Evidence of Genetic Connectivity among Lyle’s Flying Fox Populations in Thailand for Wildlife Management and One Health Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and DNA Extraction
2.2. Mitochondrial D-Loop and Cytb Sequencing
2.3. Genetic Diversity and Population Structure
2.4. Demographic History
3. Results
3.1. Haplotype Diversity and Population Structure
3.2. Demography of the Ten Bat Colonies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.S.; Li, B.; Shen, X.R.; Jiang, R.D.; Zhu, Y.; Wu, J.; Yi, F.; Herve, B.; Ben, H.; Ge, X.Y.; et al. Characterization of novel rhabdoviruses in Chinese bats. Viruses 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, B.J.; Yang, X.L.; Zeng, L.P.; Li, B.; Ouyang, S.; Shi, Z.L. Evolutionary arms race between virus and host drives genetic diversity in bat severe acute respiratory syndrome-related coronavirus spike genes. J. Virol. 2020, 94, e00902-20. [Google Scholar] [CrossRef] [PubMed]
- Wongtienchai, P.; Lapbenjakul, S.; Jangtarwan, K.; Areesirisuk, P.; Mahaprom, R.; Subpayakom, N.; Singchat, W.; Sillapaprayoon, S.; Muangmai, N.; Songchan, R.; et al. Genetic management of a water monitor lizard (Varanus salvator macromaculatus) population at Bang Kachao Peninsula as a consequence of urbanization with Varanus Farm Kamphaeng Saen as the first captive research establishment. J. Zool. Syst. Evol. Res. 2021, 59, 484–497. [Google Scholar] [CrossRef]
- Woolhouse, M.E.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Wang, L.F.; Crameri, G. Emerging zoonotic viral diseases. Rev. Sci. Tech. 2014, 33, 569–581. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 2142. [Google Scholar]
- Hutcheon, J.M.; Kirsch, J.A. A moveable face: Deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropt. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Investigating the Role of Bats in Emerging Zoonoses: Balancing Ecology, Conservation and Public Health Interests; Newman, S.H., Field, H.E., Jong de, C.E., Epstein, J.H., Eds.; FAO Animal Production and Health Manual: Rome, Italy, 2011; p. 12. [Google Scholar]
- Smith, D.R. Review a brief history of coronaviruses in Thailand. J. Virol. Methods. 2020, 289, 114034. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Duengkae, P.; Chaiyes, A.; Kaewpom, T.; Rodpan, A.; Yingsakmongkon, S.; Petcharat, S.; Phengsakul, P.; Maneeorn, P.; Hemachudha, T. Longitudinal study of age-specific pattern of coronavirus infection in Lyle’s flying fox (Pteropus lylei) in Thailand. J. Virol. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Okada, P.; Buathong, R.; Phuygun, S.; Thanadachakul, T.; Parnmen, S.; Wongboot, W.; Waicharoen, S.; Wacharapluesadee, S.; Uttayamakul, A.; Maurer-Stroh, S.; et al. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Eurosurveillance 2020, 25, 2000097. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.S.; Tuttle, M.D. Flying foxes (Chiroptera: Pteropodidae): Threatened animals of key ecological and economic importance. Conserv. Biol. 1991, 5, 455–463. [Google Scholar] [CrossRef]
- Simmons, N.B. Order Chiroptera. In Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 312–529. [Google Scholar]
- Helgen, K.M.; Helgen, L.E.; Wilson, D.E. Pacific flying foxes (Mammalia: Chiroptera): Two new species of Pteropus from Samoa, probably extinct. Am. Mus. Novit. 2009, 2009, 1–37. [Google Scholar] [CrossRef]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Mahy, B.W.J. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef]
- Bumrungsri, S.; Harbit, A.; Benzie, C.; Carmouche, K.; Sridith, K.; Racey, P. The pollination ecology of two species of Parkia (Mimosaceae) in southern Thailand. J. Trop. Ecol. 2008, 24, 467–475. [Google Scholar] [CrossRef]
- Thanapongtharm, W.; Linard, C.; Wiriyarat, W.; Chinsorn, P.; Kanchanasaka, B.; Xiao, X.; Biradar, C.; Wallace, R.G.; Gilbert, M. Spatial characterization of colonies of the flying fox bat, a carrier of Nipah Virus in Thailand. BMC Vet. Res. 2015, 11, 81. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Ruangvejvorachai, P.; Hemachudha, T. A simple method for detection of rabies viral sequences in 16-year old archival brain specimens with one-week fixation in formalin. J. Virol. Methods. 2006, 134, 267–271. [Google Scholar] [CrossRef]
- Chaiyes, A.; Duengkae, P.; Wacharapluesadee, S.; Pongpattananurak, N.; Olival, K.J.; Hemachudha, T. Assessing the distribution, roosting site characteristics, and population of Pteropus lylei in Thailand. Raffles Bull. Zool. 2017, 65, 670–680. [Google Scholar]
- Boonkird, K.; Wanghongsa, S. On the population number and distribution of flying foxes (Pteropus lylei) in central plain. Wildlife Yearbook 2004, 5, 89–100. [Google Scholar]
- Duengkae, P.; Wacharapluesadee, S.; Khumbucha, W.; Srikhunmuang, P.; Phengsakul, P.; Chaiyes, A.; Pongpattananurak, N.; Hemachudha, T. Monitoring roosting sites and population of Lyle’s Flying Fox (Pteropus lylei) in Thailand. Forestry 2015, 1, 121–127. [Google Scholar]
- Weber, N.; Duengkae, P.; Fahr, J.; Dechmann, D.K.; Phengsakul, P.; Khumbucha, W.; Siriaroonrat, B.; Wacharapluesadee, S.; Maneeorn, P.; Newman, S.; et al. High-resolution GPS tracking of Lyle’s flying fox between temples and orchards in central Thailand. J. Wildl. Manag. 2015, 79, 957–968. [Google Scholar] [CrossRef]
- Jeong, J.; McCallum, H. Using stochastic modeling to predict the effect of culling and colony dispersal of bats on zoonotic viral epidemics. Vector Borne Zoonotic Dis. 2021, 21, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hondo, E.; Inoue, N.; Maeda, K.; Rerkamnuaychoke, W.; Duengkae, P. Movement of Lyle’s flying fox (Pteropus lylei) in Central Thailand. J. Wildl. Manag. 2010, 17, 55–63. [Google Scholar]
- Brown, V.A.; Brooke, A.; Fordyce, J.A.; McCracken, G.F. Genetic analysis of populations of the threatened bat Pteropus mariannus. Conserv. Genet. Resour. 2011, 12, 933–941. [Google Scholar] [CrossRef]
- Larsen, P.A.; Hayes, C.E.; Wilkins, M.A.; Gomard, Y.; Sookhareea, R.; Yoder, A.D.; Goodman, S.M. Population genetics of the Mauritian flying fox, Pteropus niger. Acta Chiropt. 2014, 16, 293–300. [Google Scholar] [CrossRef]
- Harrison, R.G. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends. Ecol. Evol. 1989, 4, 6–11. [Google Scholar] [CrossRef]
- Roman, J.; Palumbi, S.R. Whales before whaling in the North Atlantic. Science 2003, 301, 508–510. [Google Scholar] [CrossRef]
- Sukgosa, N.; Duangjai, S.; Duengkae, P.; Wacharapluesadee, S.; Songmongkol, P.; Yingsakmongkon, S.; Olival, K.J.; Hemachudha, T. Genetic diversity and relationships among Lyle’s flying fox colonies in Thailand. Agric. Nat. Resour. 2018, 2, 607–611. [Google Scholar] [CrossRef]
- Francis, C.M. A Field Guide to the Mammals of Thailand and South-East Asia: Thailand, Peninsular Malaysia, Singapore, Myanmar, Laos, Vietnam, Cambodia; New Holland Publishers: London, UK, 2008. [Google Scholar]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. Old World Fruit Bats: An Action Plan for the Family Pteropodidae; Mickleburgh, S.P., Hutson, A.M., Racey, P.A., Eds.; IUCN: Gland, Switzerland, 1992; p. 263. [Google Scholar]
- Mickleburgh, S.; Waylen, K.; Racey, P. Bats as bushmeat: A global review. Oryx 2009, 43, 217–234. [Google Scholar] [CrossRef]
- Song, Y.; Fahs, A.; Feldman, C.; Shah, S.; Gu, Y.; Wang, Y.; Machado, R.F.; Wunderink, R.G.; Chen, J. A reliable and effective method of DNA isolation from old human blood paper cards. Springerplus 2013, 2, 616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Supikamolseni, A.; Ngaoburanawit, N.; Sumontha, M.; Chanhome, L.; Suntrarachun, S.; Peyachoknagul, S.; Srikulnath, K. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet. Mol. Res. 2015, 14, 13981–13997. [Google Scholar] [CrossRef] [PubMed]
- Beerli, P.; Palczewski, M. Unified framework to evaluate panmixia and migration direction among multiple sampling locations. Genetics 2010, 185, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Beerli, P.; Mashayekhi, S.; Sadeghi, M.; Khodaei, M.; Shaw, K. Population genetic inference with MIGRATE. Curr. Protoc. Bioinformatics 2019, 68, e87. [Google Scholar] [CrossRef] [PubMed]
- Ariyaraphong, N.; Laopichienpong, N.; Singchat, W.; Panthum, T.; Farhan Ahmad, S.; Jattawa, D.; Duengkae, P.; Muangmai, N.; Suwanasopee, T.; Koonawootrittriron, S.; et al. High-level gene flow restricts genetic differentiation in dairy cattle populations in Thailand: Insights from large-scale mt D-loop sequencing. Animals 2021, 11, 1680. [Google Scholar] [CrossRef]
- Ruedi, M.; Mayer, F. Molecular systematics of bats of the genus Myotis (Vespertilionidae) suggests deterministic ecomorphological convergences. Mol. Phylogenet. Evol. 2001, 21, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Hulva, P.; Horacek, I.; Strelkov, P.P.; Benda, P. Molecular architecture of Pipistrellus pipistrellus/Pipistrellus pygmaeus complex (Chiroptera: Vespertilionidae): Further cryptic species and Mediterranean origin of the divergence. Mol. Phylogenet. Evol. 2004, 32, 1023–1035. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Lumlertdacha, B.; Boongird, K.; Wanghongsa, S.; Chanhome, L.; Rollin, P.; Hemachudha, T. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005, 11, 1949. [Google Scholar] [CrossRef]
- Cappelle, J.; Hoem, T.; Hul, V.; Furey, N.; Nguon, K.; Prigent, S.; Dupon, L.; Ken, S.; Neung, C.; Dussart, P.; et al. Nipah virus circulation at human–bat interfaces, Cambodia. Bull. World Health Organ. 2020, 98, 539. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and disease emergence: Dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Currey, K.; Kendal, D.; Van der Ree, R.; Lentini, P.E. Land manager perspectives on conflict mitigation strategies for urban flying-fox camps. Diversity 2018, 10, 39. [Google Scholar] [CrossRef]
- Aziz, S.A.; Clements, G.R.; McConkey, K.R.; Sritongchuay, T.; Pathil, S.; Abu Yazid, M.N.H.; Campos-Arceiz, A.; Forget, P.M.; Bumrungsri, S. Pollination by the locally endangered island flying fox (Pteropus hypomelanus) enhances fruit production of the economically important durian (Durio zibethinus). Ecol. Evol. 2017, 7, 8670–8684. [Google Scholar] [CrossRef] [PubMed]
- Chaiyes, A.; Escobar, L.E.; Willcox, E.V.; Duengkae, P.; Suksavate, W.; Watcharaanantapong, P.; Pongpattananurakh, N.; Wacharapluesadee, S.; Hemachudha, T. An assessment of the niche centroid hypothesis: Pteropus lylei (Chiroptera). Ecosphere 2020, 11, e03134. [Google Scholar] [CrossRef]
- Duengkae, P.; Srikhunmuang, P.; Chaiyes, A.; Suksavate, W.; Pongpattananurak, N.; Wacharapluesadee, S.; Hemachudha, T. Patch metrics of roosting site selection by Lyle’s flying fox (Pteropus lylei Andersen, 1908) in a human-dominated landscape in Thailand. Folia Oecologica 2019, 46, 63–72. [Google Scholar] [CrossRef]
- Adams, R.A. Onset of volancy and foraging patterns of juvenile little brown bats, Myotis lucifugus. J. Mammal. 1997, 78, 239–246. [Google Scholar] [CrossRef]
- Maruthupandian, J.; Marimuthu, G. Cunnilingus apparently increases duration of copulation in the Indian flying fox, Pteropus giganteus. PLoS ONE 2013, 8, e59743. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1993, 147, 915–925. [Google Scholar] [CrossRef]
- Pichler, A.; Gast, A.; Seeler, J.S.; Dejean, A.; Melchior, F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 2002, 108, 109–120. [Google Scholar] [CrossRef]
- Fu, Y.X.; Li, W.H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [CrossRef]
- Okello, M.M. Land use changes and human–wildlife conflicts in the Amboseli Area, Kenya. Hum. Dimens. Wildl. 2005, 10, 19–28. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Harpending, H.C. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum. Biol. 1994, 66, 591–600. [Google Scholar]
- Rogers, A.R. Genetic evidence for a Pleistocene population expansion. Evolution 1995, 49, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge MA, USA, 2000; p. 447. [Google Scholar]
- Rosetti, N.; Remis, M.I. Spatial genetic structure and mitochondrial DNA phylogeography of Argentinean populations of the grasshopper Dichroplus elongatus. PLoS ONE 2012, 7, e40807. [Google Scholar] [CrossRef]
- Chan, L.M.; Goodman, S.M.; Nowak, M.D.; Weisrock, D.W.; Yoder, A.D. Increased population sampling confirms low genetic divergence among Pteropus (Chiroptera: Pteropodidae) fruit bats of Madagascar and other western Indian Ocean islands. PLoS Curr. 2011, 3, RRN1226. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Markotter, W. Studying the microbiota of bats: Accuracy of direct and indirect samplings. Ecol. Evol. 2019, 9, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Alcala, N.; Streit, D.; Goudet, J.; Vuilleumier, S. Peak and persistent excess of genetic diversity following an abrupt migration increase. Genetics 2013, 193, 953–971. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Rogers, A.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Lacki, M.J.; Amelon, S.K.; Baker, M.D. Foraging Ecology of Bats in Forests. In Bats in Forests: Conservation and Management; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; Johns Hopkins University: Baltimore, MD, USA, 2007; pp. 83–127. [Google Scholar]
- Rossiter, S.J.; Benda, P.; Dietz, C.; Zhang, S.; Jones, G. Rangewide phylogeography in the greater horseshoe bat inferred from microsatellites: Implications for population history, taxonomy and conservation. Mol. Ecol. 2007, 16, 4699–4714. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Mainzer, A.; Masiero, J.; Grav, T.; Cutri, R.; Bauer, J. Response to “An empirical examination of WISE/NEOWISE asteroid analysis and results”. arXiv 2018, arXiv:1811.01454. [Google Scholar]
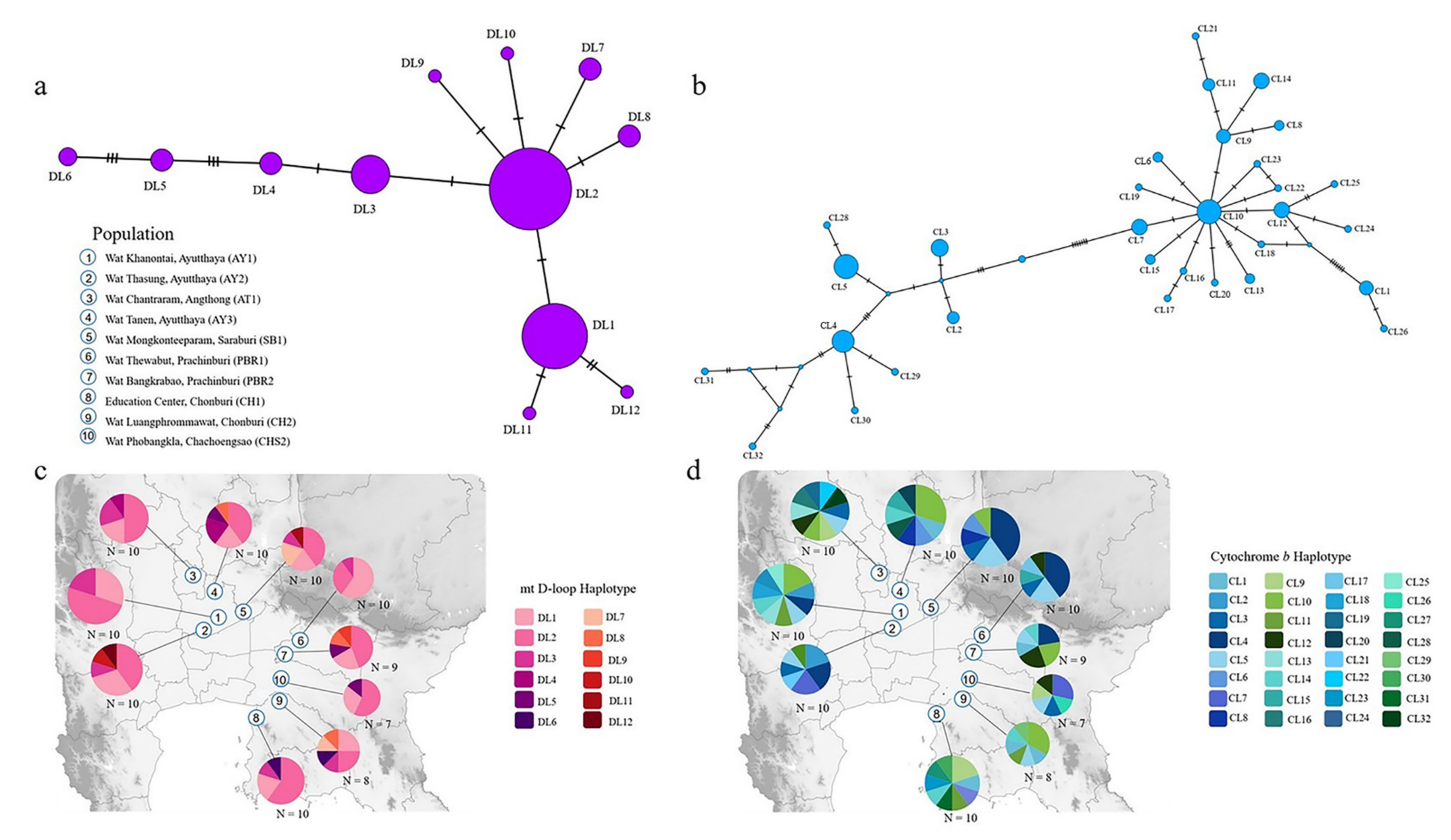
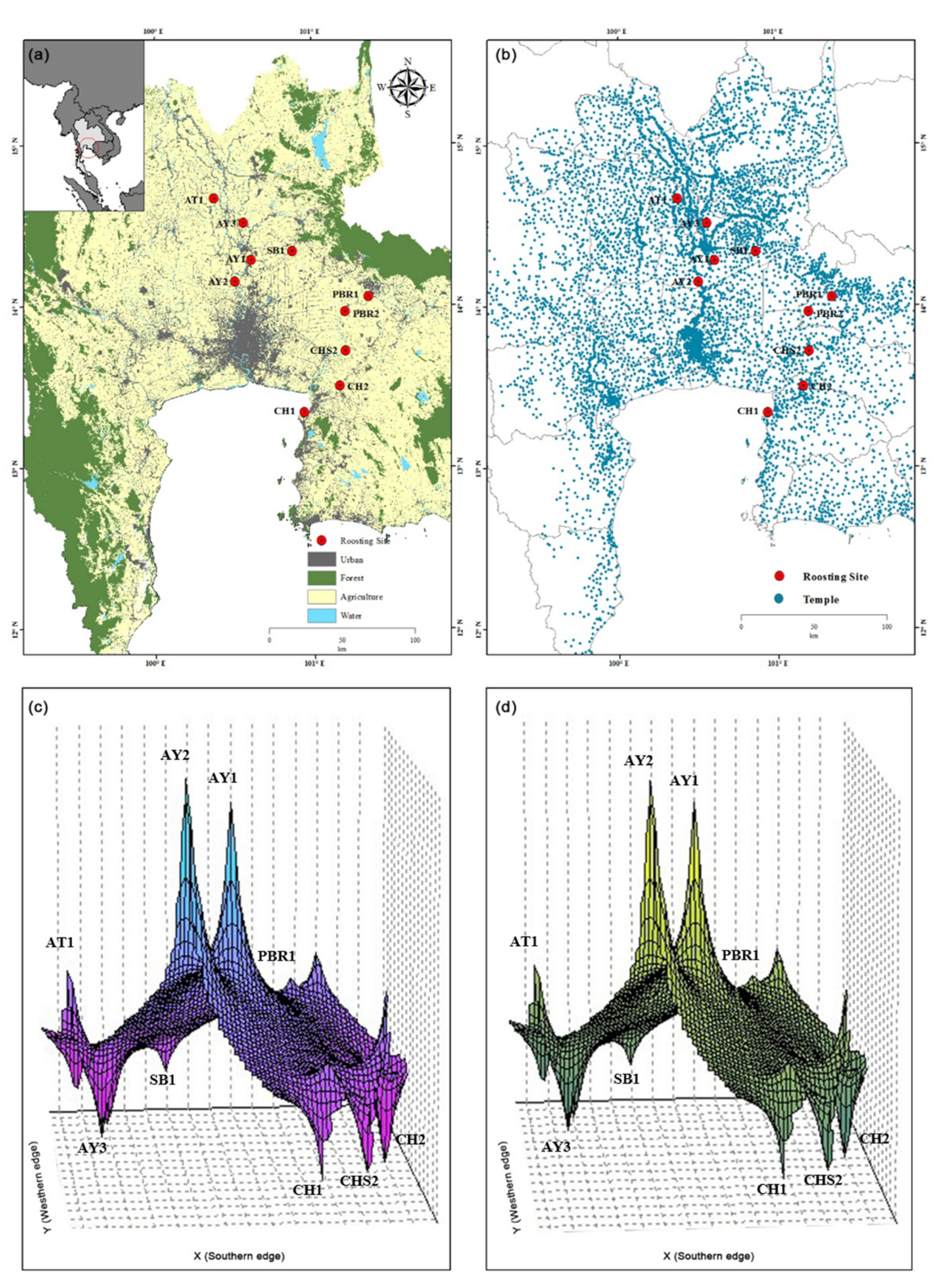
| No. | Roosting Site Code | Province of Roosting Site | Geographic Coordinates | Number of Individuals Sampled | mt D-Loop GenBank Accession Number | mt Cytochrome b GenBank Accession Number |
|---|---|---|---|---|---|---|
| 1 | AY1 | WatKhanontai: Ayutthaya | 14°17′24.0″ N 100°36′36.0″ E | 10 | LC579448–LC579457 | LC580006–LC580016 |
| 2 | AY2 | WatThasung: Ayutthaya | 14°09′00.0″ N 100°30′00.0″ E | 10 | LC579458–LC579467 | LC580017–LC580026 |
| 3 | AT1 | WatChantraram: Angthong | 14°40′12.0″ N 100°22′48.0″ E | 10 | LC579468–LC579477 | LC580027–LC580036 |
| 4 | AY3 | WatTanen: Ayutthaya | 14°31′12.0″ N 100°33′36.0″ E | 10 | LC579478–LC579487 | LC580037–LC580046 |
| 5 | SB1 | WatMongkonteeparam: Saraburi | 14°20′24.0″ N 100°52′12.0″ E | 10 | LC579488–LC579497 | LC580047–LC580056 |
| 6 | PBR1 | WatThewabut: Prachinburi | 14°03′36.0″ N 101°21′00.0″ E | 10 | LC579498–LC579507 | LC580057–LC580066 |
| 7 | PBR2 | WatBangkrabao: Prachinburi | 13°58′12.0″ N 101°12′00.0″ E | 9 | LC579508–LC579516 | LC580067–LC580075 |
| 8 | CH1 | Education Center: Chonburi | 13°20′24.0″ N 100°56′24.0″ E | 10 | LC579517–LC579526 | LC580076–LC580085 |
| 9 | CH2 | WatLuangphrommawat: Chonburi | 13°30′00.0″ N 101°10′12.0″ E | 8 | LC579527–LC579534 | LC580086–LC580093 |
| 10 | CHS2 | WatPhobangkla: Chachoengsao | 13°43′12.0″ N 101°12′00.0″ E | 7 | LC579535–LC579541 | LC580094–LC580099 |
| Colony | Sample Size | Number of Haplotypes (H) | Haplotype Diversity (h) | Nucleotide Diversity (π) | Theta (Per Site) from S | Average Number of Nucleotide Differences (k) |
|---|---|---|---|---|---|---|
| AY1 | 10 | 7.000 | 0.867 ± 0.107 | 0.011 ± 0.008 | 0.011 | 2.089 |
| AY2 | 10 | 10.000 | 1.000 ± 0.045 | 0.013 ± 0.009 | 0.035 | 15.889 |
| AT1 | 10 | 10.000 | 1.000 ± 0.045 | 0.012 ± 0.008 | 0.046 | 17.222 |
| AY3 | 10 | 9.000 | 0.978 ± 0.054 | 0.021 ± 0.013 | 0.044 | 14.600 |
| SB1 | 10 | 10.000 | 1.000 ± 0.045 | 0.022 ± 0.014 | 0.049 | 15.889 |
| PBR1 | 10 | 10.000 | 1.000 ± 0.045 | 0.010 ± 0.007 | 0.044 | 15.667 |
| PBR2 | 9 | 9.000 | 1.000 ± 0.045 | 0.020 ± 0.013 | 0.049 | 19.917 |
| CH1 | 10 | 10.000 | 1.000 ± 0.045 | 0.019 ± 0.012 | 0.048 | 15.889 |
| CH2 | 8 | 8.000 | 1.000 ± 0.045 | 0.026 ± 0.016 | 0.052 | 20.107 |
| CHS2 | 7 | 6.000 | 0.952 ± 0.096 | 0.021 ± 0.014 | 0.045 | 19.619 |
| All colonies | 94 | 12.000 | 0.727 ± 0.034 | 0.017 ± 0.010 | 0.025 | 1.376 |
| Colony | Sample Size | Number of Haplotypes (H) | Haplotype Diversity (h) | Nucleotide Diversity (π) | Theta (Per Site) from S | Average Number of Nucleotide Differences (k) |
|---|---|---|---|---|---|---|
| AY1 | 10 | 9.000 | 0.978 ± 0.054 | 0.006 ± 0.003 | 0.008 | 6.778 |
| AY2 | 10 | 7.000 | 0.933 ± 0.062 | 0.007 ± 0.004 | 0.007 | 7.556 |
| AT1 | 10 | 10.000 | 1.000 ± 0.045 | 0.006 ± 0.003 | 0.009 | 7.222 |
| AY3 | 10 | 8.000 | 0.933 ± 0.077 | 0.005 ± 0.003 | 0.007 | 5.022 |
| SB1 | 10 | 6.000 | 0.844 ± 0.103 | 0.006 ± 0.003 | 0.005 | 6.156 |
| PBR1 | 10 | 6.000 | 0.911 ± 0.077 | 0.006 ± 0.003 | 0.006 | 6.244 |
| PBR2 | 9 | 6.000 | 0.917 ± 0.073 | 0.006 ± 0.004 | 0.006 | 6.944 |
| CH1 | 10 | 9.000 | 0.978 ± 0.054 | 0.007 ± 0.004 | 0.010 | 8.244 |
| CH2 | 8 | 6.000 | 0.893 ± 0.111 | 0.006 ± 0.004 | 0.008 | 7.000 |
| CHS2 | 7 | 6.000 | 0.952 ± 0.096 | 0.007 ± 0.004 | 0.007 | 7.905 |
| All colonies | 94 | 32.000 | 0.944 ± 0.010 | 0.006 ± 0.003 | 0.011 | 7.034 |
| Colony | Tajima D | Fu D* | Fu F* | Fu’s Fs | Ewens–Watterson Test | Chakraborty’s Test | Ramos-Onsins and Rozas | Raggedness Index |
|---|---|---|---|---|---|---|---|---|
| AY1 | 1.410 ns | 0.638 ns | 0.740 ns | 1.900 ns | 0.187 ns | 0.829 ns | 0.198 | 0.601 * |
| AY2 | 0.154 ns | 0.245 ns | 0.304 ns | −0.385 ns | 0.689 ns | 0.611 ns | 0.160 | 0.304 ns |
| AT1 | 0.780 ns | −0.559 ns | −0.632 ns | 0.713 ns | 0.498 ns | 0.674 ns | 0.114 | 0.530 * |
| AY3 | −0.306 ns | −0.084 ns | −0.212 ns | 0.765 ns | 0.516 ns | 0.695 ns | 0.137 | 0.256 ns |
| SB1 | −0.895 ns | −0.030 ns | −0.027 ns | 0.922 ns | 0.495 ns | 0.695 ns | 0.144 | 0.686 * |
| PBR1 | 0.686 ns | −0.354 ns | −0.350 ns | 1.546 ns | 0.578 ns | 0.671 ns | 0.131 | 0.507 * |
| PBR2 | −1.042 ns | −0.270 ns | −0.276 ns | 0.352 ns | 0.854 ns | 0.563 ns | 0.138 | 0.133 ns |
| CH1 | −0.967 ns | −0.445 ns | −0.516 ns | 1.820 ns | 0.880 ns | 0.446 ns | 0.123 | 0.453 ns |
| CH2 | −1.084 ns | −0.545 ns | −0.608 ns | −0.706 ns | 0.655 ns | 0.772 ns | 0.126 | 0.500 * |
| CHS2 | −0.608 ns | 0.103 ns | 0.121 ns | 2.659 ns | 0.687 ns | 0.686 ns | 0.167 | 0.456 ns |
| All colonies | −1.213 ns | −0.559 ns | −1.053 ns | −0.552 ns | 0.830 ns | 0.273 ns | 0.050 | 0.367 ns |
| Colony | Tajima D | Fu D* | Fu F* | Fu’s Fs | Ewens–Watterson Test | Chakraborty’s Test | Ramos-Onsins and Rozas | Raggedness Index |
|---|---|---|---|---|---|---|---|---|
| AY1 | −0.763 ns | −0.864 ns | −0.953 ns | −0.222 ns | 1.000 ns | 0.364 ns | 0.097 | 0.126 ns |
| AY2 | 0.323 ns | 0.047 ns | 0.099 ns | 0.226 ns | 0.345 ns | 0.803 ns | 0.155 | 0.076 ns |
| AT1 | −0.697 ns | −1.302 ns | −1.412 ns | −2.995 * | 1.000 ns | 0.764 ns | 0.100 | 0.087 ns |
| AY3 | −1.695 * | −1.734 ns | −1.945 ns | −1.870 ns | 1.000 ns | 0.544 ns | 0.108 | 0.050 ns |
| SB1 | 6.324 ns | 0.156 ns | 0.207 ns | 0.963 ns | 0.902 ns | 0.525 ns | 0.161 | 0.212 ns |
| PBR1 | 0.182 ns | 0.015 ns | 0.030 ns | 0.920 ns | 0.889 ns | 0.525 ns | 0.147 | 0.162 ns |
| PBR2 | −0.033 ns | −2.388 ns | −2.171 ns | 0.726 ns | 0.265 ns | 0.813 ns | 0.156 | 0.052 ns |
| CH1 | −0.918 ns | −0.868 ns | −0.960 ns | −0.897 ns | 1.000 ns | 0.544 ns | 0.112 | 0.025 ns |
| CH2 | −0.771 ns | −0.708 ns | −0.802 ns | 1.393 ns | 1.000 ns | 0.410 ns | 0.132 | 0.199 ns |
| CHS2 | −0.098 ns | −0.081 ns | −0.113 ns | −0.331 ns | 1.000 ns | 0.767 ns | 0.161 | 0.107 ns |
| All colonies | −0.968 ns | −2.388 ns | −2.171 ns | −7.573 ns | 0.797 ns | 0.404 ns | 0.067 | 0.025 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyes, A.; Ariyaraphong, N.; Sukgosa, N.; Jangtarwan, K.; Ahmad, S.F.; Laopichienpong, N.; Singchat, W.; Panthum, T.; Duangjai, S.; Muangmai, N.; et al. Evidence of Genetic Connectivity among Lyle’s Flying Fox Populations in Thailand for Wildlife Management and One Health Framework. Sustainability 2022, 14, 10791. https://doi.org/10.3390/su141710791
Chaiyes A, Ariyaraphong N, Sukgosa N, Jangtarwan K, Ahmad SF, Laopichienpong N, Singchat W, Panthum T, Duangjai S, Muangmai N, et al. Evidence of Genetic Connectivity among Lyle’s Flying Fox Populations in Thailand for Wildlife Management and One Health Framework. Sustainability. 2022; 14(17):10791. https://doi.org/10.3390/su141710791
Chicago/Turabian StyleChaiyes, Aingorn, Nattakan Ariyaraphong, Ngamphrom Sukgosa, Kornsuang Jangtarwan, Syed Farhan Ahmad, Nararat Laopichienpong, Worapong Singchat, Thitipong Panthum, Sutee Duangjai, Narongrit Muangmai, and et al. 2022. "Evidence of Genetic Connectivity among Lyle’s Flying Fox Populations in Thailand for Wildlife Management and One Health Framework" Sustainability 14, no. 17: 10791. https://doi.org/10.3390/su141710791
APA StyleChaiyes, A., Ariyaraphong, N., Sukgosa, N., Jangtarwan, K., Ahmad, S. F., Laopichienpong, N., Singchat, W., Panthum, T., Duangjai, S., Muangmai, N., Wacharapluesadee, S., Duengkae, P., & Srikulnath, K. (2022). Evidence of Genetic Connectivity among Lyle’s Flying Fox Populations in Thailand for Wildlife Management and One Health Framework. Sustainability, 14(17), 10791. https://doi.org/10.3390/su141710791










