1. Introduction
Nutrients can be a significant source of air, soil, and water pollution, and can negatively affect biodiversity and the climate. Many European water bodies are in poor condition due to eutrophication [
1]. The European water policy aims to achieve a good ecological status in all rivers, lakes, coastal and intermediate waters by 2027 at the latest [
2]. Municipal wastewater treatment plants need to be optimized to achieve greater efficiency in removing nitrogen and phosphorus from wastewater. The efficient removal of nutrients from wastewater can only be achieved if wastewater treatment plants are expanded with the tertiary stage of wastewater treatment [
3]. Tertiary wastewater treatment is the removal of pollutants remaining after secondary (biological) treatment by filtering through various fillers and membranes, adsorption, ion exchange, coagulation–flocculation processes, and their combinations [
4,
5,
6]. Extremely high purification rates (99%) are achieved by applying membrane filtration, but this is one of the most complicated and expensive methods of wastewater treatment. Some wastewater treatment methods pose the risk of forming secondary pollutants [
4]. One of the methods of simple and convenient management and economical tertiary wastewater treatment is sorption using sorbents. Minerals and organic or inorganic materials, which are mainly used as adsorbents, have different adsorption capacities for the removal of specific contaminants from wastewater. Until now, most of the published findings involve laboratory-scale experiments. The lack of pilot-scale information is a major drawback in using cost-effective adsorbents as a substitute for activated carbon, as well as other expensive wastewater treatment technologies [
4]. When removing nitrogen from wastewater, it should be noted that it can be in the form of ammonium or nitrates. Due to their different charge, as some are cations and others are anions, these nitrogen compounds are adsorbed or ion-exchanged by different materials. Ammonium is efficiently adsorbed by zeolites, and nitrates can be removed by ion exchange using resins [
5,
6,
7]. Most of the phosphorus remaining in the effluent after biological wastewater treatment is in the form of phosphates [
8]. Phosphate phosphorus is adsorbed by biochar-based sorbents, zirconium–lanthanum-modified magnetite, calcite, and rare earth elements [
9,
10,
11,
12]. The materials used to remove nitrogen and phosphorus compounds from the effluent after biological wastewater treatment must be insoluble, harmless, and not contaminate the water while treating it in the tertiary treatment stage. When the materials’ sorption capacity is exhausted, these materials must be recovered and reused or used in another field (agriculture, soil improvement). The authors of this article aimed to select suitable materials, then form a layered filter media, and reduce nitrogen and phosphorus concentrations in wastewater by filtration in the tertiary wastewater treatment stage. Of the selected materials, two are non-traditional; they are not used for wastewater treatment. Quartz sand grains with coating are taken from drinking water treatment plants, and Purolite A502PS resin is intended for use in the food industry. The materials are selected for their ability to remove phosphates or nitrates from water. The use of these materials in one filter together with natural zeolite has not been tested. The advantage of layered filling is that, without using chemical reagents, nitrogen and phosphorus removal processes from wastewater take place in one filter (with minimal production of secondary pollutants). Further research is needed to promote the use of non-conventional adsorbents on a large scale. The pilot experiments were performed, and they are useful in the development of tertiary wastewater treatment plants.
2. Materials and Methods
The pilot experimental study was performed in a medium-sized (with a flow rate of more than 5 m
3 per day but with a population equivalent (PE) to less than 10,000) wastewater treatment plant with biological wastewater treatment. The wastewater treatment plant was built and operates in the Vilnius district of Lithuania. The removal of nitrogen and phosphorus is in the anaerobic, anoxic, and aerobic zones by the technological scheme of the treatment plant. The pilot experimental study was conducted in the period from 21 February 2022 to 29 April 2022. An experimental model with filtration columns, the scheme of which is shown in
Figure 1, was installed in the room of the wastewater treatment building.
During the pilot experimental study, a certain amount (104 L) of wastewater treated at the wastewater treatment plant was collected daily, at the same time of day (approximately 6:00 a.m.). The wastewater after biological treatment was poured into a treated wastewater tank ((2)
Figure 1), from which it was fed to two filtration columns ((5)
Figure 1) by a distribution pipe using a pump ((4)
Figure 1) to lift wastewater. Filtration was performed daily for 12 h, during which 52 L of effluent flowed out of each column. A uniform amount of wastewater was determined by the volumetric method by turning the valves equally ((6)
Figure 1). The filtering was not performed at night. Wastewater was filtered at a rate of 0.5 m/h (flow rate 4.3 L/h). Layers of sorbents were poured onto the supporting layer of fine stones in two filtration columns. The composition and height of these layers are given in
Table 1. The filter layers were submerged during the experiment, with filtration from top to bottom. Pressure losses in the columns were measured. The hydraulic surface load of each filter was maintained: 0.5 m
3/(m
2·h). The wastewater after filtration was sampled from each column twice daily (7:30 and 18:30) ((6)
Figure 1). At the same time, wastewater samples were taken from the treated wastewater tank ((2)
Figure 1). Samples were analyzed in the laboratory by daily measurements of their temperature, pH, ammonium nitrogen (NH
4-N), nitrate nitrogen (NO
3-N), and phosphate phosphorus concentrations (PO
4-P). Concentrations of BOD
7, COD, and suspended solids (TSS) were measured in the samples once a week.
The following sorbents were selected as filter media layers: quartz sand with iron and manganese oxide film (OG), Purolite A502PS resin, and natural zeolite (clinoptilolite). All three materials were used in the first column and only OG and zeolite were in the second column (
Table 1). Below is a description of each sorbent.
OG is a filter media used in drinking water treatment plants in Vilnius. During the preparation of drinking water from groundwater containing iron and manganese, deposits of iron and manganese oxides are formed on the grains of quartz sand. At pH > 6, phosphorus is retained by physical adsorption; phosphorus binds to iron (Fe) and aluminum (Al) oxides and precipitates as sparingly soluble phosphates [
13]. Previous studies have shown that OG sorbs phosphorus from wastewater [
14], so this sorbent is now selected for testing under real conditions in a multilayer filter.
Purolite A502PS resin is chosen because it can exchange ions: during filtration through the Purolite filter media, the anions in it bind to free radicals and release chloride ions. Purolite A502PS is a macroporous anion exchange resin designed to remove organic matter, color, and odor used in food production, it is noted that resins are suitable for the removal of nitrates from water [
7]. In this study, the sorbent Purolite A502PS was selected to reduce nitrate concentrations in wastewater.
Zeolite (clinoptilolite-[AlSi
5O
12]
2(K
2,Na
2,Ca)(H
2O)
8) was obtained from the Sokirnicki site (Ukraine). It was chosen because it has a natural negative charge that gives it a high cation exchange capacity. Zeolite adsorbs ammonia ions (NH
4-N) and other water-soluble cations and organic compounds [
5]. Zeolites are hydrated aluminum silicates in crystalline form. The structure of zeolites is similar to the scaffold, and it provides a larger internal and external surface on which ion exchange and chemical reactions can take place. It is observed that ammonium nitrogen may be present in the effluent after biological treatment [
15]. The concentration of ammonium nitrogen could be reduced in a multilayer filter during the pilot experimental study.
Wastewater temperature was measured using a SevenGo pro SG6 (Mettler Toledo, Switzerland). The pH was determined potentiometrically (ISO 10523:2012) by measuring the pH of the WTW production pH meter with Hamilton (Switzerland) certified reference buffers pH 7.00 ± 0.01 and pH 9.21 ± 0.02. COD, BOD7, and TSS were measured using standard methods (ISO 6060:2003; EN 1899-2:2000; EN 872:2005).
NH4-N, NO3-N, and PO4-P concentrations were measured using MERCK Spectroquant® tests. Absorbance measurements were performed by pouring test samples into cuvettes (Hellma) and measuring with a Genesys 10 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at the required wavelength. Each sample was tested three times, and the mean values of the results are given. Indices values varied by 6–12%.
The removal efficiency of the respective pollutant
Ei (%) from wastewater was calculated according to Formula (1):
where
C0 is the concentration of the respective pollutant before filtration, (mg/L), and
Ci is the concentration of the respective pollutant after filtration, (mg/L).
3. Results and Discussion
During the testing period, the temperature and pH of the wastewater entering the filtration columns from the tank of biologically treated wastewater were 18–21 °C and 7.6–8.5, respectively. The temperature of the filtrates from both columns was slightly different from the initial one, averaging 20.02 °C. The pH, which averaged 8.06 before filtration, decreased in the filtrates of the first column and averaged 7.56. Meanwhile, the average pH of the second column filtrates was 7.71. It is concluded that the sorbent aggregates slightly reduced the pH of the effluent, especially the Purolite A502PS resin used in the first column. Wastewater with a temperature not exceeding 30 °C and a pH in the range of 6.5–8.5 may be discharged into natural water bodies as established by the Regulation on Wastewater Management of the Republic of Lithuania [
16]. The results obtained during the pilot experimental study meet these limits. The average concentration of BOD
7 in biologically treated wastewater supplied to the filters was 10.1 mg/L, the COD concentration was 42.1 mg/L, the TSS concentration was 53.2 mg/L, the ammonium nitrogen concentration was 8.22 mg/L, the nitrate nitrogen concentration was 2.46 mg/L, and the phosphate phosphorus concentration was 1.56 mg/L. All biologically treated wastewater parameters, except the ammonium nitrogen concentration, meet the current requirements for medium-sized wastewater treatment (according to PE < 10,000). The concentration of ammonium nitrogen in treated wastewater discharged into the natural environment is too high when it exceeds 1–2 mg/L. Wastewater that is rich in ammonium nitrogen does great harm to the water environment [
17]. An analysis of the wastewater treatment efficiency of 11 medium-sized wastewater treatment plants performed earlier by the authors of this article showed that the concentration of NH
4-N in the total nitrogen concentration in the treated wastewater ranged from 17% to 48% [
15]. Ammonium nitrogen is removed from wastewater inefficiently when there is insufficient aeration, as well as when the residence time of activated sludge in the plant is too short, and nitrifying organisms are washed away when the treated wastewater is toxic. These are problems with wastewater treatment plants that require time and electricity to solve. On the other hand, tertiary wastewater treatment using sorbents can reduce NH
4-N concentrations in treated wastewater discharged into the natural environment.
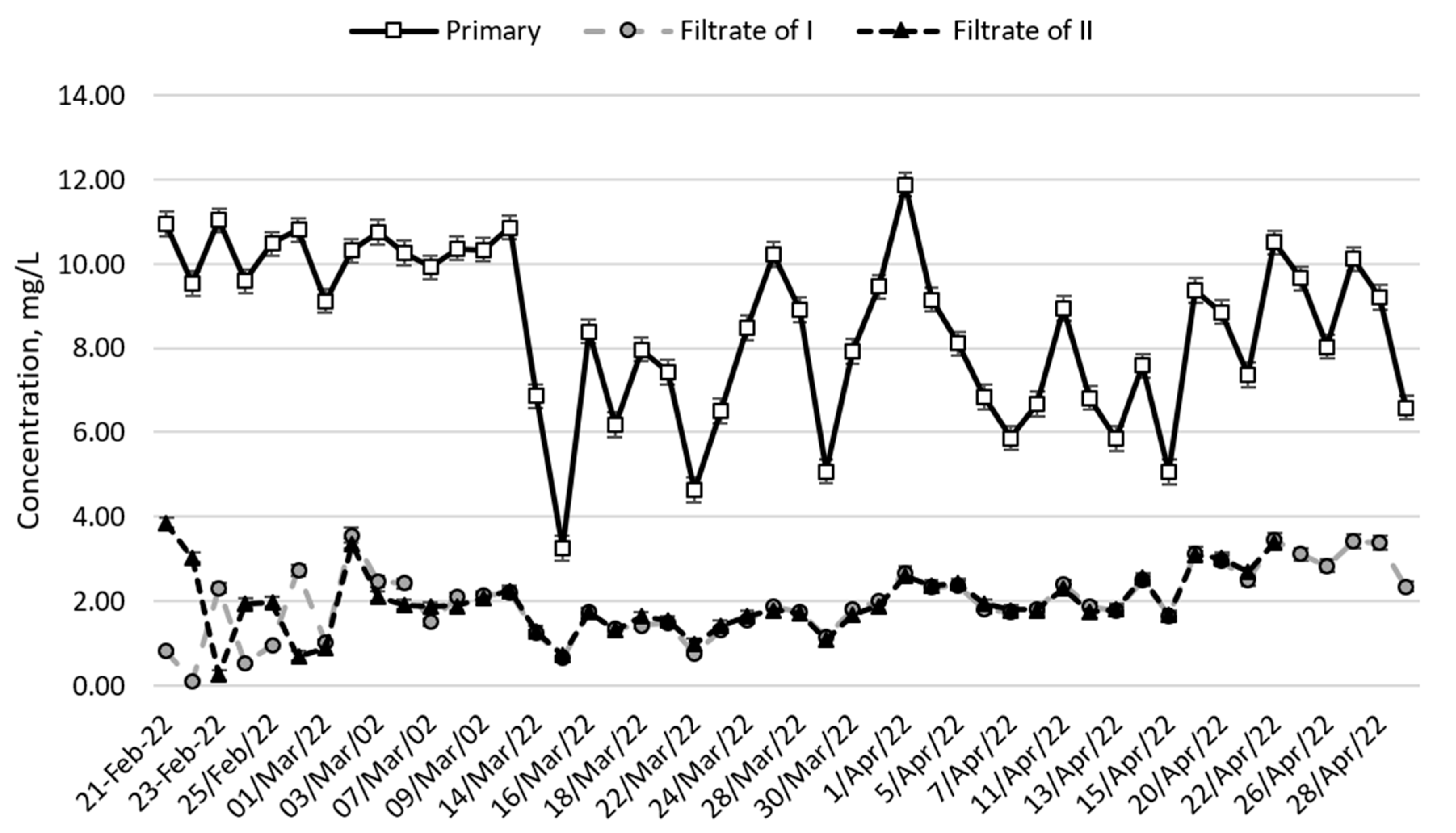
The daily concentration of NH
4-N of the biologically treated wastewater entering filtration columns is shown in
Figure 2.
Figure 2 shows that by mid-March, the initial NH
4-N concentration was around 10 mg/L, falling to 3.3 mg/L on 15 March, and then fluctuating sharply from 3.3 to 11.9 mg/L. On April 1st, NH
4-N concentrations were highest (11.9 mg/L) throughout the pilot study period. The concentration of NH4-N in the filtrates from both columns was, on average, 80–83% lower than the initial NH
4-N concentration. The average NH
4-N concentration obtained in the filtrates from the columns was 1.6 mg/L from the first column and 1.5 mg/L from the second column. These are good results (concentrations less than 2 mg/L), as the treated wastewater can be discharged into the natural environment. However, at the beginning and end of the experiment, the concentration of NH
4-N in the filtrates was higher than 2 mg/L. At the beginning of February, this may have been influenced by the high (>10 mg/L) initial concentration of ammonium nitrogen in the biologically treated wastewater. In the end, from April 15, the sorption capacity of the zeolite layer was possibly reduced due to the fact that the zeolite was used for 2 months and depleted. The efficiency of the first column to remove NH
4-N from the effluent was 92–100% at the beginning and 64–69% at the end, thus reducing the efficiency. The experiment was terminated before the sorption capacity of the zeolite layer was completely depleted due to increased pressure loss due to the filter becoming clogged. The average pressure loss in each column was about 13 cm. The pressure loss in the second filter grew faster than in the first, so it clogged earlier, on April 21. According to the authors, the filters were clogged with too high of a concentration of TSS in the biologically treated wastewater (average 53.2 mg/L), before the sorption properties of the layers were completely exhausted. The efficiency of the second column to remove NH
4-N from wastewater was 64–95% at the beginning and 66–67% at the end of the experiment, so the zeolite layer could still sorb NH
4-N.
The daily concentration of NO
3-N of the biologically treated wastewater entering filtration columns is shown in
Figure 3.
Figure 3 shows that only the first column containing the Purolite layer removed nitrates from the biologically treated wastewater. This layer was characterized by ion exchange, where nitrates are removed from wastewater and chloride ions are released into the environment. Chloride ions released during wastewater treatment are not pollutants, because, according to the Regulation on Wastewater Management of the Republic of Lithuania [
16], the maximum allowable concentration of chlorides in the wastewater discharged into the natural environment is 1000 mg/L, and this concentration has not been reached. The first column removed nitrates with an average efficiency of 82%, and the ion exchange capacity of the Purolite layer was not completely depleted at the end of the experiment (efficiency of 74%). The filtrate from the second column remained similar to the initial NO
3-N concentration (
Figure 3), and it is concluded that the layers of natural zeolite and OG did not remove nitrate nitrogen from the biologically treated wastewater.
The daily concentration of PO
4-P of the biologically treated wastewater entering filtration columns is shown in
Figure 4.
Figure 4 shows that in winter and spring until mid-March, the initial concentration of PO
4-P in wastewater was higher than allowed (2 mg/L). The results of the pilot study confirm the observation of the authors [
18,
19] that phosphorus removal from the biologically treated wastewater in the cold period is not as efficient as in the warm. Phosphate concentrations above 2 mg/L were observed throughout the pilot study period on 11 April (2.21 mg/L). The OG filter media layers of both columns removed PO
4-P from the effluent with 65–67% efficiency. The filtrates from columns I and II had average concentrations of PO
4-P of 0.48 mg/L and 0.45 mg/L, respectively. It was observed that the OG layer in both the first and second columns did not remove phosphorus from the wastewater as efficiently during the first two days of the experiment as later. This is considered to be an adaptation period after which the efficiency of the OG filter media layers of both columns in removing PO
4-P was 96–99%. Over time, the sorption capacity of the OG layers decreased and at the end of the experiment, the phosphorus removal efficiency was 25% in the first column and 24% in the second. When the columns were clogged, the sorption capacity of OG was not yet exhausted. It is concluded that both columns and the sorption layers in them could have reduced the concentrations of nitrogen and phosphorus in biologically treated wastewater for some time if the biologically treated wastewater had a lower concentration of suspended solids. At the end of the filtration, the sorbent layers were removed from the columns and inspected, but no changes or signs of disintegration were observed. So, the sorbents themselves and their particles were not the cause of clogging during filtration.
A two-month pilot study has shown that additional wastewater treatment using layers of sorbents is effective: the quality of the treated wastewater meets not only the requirements for medium-sized wastewater treatment plants, but also the stricter requirements for large (>100,000 PE) treatment plants. Comparing the initial (after biological treatment in the treatment plant) and final (after additional filtration) average concentrations, it was observed that column I reduced NH
4-N concentrations by 5 times (from 8.22 to 1.5 mg/L), NO
3-N by 6 times (from 2.46 to 0.44 mg/L), and PO
4-P by 3 times (from 1.56 to 0.45 mg/L). Column II reduced NH
4-N concentrations by 5 times, and PO
4-P by 3 times. Compared to other wastewater treatment technologies, a good result was achieved. Horizontal flow vegetated filters (constructed wetlands) filled with sand or crushed stone (20–30 mm) remove the total nitrogen from wastewater with an efficiency of 15–40% and total phosphorus with an efficiency of 30–45% [
20]. When applying underground infiltration (for loam soil), nitrogen can be removed with 30–60% efficiency, and phosphorus with 60–90% efficiency [
21]. In addition, sequencing batch-constructed wetlands, bioretention systems have disadvantages: pumps and siphons are needed for wastewater distribution, a lot of space is needed, they can only be applied in the presence of suitable soils, and they require a certain unsaturated soil depth (above the groundwater level). The commercial material Filtralite P used for phosphorus removal from wastewater is effective (>90% efficiency), but requires large amounts of this material and increases the pH of the wastewater [
14]. The layered filter media tested in this experiment does not have such disadvantages. The filter media could have worked longer if the wastewater after biological treatment had a concentration of TSS of only 10–15 mg/L, which is usually ensured by well-functioning WWTPs (their efficiency in removing TSS from wastewater is 97–99%). Previous laboratory tests were carried out by filtering wastewater with a TSS concentration of <15 mg/L, so no filter clogging was observed [
14]. The experiments described in this work were carried out at the real wastewater treatment plant, during the winter–spring period, when the real conditions may not have corresponded to the design ones. This circumstance highlighted the importance of proper biological wastewater treatment for TSS removal in tertiary treatment.
According to the authors, the tested sorbent layers did not deteriorate the wastewater quality. Zeolite (clinoptilolite) is known to be an environmentally friendly substance [
22]. Purolite A502PS is used in food production, its manufacturers report that Purolite A502PS is a thermally stable, osmotic-resistant, granular, macroporous material that is not classified as hazardous. Chloride ions that enter the environment due to ion exchange do not exceed the allowable concentrations for wastewater. OG, quartz sand from filters in drinking water treatment plants, is also non-hazardous. The shell of iron and manganese oxides on the surface of the OG is strong and non-crumbly. Oxides of iron and manganese sorb phosphorus according to the equation given in [
23]:
In previous laboratory studies, environmentally friendly materials for removing nitrogen or phosphorus were tested separately as single-layer filter media [
14,
24,
25]. The novelty of this study is that ammonium and nitrate nitrogen and phosphate phosphorus were removed in the same filter by single-stage filtration. Ammonium or nitrate nitrogen concentrations in wastewater after biological treatment are unstable [
15], and both nitrogen compounds (NH
4+ and NO
3−) are often present, so it is irrational to use only one selected filler to remove only ammonium or only nitrates from wastewater. Phosphorus, which biological treatment does not completely remove from wastewater, must also be removed by filter media. Therefore, a layered filter media was formed, in which processes of nitrogen and phosphorus removal from wastewater take place, filtering the wastewater at a speed of 0.5 m/h. It is important that after filtering through the tested fillers, the pH of the water remains neutral (7.6–7.7) and its temperature does not change (remains ~20 °C), so the water may be discharged into natural water bodies [
16].
Summarizing the obtained results, it can be seen that the tested sorbent layers effectively treat wastewater from nitrogen and phosphorus and do not pollute the wastewater with environmentally hazardous substances, therefore it can be used in the development of tertiary wastewater treatment plants. Filters filled with these layers must be protected from clogging by suspended solids, therefore preliminary removal of TSS from wastewater (by precipitation or filtration through grain materials) is recommended. According to the authors, after reducing the concentrations of TSS in the biologically treated wastewater, it is recommended to carry out longer-term studies in order to assess the full sorption capacity of the materials and the further use of recovered sorbents.