Plant Diversity and Aboveground Biomass Interact with Abiotic Factors to Drive Soil Organic Carbon in Beijing Mountainous Areas
Abstract
:1. Introduction
2. Materials and Methods
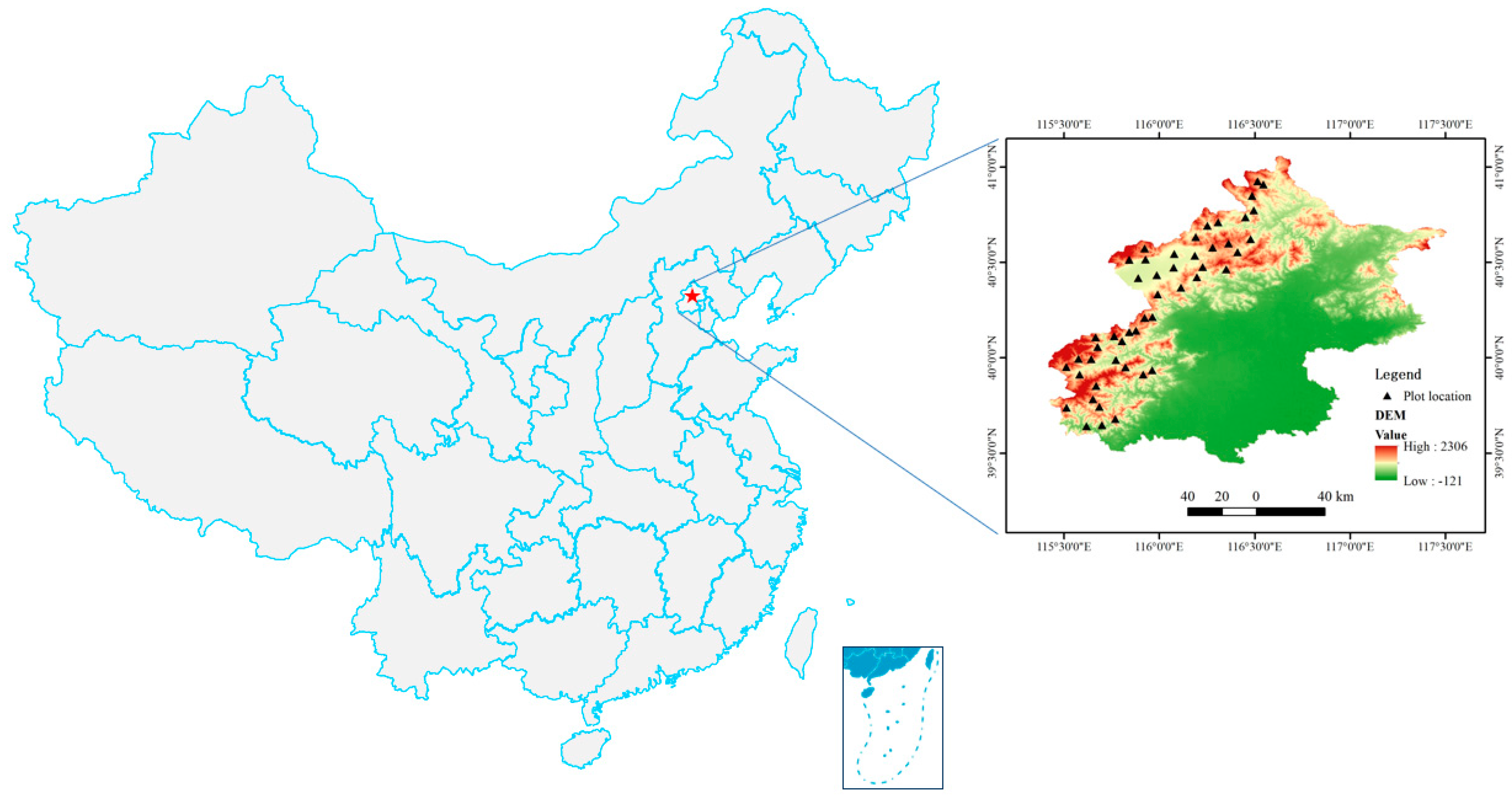
2.1. Study Sites
2.2. Data Sampling
2.3. Data Analyses and Modeling
2.3.1. Plant Diversity Index
2.3.2. Aboveground Biomass
2.3.3. Multiple Linear Regression Analysis
2.3.4. Structural Equation Model
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houghton, R.A. Balancing the Global Carbon Budget. Annu. Rev. Earth Planet. Sci. 2007, 35, 313–317. [Google Scholar] [CrossRef]
- Pete, S. Soil carbon sequestration and biochar as negative emission technologies. Glob. Change Biol. 2016, 22, 1315–1324. [Google Scholar]
- Crowther, T.W. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Cornelia, R.; Farshad, A.; Lydie-Stella, K.; Pete, S. Put more carbon in soils to meet Paris climate pledges. Nature 2018, 564, 32–34. [Google Scholar]
- De, D.G.; Cornelissen, S.M.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting. Ecol. Lett. 2008, 11, 516–531. [Google Scholar]
- Markus, L.; Nico, E.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 1–8. [Google Scholar]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Francesca, C.M.; Wallenstein, M.D. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–1005. [Google Scholar]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V.J. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Change 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Cusack, D.F.; Halterman, S.M.; Tanner, E.V.J. Decadal-scale litter manipulation alters the biochemical and physical character of tropical forest soil carbon. Soil Biol. Biochem. 2018, 124, 199–209. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant Functional Composition Influences Rates of Soil Carbon and Nitrogen Accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S.; Chareonwong, U. Factors controlling soil organic carbon sequestration of highland agricultural areas in the Mae Chaem Basin, Northern Thailand. Agronomy 2020, 10, 305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.C.; Shi, X.Z.; Lu, X.X.; Yu, D.S.; Wang, H.J.; Sun, W.X.; Darilek, J.L. Variation of soil organic carbon estimates in mountain regions: A case study from Southwest China. Geoderma 2008, 146, 449–456. [Google Scholar] [CrossRef]
- Carvalhais, N.; Forkel, M.; Khomik, M. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.Y.; Li, J.P.; Wei, X. Effects of stand spatial structure on herbaceous species diversity in forests based on structural equation modeling. Acta Ecol. Sin. 2022, 42, 1–11. [Google Scholar]
- Wang, M.Y.; Yang, J.; Gao, H.L.; Xu, W.S.; Dong, M.Q.; Shen, G.C.; Xu, J.; Xu, X.L.; Xue, J.M.; Xu, C.Y.; et al. Interspecific plant competition increases soil labile organic carbon and nitrogen contents. For. Ecol. Manag. 2020, 462, 117991. [Google Scholar] [CrossRef]
- Haghverdi, K.; Kooch, Y. Effects of diversity of tree species on nutrient cycling and soil-related processes. Catena 2019, 178, 335–344. [Google Scholar] [CrossRef]
- He, Y.; Qin, L.; Li, Z.Y.; Liang, X.Y.; Shao, M.X.; Tan, L. Carbon storage capacity of monoculture and mixed-species plantations in subtropical China. For. Ecol. Manag. 2013, 295, 193–198. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Review: Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Morgan, J.A.; Blumenthal, D. Water limitation and plant inter-specific competition reduce rhizosphere-induced C decomposition and plant N uptake. Soil Biol. Biochem. 2010, 42, 1073–1082. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Duan, B.L.; Xian, J.R.; Helena, K.; Li, C.Y. Links between plant diversity, carbon stocks and environmental factors along a successional gradient in a subalpine coniferous forest in Southwest China. For. Ecol. Manag. 2011, 262, 361–369. [Google Scholar] [CrossRef]
- Jobbagy, E.G. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Laurent, A.; Antra, B. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar]
- Osborne, J.F. Comparisons of Soil Analytical Methods. East Afr. Agric. For. J. 2015, 39, 265–271. [Google Scholar] [CrossRef]
- Fang, J.Y.; Liu, G.H.; Xu, H.L. Biomass and Net Production of Forest Vegetation in China. Acta Ecol. Sin. 1996, 16, 497–508. [Google Scholar]
- Wei, X.; Bi, H.X.; Liang, W. Relationship between Soil Characteristics and Stand Structure of Robinia pseudoacacia L. and Pinus tabulaeformis Carr. Mixed Plantations in the Caijiachuan Watershed: An Application of Structural Equation Modeling. Forests 2018, 9, 124. [Google Scholar] [CrossRef]
- Hou, G.R.; Bi, H.X.; Huo, Y.M.; Wei, X.Y.; Zhu, Y.J.; Wang, X.X.; Liao, W.C. Determining the optimal vegetation coverage for controlling soil erosion in Cynodon dactylon grassland in North China. J. Clean. Prod. 2020, 244, 118771. [Google Scholar] [CrossRef]
- You, H.Z.; Bi, J.; Wang, C.; Ren, Q.W.; Li, L.D. Distribution characteristics and influencing factors of soil organic carbon density of Betula platyphylla forest at different altitudes in Xiaowutai Mountain, Hebei Province. J. Ecol. Environ. 2018, 27, 432–437. [Google Scholar]
- Hong, X.J. Study on soil organic carbon density and influencing factors of main forest community types in Da and Xiao Xing’an Mountains. Northeast. For. Univ. 2012, 12, 22–30. [Google Scholar]
- Zhang, H.Q.; Lin, C.; Chen, H.; Jing, C.S.; Xu, Z.K.; Wei, Z.C.; Ma, Q.X. Characteristics and influencing factors of soil organic carbon in Phyllostachys pubescens forests with different altitude gradients in Wuyi Mountain. Soil 2019, 51, 821–828. [Google Scholar]
- Liang, J.J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Zhang, X.P.; He, L. Carbon sequestration characteristics of different Restored Vegetation Types in Loess Hilly Region. Environ. Sci. 2022, 43, 1–16. [Google Scholar]
- Xu, G.P.; Li, Y.Q.; Shen, Y.Y.; Zhang, D.N.; Sun, Y.J.; Zhang, Z.F.; Zhou, L.W.; Duan, C.Y. Characteristics of soil organic carbon and its components of different plant communities under water level gradient in Huixian karst wetland, Guilin. Environ. Sci. 2019, 40, 1491–1503. [Google Scholar]
- Zhao, Q.; Liu, S.; Chen, K.; Wang, S.J.; Wu, C.Z.; Li, J.; Lin, Y.M. Change characteristics and influencing factors of soil organic carbon in Castanopsis eyrei natural forests at different altitudes in Wuyishan Nature Reserve. J. Ecol. 2021, 41, 5328–5339. [Google Scholar]
- Sheikh; Munesh, K.; Rainer, B. Altitudinal variation in soil organic carbon stock in coniferous subtropical and broadleaf temperate forests in Garhwal Himalaya. Carbon Balance Manag. 2009, 4, 6. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Pan, D.B.; Zhu, Z.Y.; Liu, L.Z.; Wu, M.Y.; Li, X.B. Distribution law and influencing factors of soil organic carbon of Larix principis rupprechtii along altitude gradient in Liupan Mountain. J. Ecol. 2021, 41, 6773–6785. [Google Scholar]
- Zhou, G.Y.; Xu, S.; Ciais, P.; Manzoni, S.; Fang, J.Y.; Yu, G.R.; Tang, X.L.; Zhou, P.; Wang, W.T.; Yan, J.H.; et al. Climate and litter C/N ratio constrain soil organic carbon accumulation. Natl. Sci. Rev. 2019, 6, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Wang, R.R.; Gong, Y.L.; Cai, J.Y. Effects of community species and structural diversity on Forest Aboveground Biomass and its scale effects: A case study of BCI sample plot in Panama. Biodiversity 2017, 25, 1054–1064. [Google Scholar]
- Chen, L.F.; He, Z.B.; Du, J.; Zhu, X. Patterns and environmental controls of soil organic carbon and total nitrogen in alpine ecosystems of northwestern China. Catena 2016, 137, 37–43. [Google Scholar] [CrossRef]
- Zhao, P.P.; Zhou, J.H.; Lin, K.D.; Lin, W.S.; Yuan, P.; Zeng, X.; Su, Y.; Xu, J.; Chen, Y.; Yang, Y.; et al. Effects of different altitudes on soil microbial biomass and soil enzyme activity of Huangshan pine forest in Daiyun mountain, Fujian. J. Ecol. 2019, 39, 2676–2686. [Google Scholar]
- Wu, B.C.; Zhou, L.J.; Qi, S.; Jin, M.L.; Hu, J.; Lu, J.S. Effect of habitat factors on the understory plant diversity of Platycladus orientalis plantations in Beijing mountainous areas based on MaxEnt model. Ecol. Indic. 2021, 129, 107917. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Y.X.; Ye, J.C.; Gong, Y.Z.; Zeng, S.C. Soil organic carbon density and its influencing factors of Eucalyptus Plantation in Guangdong Province. J. Appl. Ecol. 2010, 21, 1981–1985. [Google Scholar]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Turner, B.L.; Zhou, G.Y.; Mo, J.M. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Xiang, S.G.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and rewetting effects on C and N mineralization and microbial activity in surface and subsurface California grassland soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Wen, X.; Lu, X.M.; Ying, J.Y.; Lan, Z.C.; Chen, D.; Bai, Y. Disentangling the effects of nitrogen availability and soil acidification on microbial taxa and soil carbon dynamics in natural grasslands. Soil Biol. Biochem. 2022, 164, 108495. [Google Scholar]
| Index | Formula | Mean | Range |
|---|---|---|---|
| Species Shannon index | 1.13 | 0.12–2.01 | |
| Species Simpson index | 0.59 | 0.05–0.84 | |
| Species Margalef index | 1.09 | 0.27–2.66 | |
| DBH Shannon index | 1.79 | 1.12–2.21 | |
| DBH Simpson index | 0.81 | 0.62–0.94 | |
| Tree height Shannon index | 1.57 | 0.94–2.06 | |
| Tree height Simpson index | 0.76 | 0.56–0.88 |
| Depth of Soil Laye(cm) | Explanatory Variables | a1 | a2 | R2 |
|---|---|---|---|---|
| 0–10 | Hs + Hd | 0.049 | −0.586 | 0.353 |
| Hs + Dd | 0.060 | −0.557 | 0.320 | |
| Hs + Hh | −0.092 | −0.628 | 0.364 | |
| Hs + Dh | −0.040 | −0.545 | 0.286 | |
| Ds + Hd | 0.090 | −0.592 | 0.351 | |
| Ds + Dd | 0.046 | −0.564 | 0.346 | |
| Ds + Hh | −0.111 | −0.623 | 0.368 | |
| Ds + Dh | −0.080 | −0.550 | 0.291 | |
| S + Hd | 0.045 | −0.586 | 0.352 | |
| S + Dd | 0.040 | −0.557 | 0.318 | |
| S + Hh | −0.087 | −0.627 | 0.364 | |
| S + Dh | −0.020 | −0.539 | 0.285 | |
| 10–20 | Hs + Hd | −0.089 | −0.271 | 0.076 |
| Hs + Dd | −0.091 | −0.329 | 0.110 | |
| Hs + Hh | −0.093 | −0.107 | 0.014 | |
| Hs + Dh | −0.107 | −0.172 | 0.030 | |
| Ds + Hd | −0.041 | −0.262 | 0.070 | |
| Ds + Dd | −0.028 | −0.319 | 0.103 | |
| Ds + Hh | −0.055 | −0.089 | 0.005 | |
| Ds + Dh | −0.067 | −0.155 | 0.024 | |
| S + Hd | −0.066 | −0.270 | 0.072 | |
| S + Dd | −0.079 | − 0.332 | 0.108 | |
| S + Hh | −0.061 | −0.097 | 0.009 | |
| S + Dh | −0.071 | −0.160 | 0.025 | |
| 20–30 | Hs + Hd | 0.021 | −0.249 | 0.063 |
| Hs + Dd | 0.026 | −0.232 | 0.056 | |
| Hs + Hh | 0.015 | −0.102 | 0.012 | |
| Hs + Dh | 0.008 | −0.143 | 0.021 | |
| Ds + Hd | 0.083 | −0.249 | 0.070 | |
| Ds + Dd | 0.095 | −0.237 | 0.064 | |
| Ds + Hh | 0.068 | −0.091 | 0.016 | |
| Ds + Dh | 0.062 | −0.133 | 0.025 | |
| S + Hd | 0.074 | −0.261 | 0.068 | |
| S + Dd | −0.075 | −0.247 | 0.061 | |
| S + Hh | −0.083 | −0.136 | 0.018 | |
| S + Dh | −0.083 | −0.168 | 0.027 |
| Depth of Soil Laye | Aboveground Biomass | Altitude | Slope Degree | Total Nitrogen | Total Phosphorus | Total Potassium | pH |
|---|---|---|---|---|---|---|---|
| 0–10 | 0.596 ** | 0.239 * | 0.190 | 0.936 ** | 0.132 | −0.274 * | −0.080 |
| 10–20 | 0.105 | 0.474 ** | 0.038 | 0.915 ** | 0.179 | −0.185 | −0.122 |
| 20–30 | 0.207 | 0.537 ** | 0.004 | 0.915 ** | 0.131 | −0.178 | −0.277 * |
| Depth of Soil Laye | Predictor | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|---|
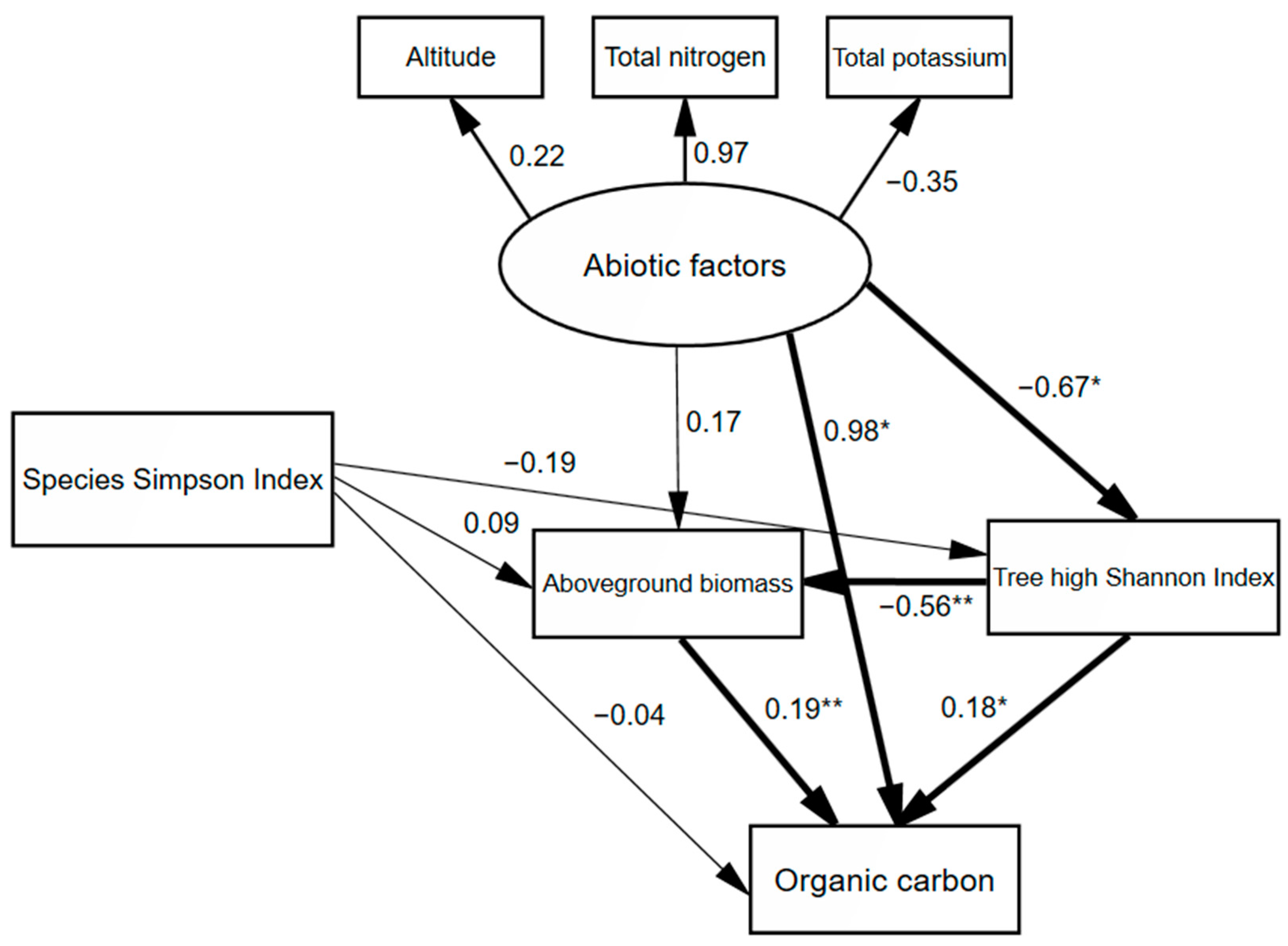
| 0–10 | Abiotic factors | 0.985 | −0.021 | 0.964 |
| Species Simpson index | −0.036 | 0.002 | −0.034 | |
| Tree height Shannon indexAboveground biomass | 0.184 0.188 | −0.015 − | 0.169 0.188 | |
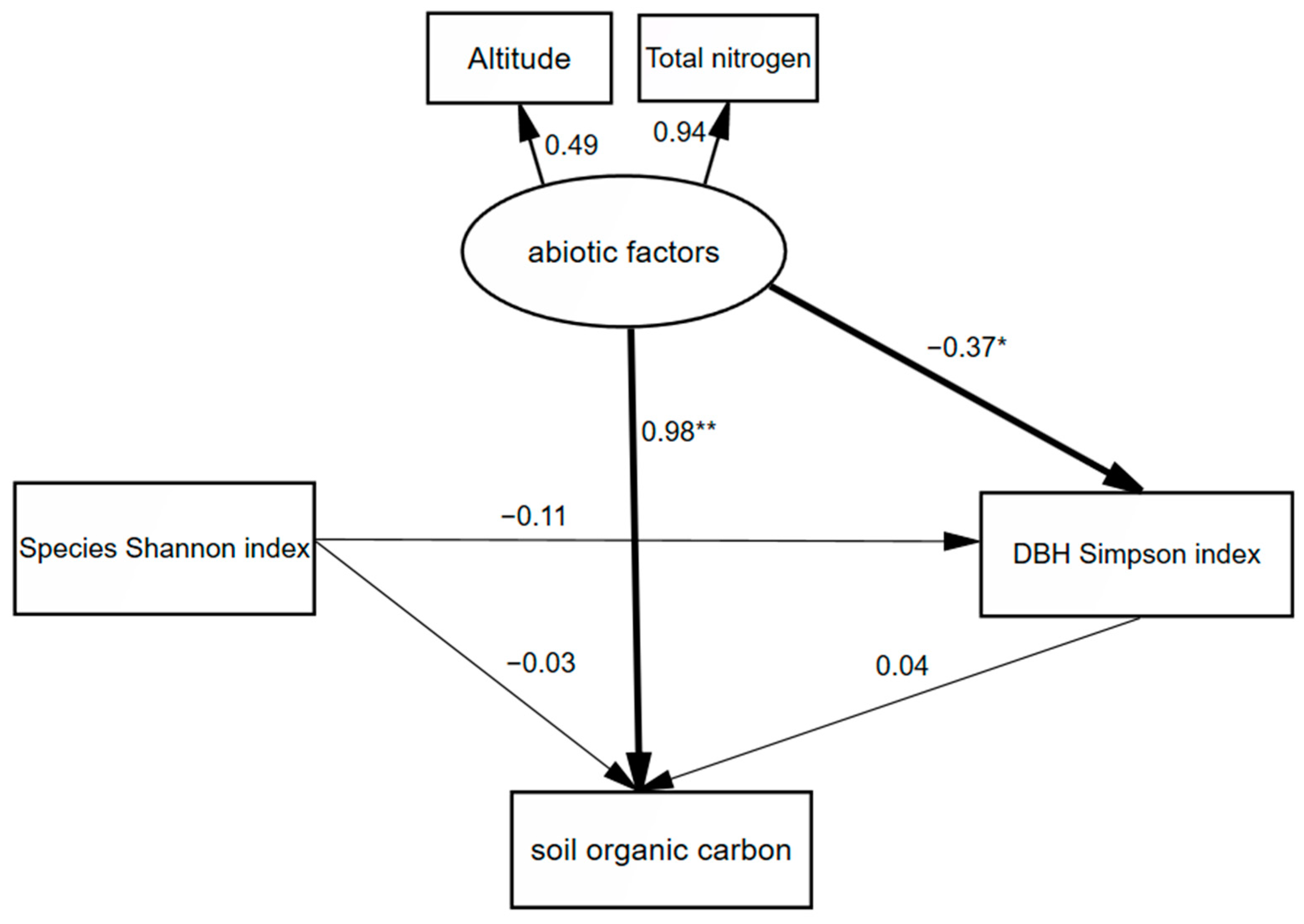
| 10–20 | Abiotic factors | 0.98 | −0.015 | 0.965 |
| Species Shannon index | −0.03 | −0.005 | −0.035 | |
| DBH Simpson index | −0.04 | − | −0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Zhang, L.; Qi, S. Plant Diversity and Aboveground Biomass Interact with Abiotic Factors to Drive Soil Organic Carbon in Beijing Mountainous Areas. Sustainability 2022, 14, 10655. https://doi.org/10.3390/su141710655
Zhou P, Zhang L, Qi S. Plant Diversity and Aboveground Biomass Interact with Abiotic Factors to Drive Soil Organic Carbon in Beijing Mountainous Areas. Sustainability. 2022; 14(17):10655. https://doi.org/10.3390/su141710655
Chicago/Turabian StyleZhou, Piao, Lin Zhang, and Shi Qi. 2022. "Plant Diversity and Aboveground Biomass Interact with Abiotic Factors to Drive Soil Organic Carbon in Beijing Mountainous Areas" Sustainability 14, no. 17: 10655. https://doi.org/10.3390/su141710655
APA StyleZhou, P., Zhang, L., & Qi, S. (2022). Plant Diversity and Aboveground Biomass Interact with Abiotic Factors to Drive Soil Organic Carbon in Beijing Mountainous Areas. Sustainability, 14(17), 10655. https://doi.org/10.3390/su141710655





