable Development of the Copper Industry. Theory. Experiment. Practice; SSA Production: Almaty, Kazakhstan, 2021; 308p. [Google Scholar]
- Swinburne, D.R.; Ko, T.S. Computational Thermodynamics Modeling of Minor Element Distribution During Copper Flash Converting. Metall. Mater. Trans. B 2012, 43, 823–829. [Google Scholar] [CrossRef]
- Chan, C.; Zhang, L.; Jahanshahi, S. Thermodynamic Modeling of Arsenic in Copper Smelting Processes. Metall. Mater. Trans. B 2010, 41, 1175–1185. [Google Scholar] [CrossRef]
- Sofra, J.; Hughes, R.A. Application of the Ausmelt process in smelting plants. In Proceedings of the Lead-Zinc 2005 Conference, Kyoto, Japan, 17–19 October 2005. [Google Scholar]
- Dosmukhamedov, N.K.; Zholdasbay, E.E.; Kabylbekov, Z.Z.; Kurmanseitov, M.B. Composition of the initial charge of the process of mine contractile melting. Min. J. Kazakhstan 2013, 9, 32–34. [Google Scholar]
- Zholdasbay, E.E.; Kabylbekov, Z.; Fedorov, A.N.; Dosmukhamedov, N.K. Features of the transition of copper, arsenic and antimony from copper-lead matte to rough lead. Non-Ferr. Met. 2015, 3, 48–52. [Google Scholar]
- Tymbaeva, A.A.; Kulenova, N.A.; Mamyachenkov, S.V. Waste management of arsenic deposition process in the form of sulfide cake from sulfur-alkaline arsenic-containing solutions. Bull. Natl. Eng. Acad. Repub. Kazakhstan 2020, 2, 193–199. [Google Scholar]
- Ojeda, M.W.; Perino, E.; Ruiz, M.C. Gold extraction by chlorination using a pyrometallurgical process. Miner. Eng. 2009, 22, 409–411. [Google Scholar] [CrossRef]
- Samikhov, S.R.; Zinchenko, Z.A.; Azizkulov, Y.B. Research of the Influence of Chlorinators on the Chlorine Dispersion Process of Gold-Copper-Arsenic-Containing Concentrates of the Taron Deposit. Bull. Tajik Natl. Univ. 2012, 2012, 153–157. [Google Scholar]
- Samikhov, S.R.; Zinchenko, Z.A. Kinetics of the Chlorine Dispersion Process of the Chore Deposit Concentrates. Bull. Tajik Natl. Univ. 2013, 2013, 181–184. [Google Scholar]
- Chmilenko, F.; Derkach, T.M.; Kurganov, D.V. The use of chloride sublimation in the analysis of gold-bearing raw materials. Probl. Chem. Chem. Technol. 2001, 5, 9–10. [Google Scholar]
- Dosmukhamedov, N.K. Theoretical and Technological Features of Processing of Industrial Products and Recycled Materials of Non-Ferrous Metallurgy; DPS Publishing House: Almaty, Kazakhstan, 2008; 360p. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980; 462p. [Google Scholar]
- Lidin, P.A.; Molochko, V.A.; Andreeva, L.L. Chemical Properties of Inorganic Materials; Chemistry: Moscow, Russia, 1997. [Google Scholar]
| Cu-Au-As Concentrate (Berezovsky Field, East Kazakhstan Region) | ||
|---|---|---|
| Elements | Unit of Measurements | Value |
| Cu | % | 20.9 |
| Fe | % | 20.4 |
| Pb | % | 2.5 |
| Zn | % | 2.6 |
| S | % | 10.8 |
| Au | ppm | 11 |
| Ag | ppm | 85 |
| As | % | 6.3 |
| Re | ppm | 35 |
| Sb | % | 0.30 |
| Al | % | 0.3 |
| Ba | % | 0.004 |
| Ca | % | 0.1 |
| Si | % | 2.0 |
| K | % | 0.1 |
| Mg | % | 0.02 |
| Mn | % | 0.01 |
| Na | % | 0.2 |
| P | % | 0.02 |
| Sn | % | 0.28 |
| Excess Air Coefficient (% from Stoichiometric Air) | Composition of the Initial Gas Mixture (Volume %) | |||
|---|---|---|---|---|
| CO2 | H2O | N2 | O2 | |
| 100 | 9.7 | 18.8 | 71.7 | 0 |
| 150 | 6.4 | 12.5 | 71.6 | 9.5 |
| 170 | 5.6 | 10.9 | 70.4 | 13.1 |
| No. | Reactions | Gibbs Energy (ΔG), kJ/mol | |||||
|---|---|---|---|---|---|---|---|
| Temperature, K | |||||||
| 473 | 673 | 873 | 1073 | 1273 | 1473 | ||
| Group 1 | |||||||
| 1 | FeAsS(s) + 2.5 O2(g) = 0.5 Fe2O3(s) + 0.5 As2O3 (g) + SO2(g) | −753 | −721 | −688 | −654 | −620 | −585 |
| 2 | As2S3 + 4.5O2 (g) = As2O3 (g) + 3SO2 (g) | −1133 | −1128 | −1113 | −1095 | −1072 | −1056 |
| 3 | As2S3 + 5.5O2 (g) = As2O5 (g) + 3SO2 (g) | −1428 | −1335 | −1240 | −1147 | −1056 | −968 |
| 4 | ReS2(s) + 3.75 O2(g) = 0.5 Re2O7(g) + 2SO2(g) | −926 | −898 | −871 | −843 | −816 | −788 |
| 5 | Cu2S(s) + 1.5O2(g) = Cu2O(s) + SO2(g) | −343 | −321 | −298 | −276 | −254 | −231 |
| Group 2 | |||||||
| 6 | FeAsS(s) + 0.5 CH4(g) + 3.25 O2(g) = FeO (s) + 0.5 As2O3 (g) + SO2(g) + 0.5 CO2(g) + H2O(g) | −1040 | −1020 | −1000 | −978 | −956 | −932 |
| 7 | FeAsS (s) + 0.5 CH4(g) + 3O2(g) = FeO + 0.5 As2O3(g) + SO2(g) + 0.5 CO(g) + H2O(g) | −219 | −217 | −214 | −211 | −207 | −204 |
| 8 | As2S3 + 1.5CH4(g) + 3O2(g) = As2O3(g) + 1.5CO2(g) + 3H2S(g) | −888 | −931 | −963 | −991 | −1016 | −1032 |
| 9 | As2S3 + 1.5CH4(g) + 2.25O2(g) = As2O3(g) + 1.5CO(g) + 3H2S(g) | −125 | −142 | −156 | −169 | −181 | −192 |
| 10 | ReS2(s) + 0.5CH4(g) + 4.75O2(g) = 0.5Re2O7(g) + 0.5CO2(g) + 2SO2(g) + H2O(g) | −1326 | −1299 | −1271 | −1244 | −1216 | −1187 |
| 11 | ReS2 + 0.5 CH4(g) + 4.5O2(g) = 0.5 Re2O7(g) + 0.5 CO(g) + 2SO2(g) + H2O(g) | −287 | −283 | −279 | −274 | −270 | −265 |
| 12 | Cu2S(s) + 0.5 CH4(g) + 0.5 O2(g) = 2Cu(s) + H2S(g) + 0.5 CO2(g) | −128 | −136 | −143 | −149 | −155 | −162 |
| Group 3 | |||||||
| 13 | FeAsS(s) + 3Cl2(g) + O2(g) = FeCl3(s) + AsCl3(g) + SO2(g) | −784 | −738 | −701 | −665 | −629 | −594 |
| 14 | As2S3 + 3Cl2(g) + 3O2(g) = 2AsCl3(g) + 3SO2(g) | −1296 | −1278 | −1250 | −1220 | −1186 | −1150 |
| 15 | ReS2(s) + 1.5Cl2(g) + 2O2(g) = ReCl3(s) + 2SO2(g) | −591 | −556 | −521 | −488 | −456 | −425 |
| 16 | Au (s) + 1.5Cl2(g) = AuCl3(g) | −16 | 23 | 61 | 97 | 130 | 163 |
| 17 | Au (s) + 3 Cl(g) = AuCl3(g) | −304 | −230 | −157 | −86 | −17 | 52 |
| 18 | Cu2S(s) + 2Cl2(g) = 2CuCl2(s) + 0.5 S2(g) | −179 | −131 | −84 | −45 | −9 | 26 |
| Group 4 | |||||||
| 19 | FeAsS(s) + 3CaCl2(s) + 2.5O2(g) + 3SiO2(s) = FeCl3(s) + AsCl3(g) + 3CaSiO3(s) + SO2(g) | −643 | −625 | −614 | −600 | −568 | −535 |
| 20 | As2S3 (s) + 3CaCl2 (s) + 4.5O2(g) + 3SiO2(s) = 2AsCl3(g) + 3CaSiO3 (s) + 3SO2(g) | −1156 | −1165 | −1164 | −1154 | −1125 | −1091 |
| 21 | ReS2(s) + 1.5CaCl2(s) + 2.75O2(g) = ReCl3(s) + 1.5CaO (s) + 2SO2(g) | −386 | −363 | −341 | −319 | −289 | −260 |
| 22 | Au (s) + 1.5 CaCl2(s) + 0.75 O2(g) = AuCl3(g) + 1.5CaO | 189 | 216 | 241 | 266 | 297 | 329 |
| 23 | Au (s) + 1.5CaCl2(s) + 1.5SiO2(s) + 0.75O2(g) = AuCl3 (g) + 1.5CaSiO3(s) | 54 | 80 | 104 | 129 | 161 | 192 |
| 24 | Cu2S(s) + 2CaCl2(s) + 2SiO2(s) + 2O2(g) = 2CuCl2(g) + 2CaSiO3(g) + SO2(g) | −413 | −369 | −324 | −286 | −237 | −189 |
| Procedure | T, K | Heating | Reagent | CaCl2, % from Cu-Au-As Concentrate Quantity | SiO2, % from Cu-Au-As Concentrate Quantity | Gold Extraction Yield, % |
|---|---|---|---|---|---|---|
| Cu-Au-As concentrate | 523 | Slow | Chlorine | - | - | 51.3 |
| Cu-Au-As concentrate | 1223 | Slow | CaCl2 + SiO2 | 37.5 | 6.4 | 58.7 |
| Cu-Au-As concentrate | 1223 | Slow | Chlorine | - | - | 99.4 |
| Cu-Au-As concentrate | 1223 | Fast | CaCl2 + SiO2 | 43.2 | 10.1 | 56.5 |
| Cu/Au/As concentrate following oxidation sintering at 873 K | 523 | Slow | Chlorine | - | - | 74.3 |
| Cu/Au/As concentrate following oxidation sintering at 873 K | 1223 | Slow | CaCl2 + SiO2 | 40.0 | 10.5 | 79.1 |
| Cu/Au/As concentrate following oxidation sintering at 873 K | 1223 | Fast | CaCl2 + SiO2 | 40.0 | 10.5 | 78.3 |
| Cu-Au-As concentrate following reduction sintering at 873 K | 523 | Slow | Chlorine | - | - | 85.7 |
| Cu-Au-As concentrate following reduction sintering at 873 K | 1223 | Slow | CaCl2 + SiO2 | 40.0 | 10.5 | 88.3 |
| Cu-Au-As concentrate following reduction sintering at 873 K | 1223 | Fast | CaCl2 + SiO2 | 40.0 | 10.5 | 88.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosmukhamedov, N.; Kaplan, V.; Zholdasbay, E.; Argyn, A.; Kuldeyev, E.; Koishina, G.; Tazhiev, Y. Chlorination Treatment for Gold Extraction from Refractory Gold-Copper-Arsenic-Bearing Concentrates. Sustainability 2022, 14, 11019. https://doi.org/10.3390/su141711019
Dosmukhamedov N, Kaplan V, Zholdasbay E, Argyn A, Kuldeyev E, Koishina G, Tazhiev Y. Chlorination Treatment for Gold Extraction from Refractory Gold-Copper-Arsenic-Bearing Concentrates. Sustainability. 2022; 14(17):11019. https://doi.org/10.3390/su141711019
Chicago/Turabian StyleDosmukhamedov, Nurlan, Valery Kaplan, Erzhan Zholdasbay, Aidar Argyn, Erzhan Kuldeyev, Gulzada Koishina, and Yeleussiz Tazhiev. 2022. "Chlorination Treatment for Gold Extraction from Refractory Gold-Copper-Arsenic-Bearing Concentrates" Sustainability 14, no. 17: 11019. https://doi.org/10.3390/su141711019
APA StyleDosmukhamedov, N., Kaplan, V., Zholdasbay, E., Argyn, A., Kuldeyev, E., Koishina, G., & Tazhiev, Y. (2022). Chlorination Treatment for Gold Extraction from Refractory Gold-Copper-Arsenic-Bearing Concentrates. Sustainability, 14(17), 11019. https://doi.org/10.3390/su141711019







