Adsorption of Copper and Lead Ions in a Binary System onto Orange Peels: Optimization, Equilibrium, and Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bio-Sorbent and Characterization
2.2. Preparation of the Synthetic Solution
2.3. Batch Adsorption Studies
2.4. Bio-Sorption Equilibrium
2.4.1. Langmuir Isotherm
2.4.2. Freundlich Isotherm
2.5. Adsorption Kinetic Studies
2.6. Central Composite Design (CCD)
3. Results and Discussion
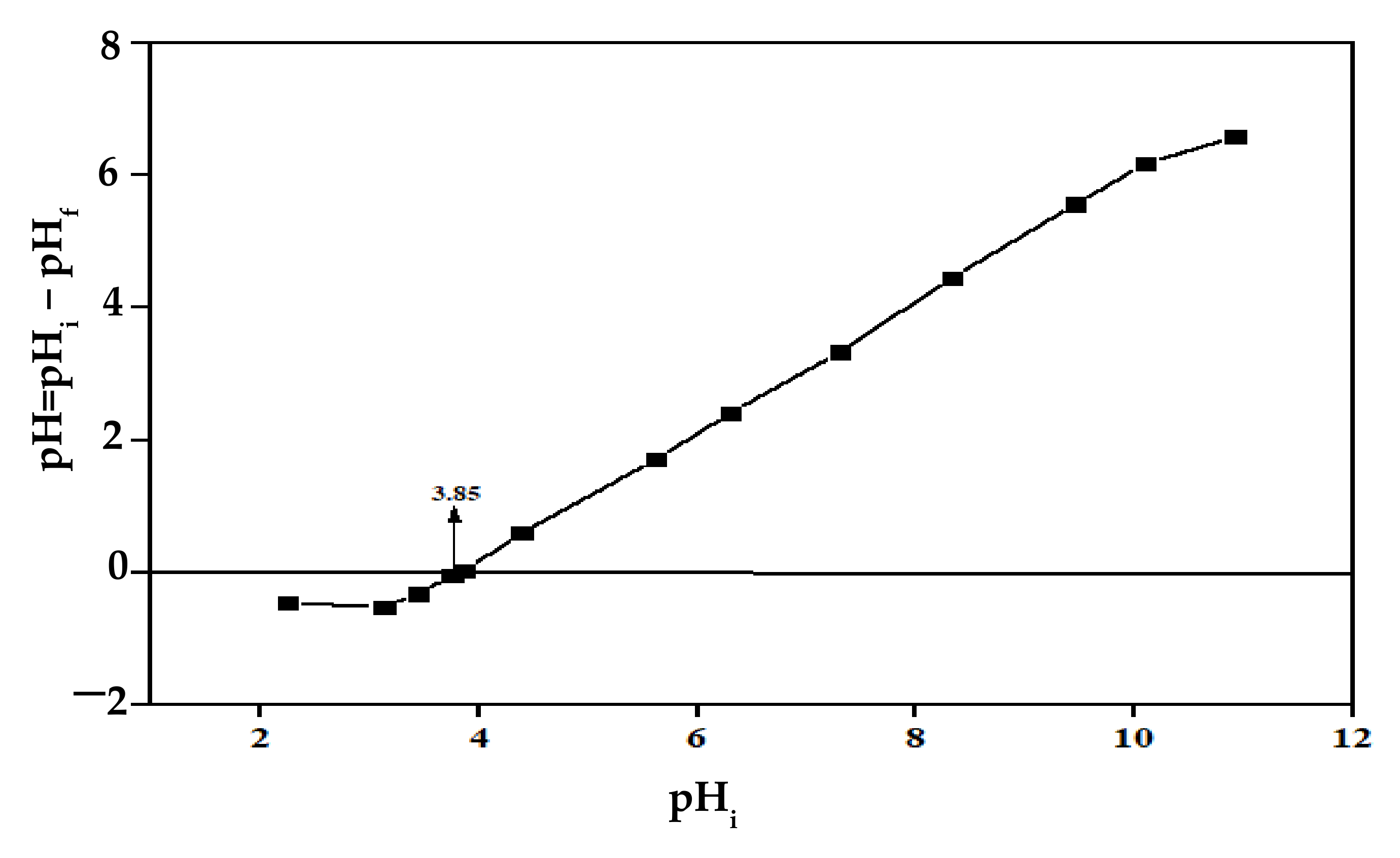
3.1. Point of Zero Charges of Orange Peels (pHpzc)
3.2. Characterization of Bio-Sorbent
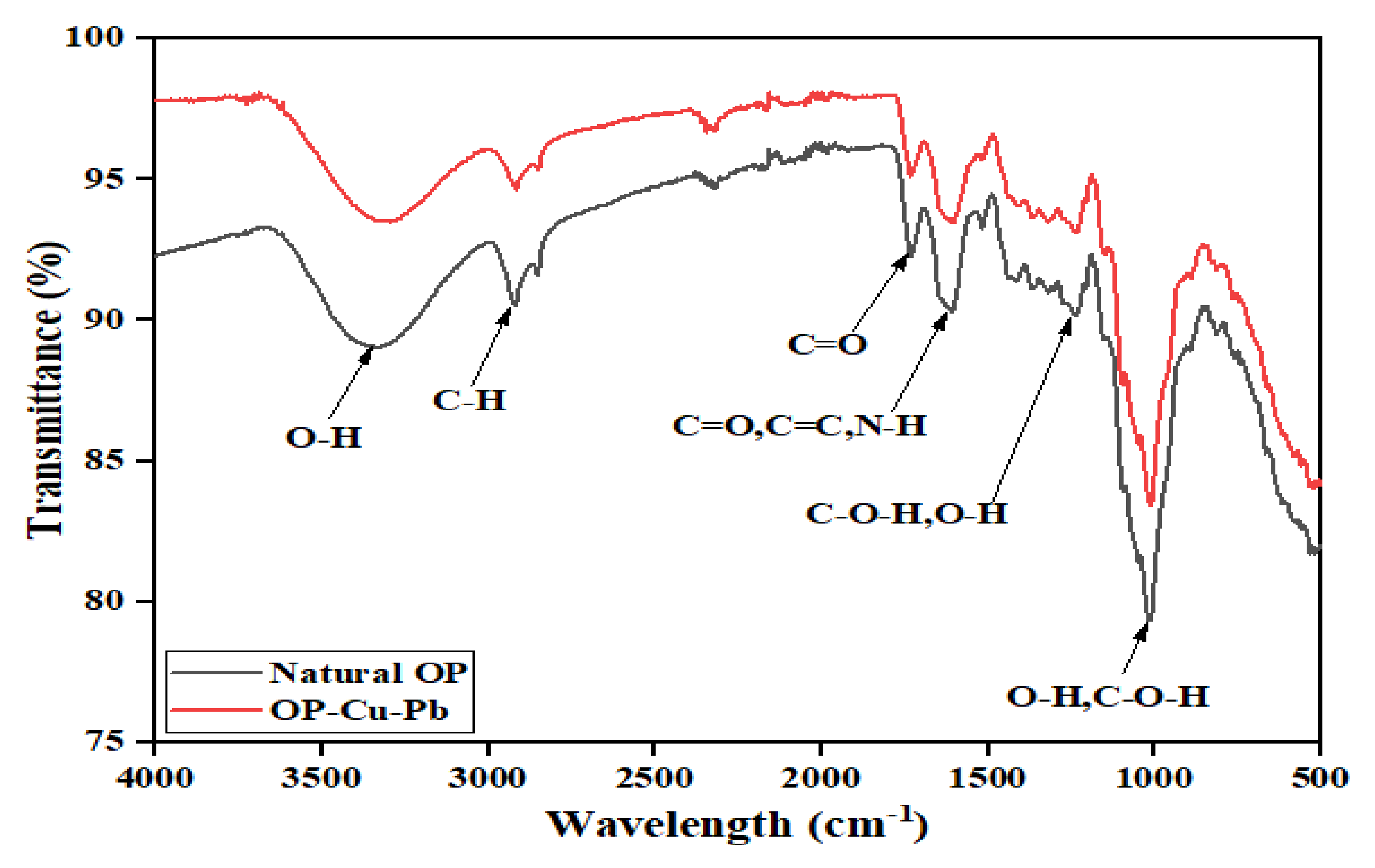
3.2.1. FTIR Spectroscopy Analysis
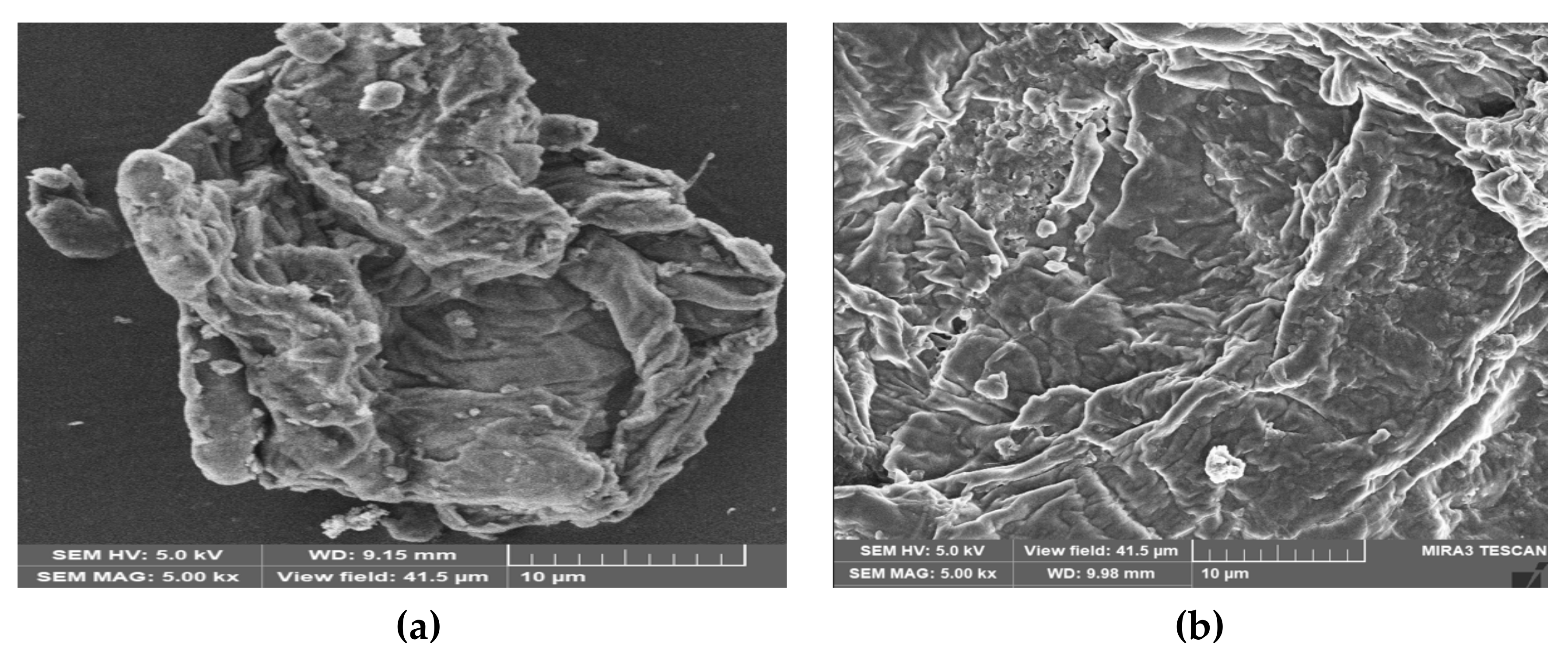
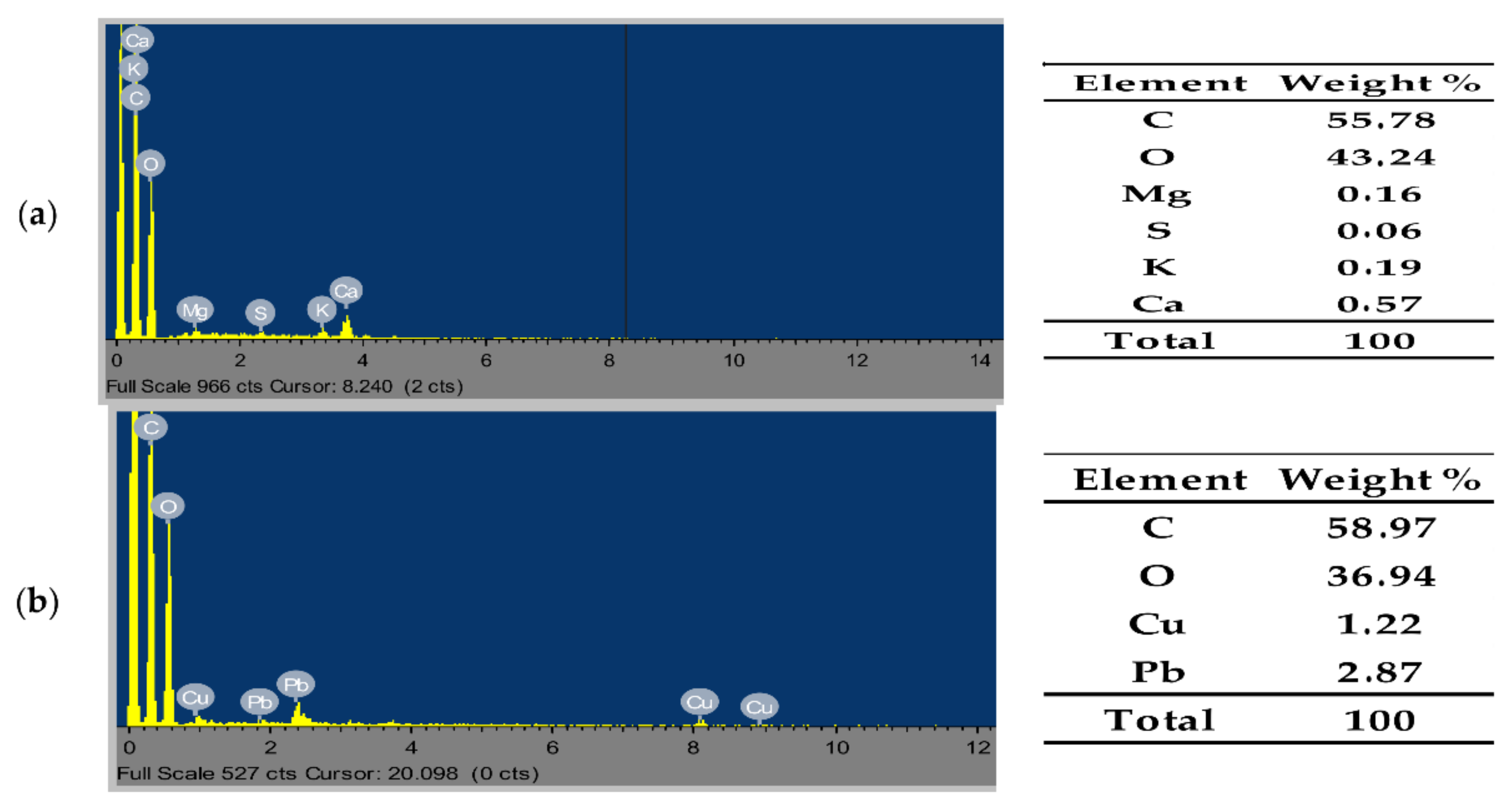
3.2.2. Scanning Electron Microscope-Energy Dispersive X-ray (SEM-EDX)
3.3. Experimental Design
3.3.1. Adsorption of Cu2+ and Pb2+ onto Orange Peels in Binary Solute Using CCD
3.3.2. Analysis of Variance (ANOVA) for the Models
3.3.3. D Representation of the Interactive Effects on the Responses
3.3.4. Optimization of the Adsorption Process
3.3.5. Mechanism of Adsorption of Cu2+ and Pb2+ Ions
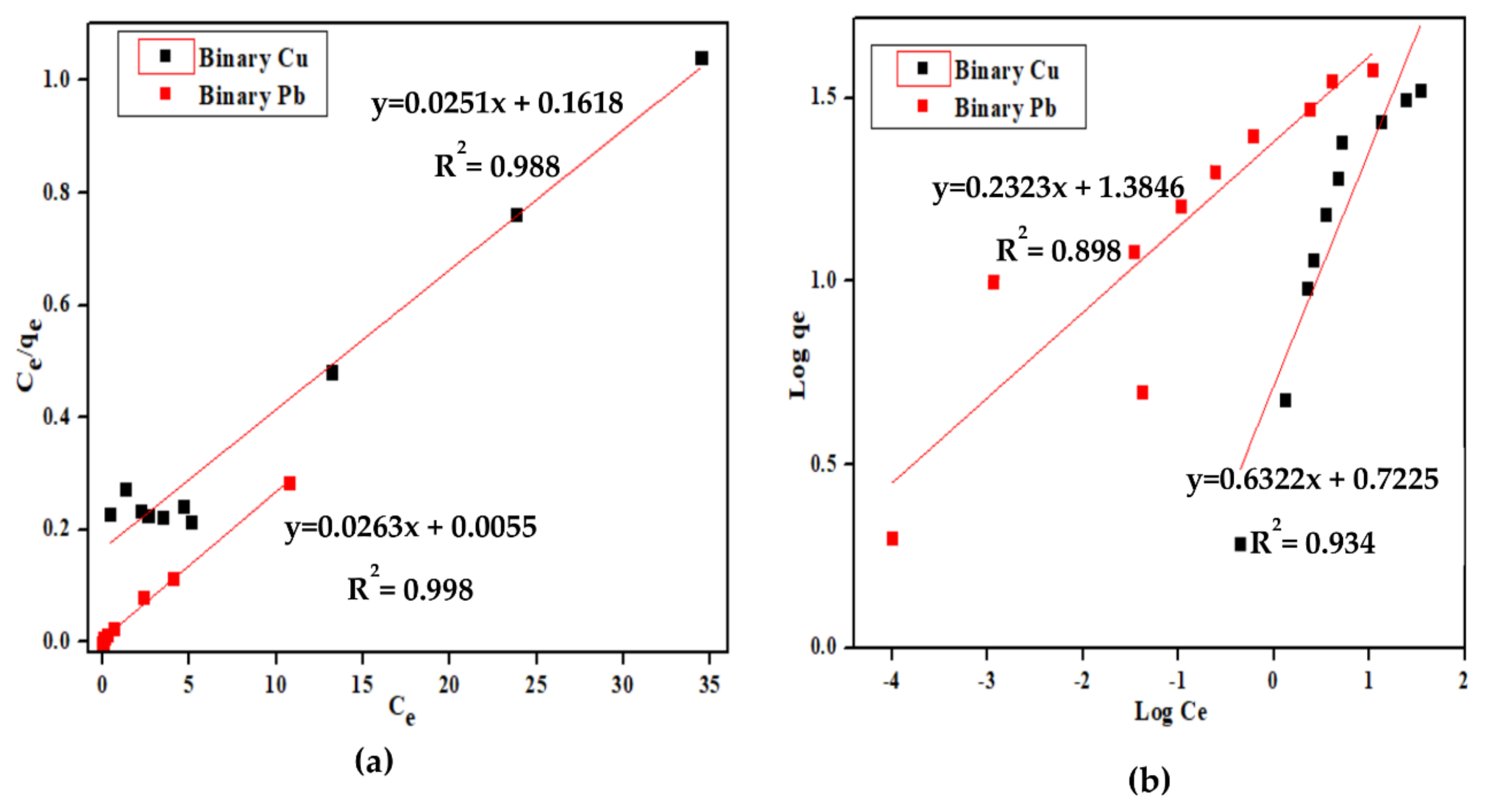
3.4. Equilibrium Study
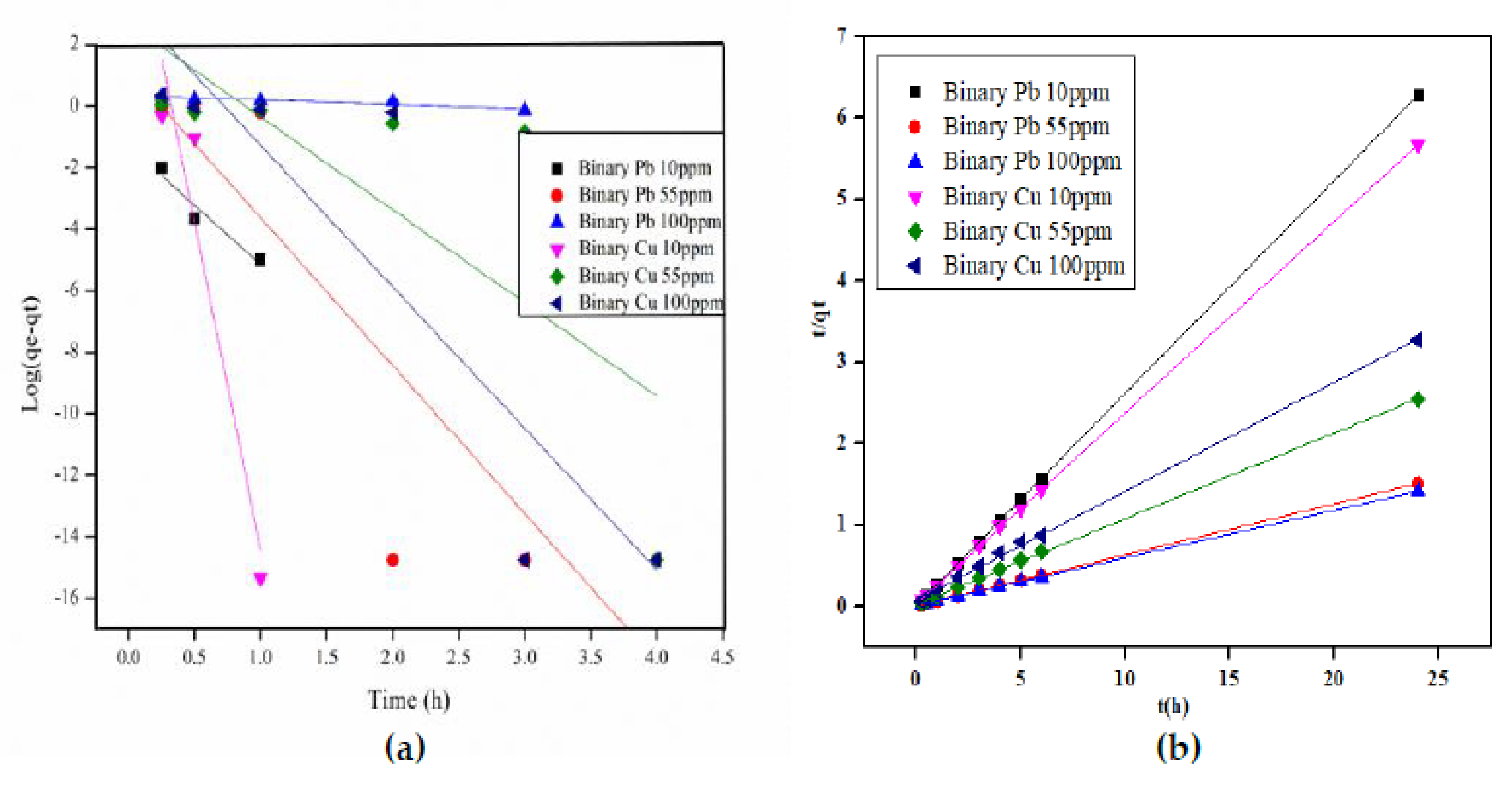
3.5. Kinetic Modelling of Pb2+ and Cu2+ onto Orange Peels
3.6. Comparison of Cu2+ and Pb2+ Adsorption Capacity Using Orange Peels with Other Bio-Sorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.Y.; Li, M.; Huang, C. Hazardous Waste Treatment Technologies. Water Environ. Res. 2016, 88, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of Removing Heavy Metals from Industrial Wastewater. J. Methods 2015, 1, 12–18. [Google Scholar]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Evaluation of Lead (II) Removal from Wastewater Using Banana Peels: Optimization Study. Pol. J. Environ. Stud. 2021, 30, 1487–1496. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Fonseca-Cantabrana, Á.; Blancas-Benitez, F.J.; González-Estrada, R.R.; Sanchez-Silva, J.M. Biosorption of heavy metals by agroindustrial byproducts. TIP Rev. Espec. Cienc. Quím.-Biol. 2020, 23, 1–18. [Google Scholar]
- Singh, N.B.; Nagpal, G.; Agrawal, S.; Rachna. Water purification by using Adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Hwan Cho, M.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Application of the Response Surface Methodology in the Removal of Cu2+ and Pb2+ from Aqueous Solutions Using Orange Peels. Sci. Afr. 2021, 13, e00931. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Babapoor, A.; Rafiei, O.M.; Yousef, A.; Mohammad, M.P.; Meysam, N.; Ayat, K.W. Comparison and Optimization of Operational Parameters in Removal of Heavy Metal Ions from Aqueous Solutions by Low-Cost Adsorbents. Int. J. Chem. Eng. 2022, 2022, 3282448. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J. Clean. Prod. 2017, 150, 58–64. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Calero, M.; Almendros, A.I.; Ronda, A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fixed bed columns. Int. J. Miner. Process. 2016, 148, 72–82. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hossain, M.; Ngo, H.H.; Guo, W.; Nguyen, T. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Int. J. GEOMATE 2012, 2, 227–234. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nghiem, L.D.; Hai, F.I.; Vigneswaran, S.; Nguyen, T.V. Competitive adsorption of metals on cabbage waste from multi-metal solutions. Bioresour. Technol. 2014, 160, 79–88. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubini-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Ashraf, M.A.; Wajid, A.; Mahmood, K.; Maah, M.J.; Yusoff, I. Removal of heavy metals from aqueous solution by using mango biomass. Afr. J. Biotechnol. 2011, 10, 2163–2177. [Google Scholar]
- Arunakumara, K.; Walpola, B.C.; Yoon, M.-H. Banana Peel: A Green Solution for Metal Removal from Contaminated Waters. Korean J. Environ. Agric. 2013, 32, 108–116. [Google Scholar] [CrossRef]
- Iqbal, M.; Khera, R.A. Adsorption of copper and lead in single and binary metal system onto Fumaria indica biomass. Chem. Int. 2015, 1, 157b–163b. [Google Scholar]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Bio-sorption of a bi-solute system of copper and lead ions onto banana peels: Characterization and optimization. J. Environ. Health Sci. Eng. 2021, 19, 613–624. [Google Scholar] [CrossRef]
- Kumar, K.; Patavardhan, S.S.; Lobo, S.; Gonsalves, R. Equilibrium study of dried orange peel for its efficiency in removal of cupric ions from water. Int. J. Phytoremediat. 2018, 20, 593–598. [Google Scholar] [CrossRef]
- Safari, E.; Rahemi, N.; Kahforoushan, D.; Allahyari, S. Copper adsorptive removal from aqueous solution by orange peel residue carbon nanoparticles synthesized by combustion method using response surface methodology. J. Environ. Chem. Eng. 2019, 7, 102847. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; Gonzalez-Gutierrez, L.V.; Baldenegro-Perez, L.A. Biosorbents prepared from orange peels using Instant Controlled Pressure Drop for Cu(II) and phenol removal. Ind. Crop. Prod. 2016, 84, 344–349. [Google Scholar] [CrossRef]

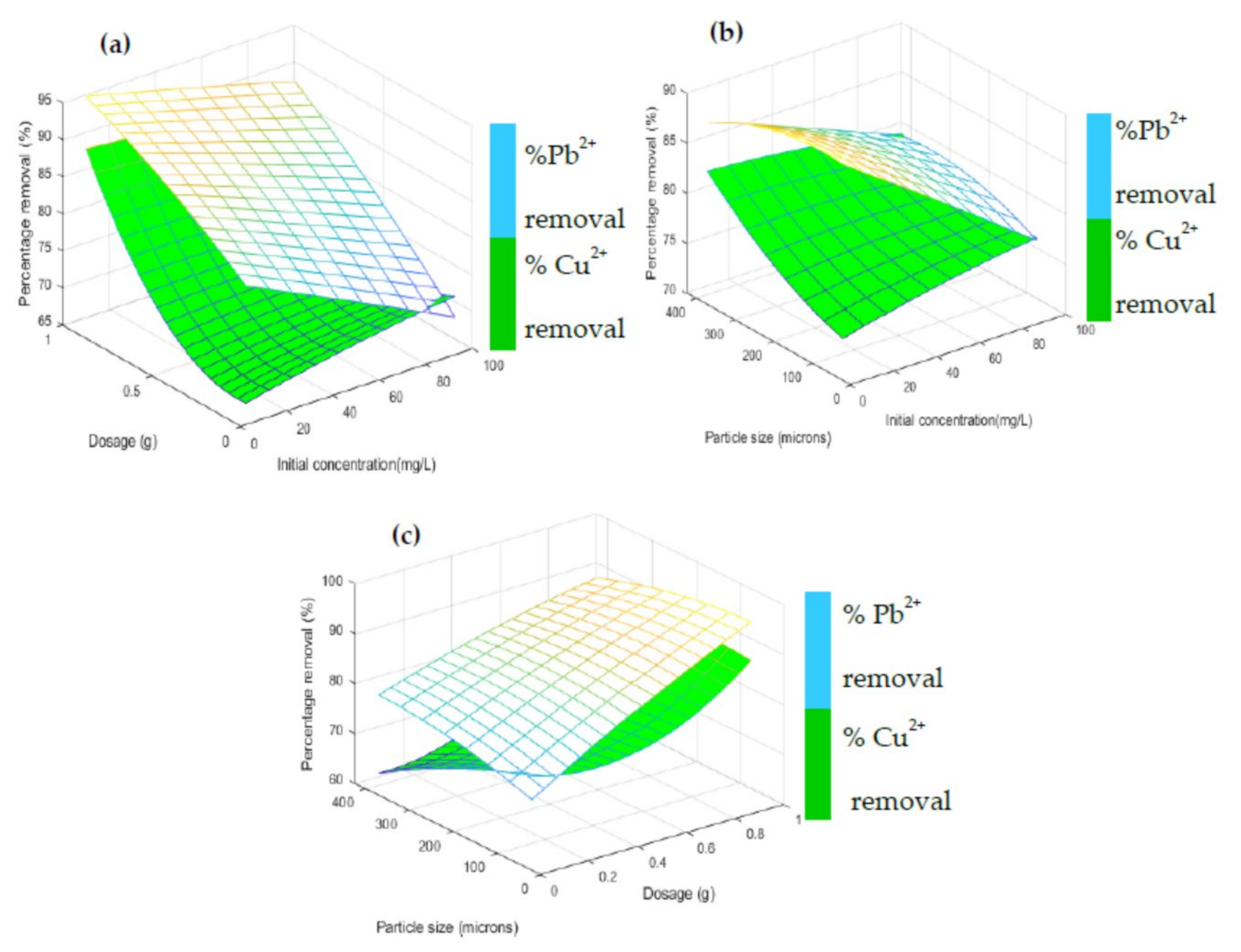
| Parameters | Factor | Range and Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Initial concentration | X1 | 10 | 55 | 100 |
| Adsorbent dosage | X2 | 0.1 | 0.55 | 1 |
| Particle size | X3 | 75 | 265 | 455 |
| Std | Run | Initial Conc. (mg/L) | Adsorbent Dosage (g) | Particle Size (µm) | Responses | |||
|---|---|---|---|---|---|---|---|---|
| Lead % Removal | Copper % Removal | |||||||
| Exp | Pred | Exp | Pred | |||||
| 15 | 1 | 55 | 0.55 | 265 | 83.75 | 83.93 | 72.89 | 71.48 |
| 5 | 2 | 10 | 0.1 | 455 | 80.34 | 80.56 | 59.1 | 59.06 |
| 2 | 3 | 100 | 0.1 | 75 | 62.07 | 61.95 | 79.5 | 78.69 |
| 17 | 4 | 55 | 0.55 | 265 | 82.86 | 83.93 | 70.17 | 71.48 |
| 12 | 5 | 55 | 1 | 265 | 90.47 | 91.18 | 85.06 | 86.55 |
| 4 | 6 | 100 | 1 | 75 | 88.12 | 88.04 | 84.72 | 84.77 |
| 6 | 7 | 100 | 0.1 | 455 | 70.86 | 71.13 | 59.95 | 60.57 |
| 20 | 8 | 55 | 0.55 | 265 | 84.15 | 83.93 | 73.72 | 71.48 |
| 9 | 9 | 10 | 0.55 | 265 | 90.12 | 88.88 | 72.82 | 70.86 |
| 3 | 10 | 100 | 1 | 75 | 98.85 | 98.71 | 86.2 | 85.60 |
| 16 | 11 | 55 | 0.55 | 265 | 84.45 | 83.93 | 72.1 | 71.48 |
| 1 | 12 | 10 | 0.1 | 75 | 77.97 | 78.86 | 68.98 | 70.76 |
| 19 | 13 | 55 | 0.55 | 265 | 83.21 | 83.93 | 69.65 | 71.48 |
| 8 | 14 | 10 | 1 | 455 | 84.78 | 84.03 | 82.65 | 80.89 |
| 18 | 15 | 55 | 0.55 | 265 | 84.05 | 83.93 | 70.25 | 71.48 |
| 10 | 16 | 10 | 0.55 | 265 | 78.15 | 78.83 | 69.29 | 71.19 |
| 14 | 17 | 55 | 0.55 | 455 | 81.75 | 81.75 | 65.98 | 66.34 |
| 13 | 18 | 55 | 0.55 | 75 | 83.45 | 82.90 | 74.55 | 74.13 |
| 11 | 19 | 55 | 0.1 | 265 | 76.06 | 74.80 | 70.53 | 68.98 |
| 7 | 20 | 100 | 1 | 455 | 86.95 | 87.21 | 87.32 | 88.14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | Comments |
|---|---|---|---|---|---|---|
| Pb Model | 1088.25 | 9 | 120.92 | 151.21 | <0.0001 | significant |
| A-Initial concentration | 252.51 | 1 | 252.51 | 315.76 | <0.0001 | |
| B-Adsorbent dosage | 670.27 | 1 | 670.27 | 838.17 | <0.0001 | |
| C-Particle size | 3.34 | 1 | 3.34 | 4.18 | 0.0682 | |
| AB | 19.47 | 1 | 19.47 | 24.35 | 0.0006 | |
| AC | 28.05 | 1 | 28.05 | 35.08 | 0.0001 | |
| BC | 87.12 | 1 | 87.12 | 108.94 | <0.0001 | |
| A2 | 0.0138 | 1 | 0.0138 | 0.0173 | 0.8980 | |
| B2 | 2.43 | 1 | 2.43 | 3.04 | 0.1116 | |
| C2 | 7.09 | 1 | 7.09 | 8.87 | 0.0139 | |
| Residual | 8.00 | 10 | 0.7997 | |||
| Lack of Fit | 6.17 | 5 | 1.23 | 3.39 | 0.1034 | not significant |
| Pure Error | 1.82 | 5 | 0.3647 | |||
| Cor Total | 1096.25 | 19 | ||||
| Std. Dev. 0.8942 | R2 0.9927 | Adjusted R2 0.9861 | Predicted R2 0.9483 | Adeq. Precision 58.15 | Mean 82.62 | C. V.% 1.08 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | Comments |
|---|---|---|---|---|---|---|
| Cu Model | 1230.20 | 9 | 136.69 | 39.32 | <0.0001 | significant |
| A-Initial concentration | 0.2856 | 1 | 0.2856 | 0.0822 | 0.7802 | |
| B-Adsorbent dosage | 772.47 | 1 | 772.47 | 222.22 | <0.0001 | |
| C-Particle size | 151.71 | 1 | 151.71 | 43.64 | <0.0001 | |
| AB | 38.37 | 1 | 38.37 | 11.04 | 0.0077 | |
| AC | 20.67 | 1 | 20.67 | 5.95 | 0.0349 | |
| BC | 101.39 | 1 | 101.39 | 29.17 | 0.0003 | |
| A2 | 0.5762 | 1 | 0.5762 | 0.1658 | 0.6925 | |
| B2 | 108.53 | 1 | 108.53 | 31.22 | 0.0002 | |
| C2 | 4.28 | 1 | 4.28 | 1.23 | 0.2931 | |
| Residual | 34.76 | 10 | 3.48 | |||
| Lack of Fit | 20.79 | 5 | 4.16 | 1.49 | 0.3365 | not significant |
| Pure Error | 13.97 | 5 | 2.79 | |||
| Cor Total | 1264.96 | 19 | ||||
| Std. Dev. 1.86 | R2 0.9725 | Adjusted R2 0.9478 | Predicted R2 0.7934 | Adeq. Precision 22.06 | Mean 73.77 | C. V.% 2.53 |
| Ion | System | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| b | qm | R2 | RL | Kf | n | R2 | ||
| (L/mg) | (mg/g) | |||||||
| Cu2+ | Binary | 0.15 | 38.18 | 0.988 | 0.4 | 5.19 | 1.56 | 0.934 |
| Pb2+ | Binary | 0.77 | 40.05 | 0.998 | 0.02 | 24.06 | 4.29 | 0.898 |
| Metal Ion Concentration | Cu | Pb | |||||
|---|---|---|---|---|---|---|---|
| 10 mg/L | 55 mg/L | 100 mg/L | 10 mg/L | 55 mg/L | 100 mg/L | ||
| Pseudo-first order | Binary | ||||||
| qe | 6.83E6 | 465.27 | 2087.37 | 0.05 | 14.72 | 2.18 | |
| K1 | 48.96 | 6.96 | 10.59 | 8.83 | 11.06 | 0.33 | |
| R2 | 0.958 | 0.762 | 0.897 | 0.970 | 0.891 | 0.924 | |
| Pseudo-second order | Binary | ||||||
| qe | 42.48 | 9.49 | 13.39 | 3.82 | 15.96 | 17.09 | |
| K2 | 0.02 | 0.44 | 0.04 | 10.71 | 0.55 | 0.30 | |
| R2 | 0.999 | 0.999 | 0.999 | 1.000 | 0.999 | 0.999 | |
| Bio-Sorbent | Cu2+ Uptake (mg/g) | Pb2+ Uptake (mg/g) | Reference |
|---|---|---|---|
| Orange peels | 38.18 | 40.05 | This study |
| Mango plant | 22.51 | 24.4 | [20] |
| Banana peels | 28 | 7.97 | [21] |
| Fumaria Indica | 6.62 | 9.15 | [22] |
| Banana peels | 29.26 | 39.32 | [23] |
| Cabbage waste | 12.96 | 61.27 | [18] |
| Orange peels | 33.99 | [24] | |
| Orange peels | 16.64 | [25] | |
| Orange peels | 31.79 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, F.O.; Musonge, P.; Bakare, B.F. Adsorption of Copper and Lead Ions in a Binary System onto Orange Peels: Optimization, Equilibrium, and Kinetic Study. Sustainability 2022, 14, 10860. https://doi.org/10.3390/su141710860
Afolabi FO, Musonge P, Bakare BF. Adsorption of Copper and Lead Ions in a Binary System onto Orange Peels: Optimization, Equilibrium, and Kinetic Study. Sustainability. 2022; 14(17):10860. https://doi.org/10.3390/su141710860
Chicago/Turabian StyleAfolabi, Felicia Omolara, Paul Musonge, and Babatunde Femi Bakare. 2022. "Adsorption of Copper and Lead Ions in a Binary System onto Orange Peels: Optimization, Equilibrium, and Kinetic Study" Sustainability 14, no. 17: 10860. https://doi.org/10.3390/su141710860
APA StyleAfolabi, F. O., Musonge, P., & Bakare, B. F. (2022). Adsorption of Copper and Lead Ions in a Binary System onto Orange Peels: Optimization, Equilibrium, and Kinetic Study. Sustainability, 14(17), 10860. https://doi.org/10.3390/su141710860







