Decontamination and Remediation of Underground Holes and Testing of Cleaning Techniques Based on the Use of Liquid Cold Decontaminant
Abstract
:1. Introduction
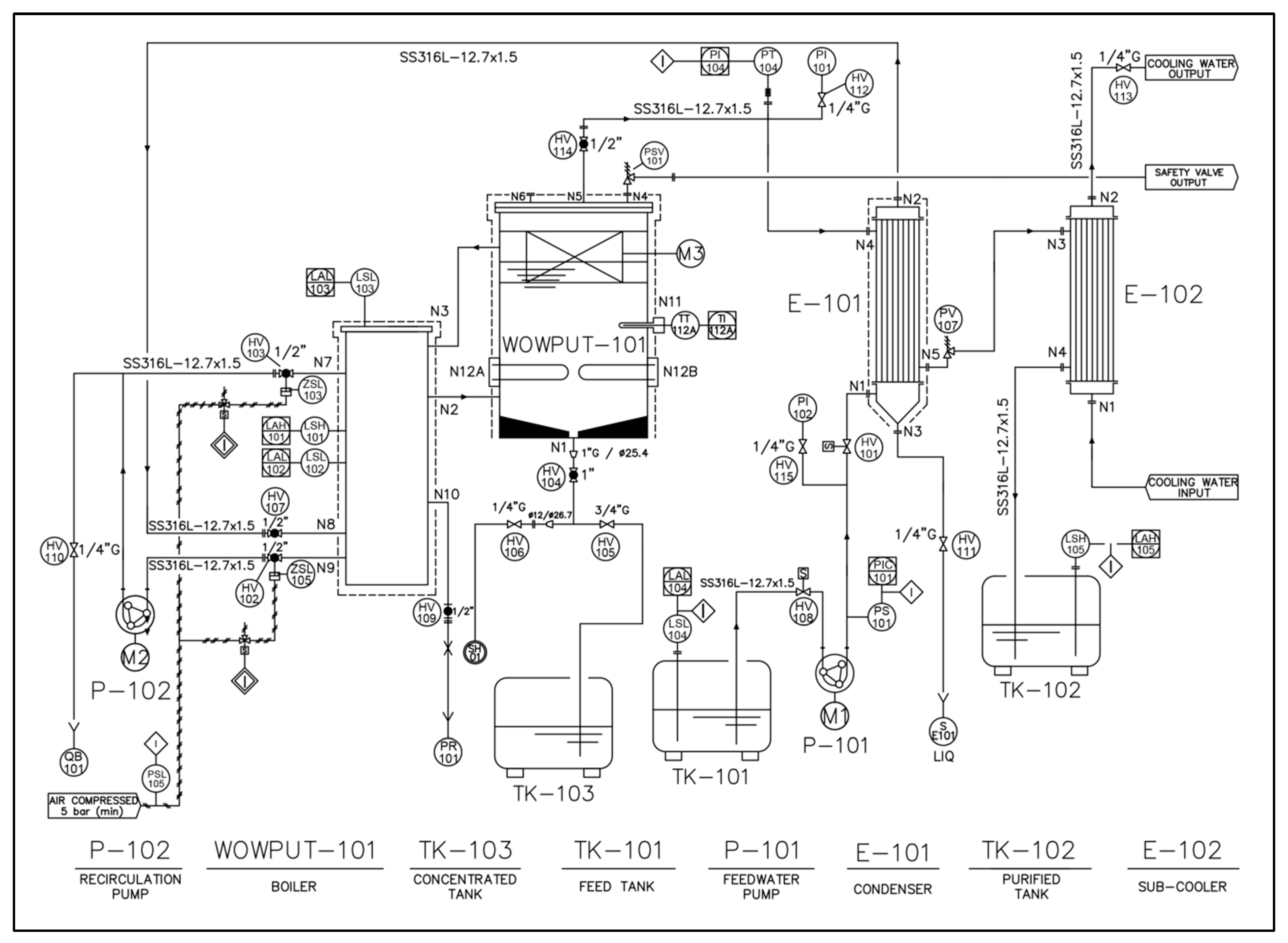
2. Description of the Work Progress
3. Results
3.1. Holes Decontamination Results
3.1.1. Holes Results
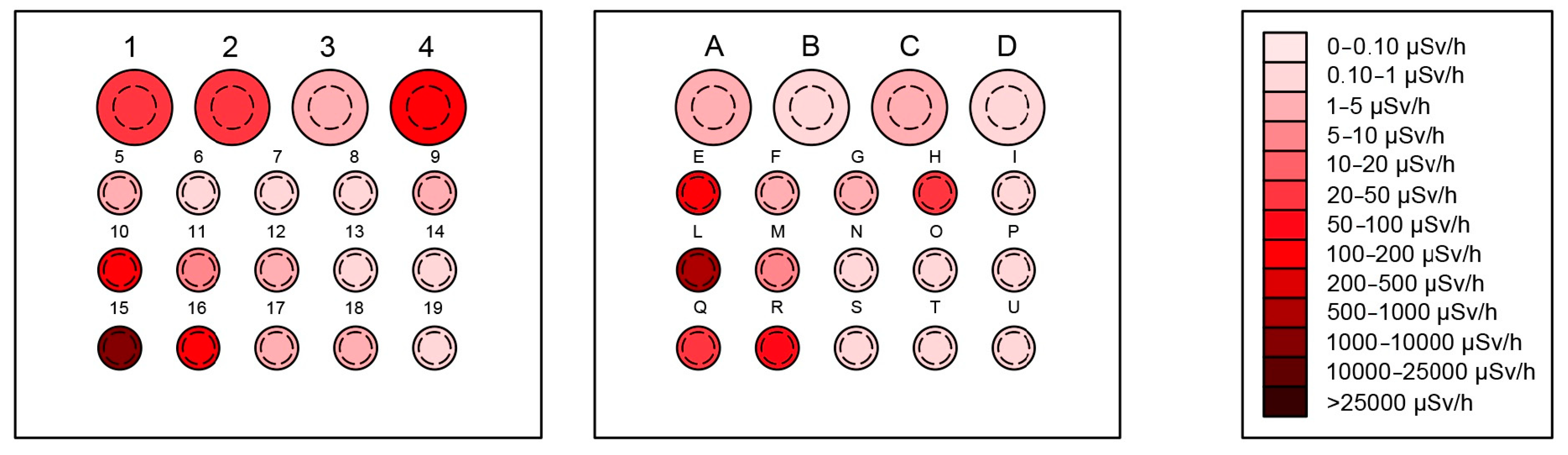
3.1.2. Specific Holes Results
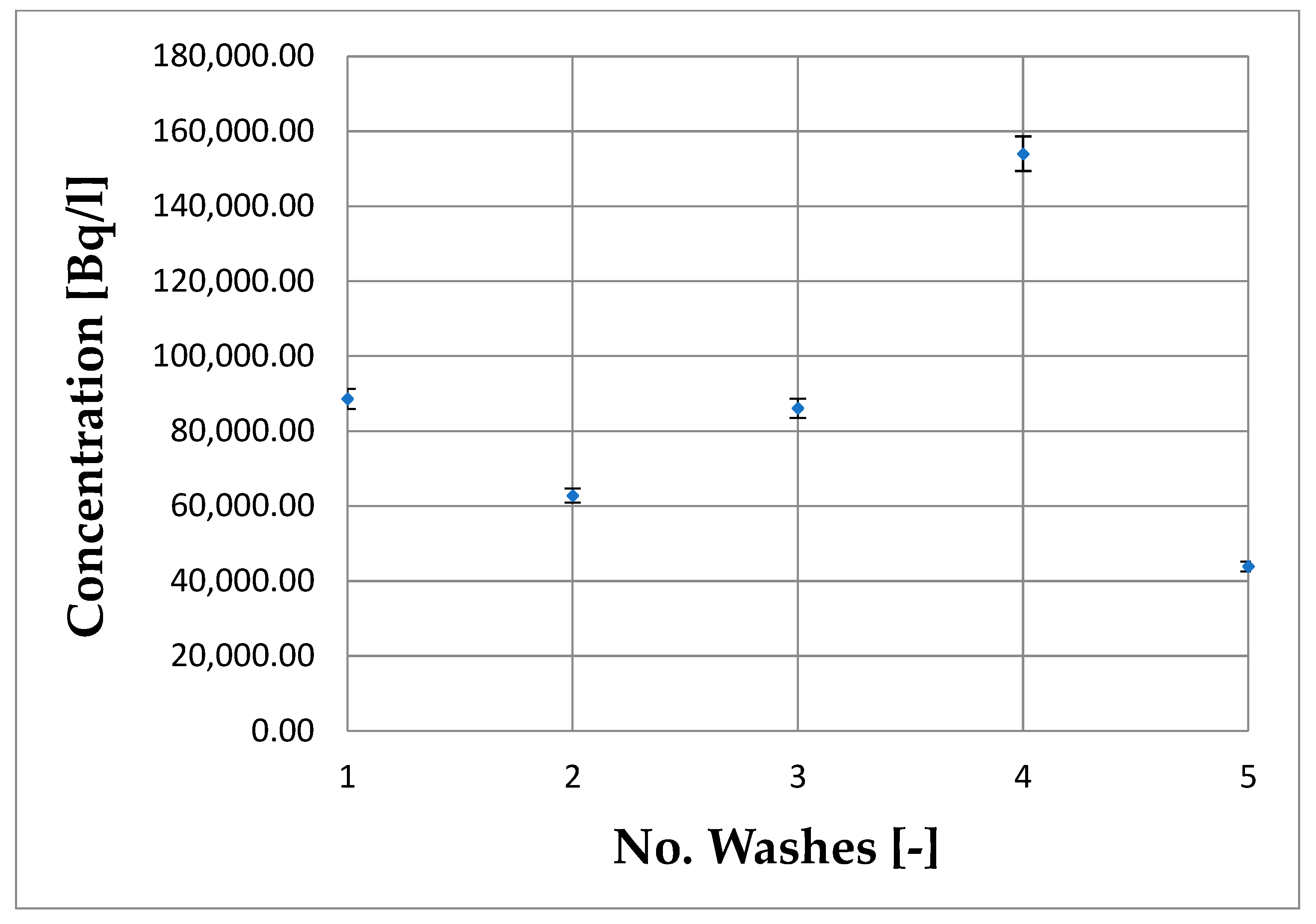
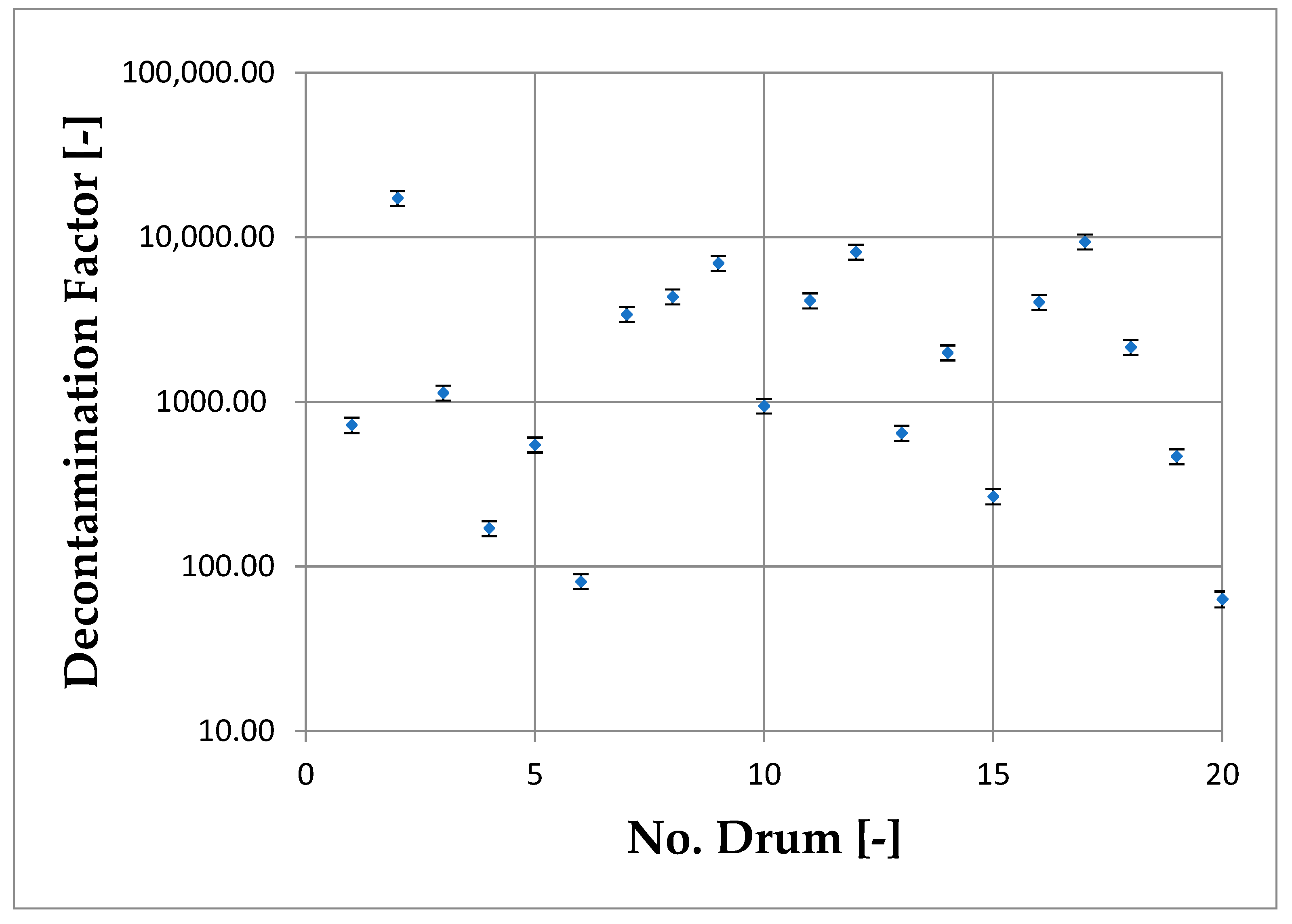
3.2. Evaluation of WOW Machine Decontamination Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; He, Y.; Xie, H.; Ge, Y.; Lin, Y.; Yao, Z.; Jin, M.; Liu, J.; Chen, X.; Sun, Y.; et al. A State-of-the-Art Review of Radioactive Decontamination Technologies: Facing the Upcoming Wave of Decommissioning and Dismantling of Nuclear Facilities. Sustainability 2022, 14, 4021. [Google Scholar] [CrossRef]
- Dickerson, K.S.; Wilson-Nichols, M.J.; Morris, M.I. Chapter 3, Candidate Technologies for Concrete Decontamination. In Contaminated Concrete: Occurrence and Emerging Technologies for DOE Decontamination, 1st ed.; U.S. Department of Energy Office of Environmental Restoration and Waste Management Office of Technology Development: Washington, DC, USA, 1995. [Google Scholar]
- Laraia, M. 14—Innovative and conventional decontamination techniques for cementitious structures. In Sustainability of Life Cycle Management for Nuclear Cementation-Based Technologies; Woodhead Publishing Series in Energy; Woodhead Publishing: Thorston, UK, 2021; pp. 455–512. [Google Scholar] [CrossRef]
- Prakash, D.; Gabani, P.; Chandel, A.K.; Ronen, Z.; Singh, O.V. Bioremediation: A genuine technology to remediate radionuclides from the environment. Microb. Biotechnol. 2013, 6, 349–360. [Google Scholar] [CrossRef]
- Arnal, J.M.; Sancho, M.; García-Fayos, B. Treatment of 137Cs contaminated water by selective adsorption. Desalination 2013, 321, 22–27. [Google Scholar] [CrossRef]
- Awual, M.R.; Suzuki, S.; Taguchi, T.; Shiwaku, H.; Okamoto, Y.; Yaita, T. Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 2014, 242, 127–135. [Google Scholar] [CrossRef]
- Oh, M.; Lee, K.; Foster, R.I.; Lee, C.H. Feasibility study on the volume reduction of radioactive concrete wastes using thermomechanical and chemical sequential process. J. Environ. Chem. Eng. 2021, 9, 105742. [Google Scholar] [CrossRef]
- Jang, S.C.; Hong, S.B.; Yang, H.M.; Lee, K.W.; Moon, J.K.; Seo, B.K.; Huh, Y.S.; Roh, C. Removal of Radioactive Cesium Using Prussian Blue Magnetic Nanoparticles. Nanomaterials 2014, 4, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.H.; Lim, J.M.; Kim, H.; Kim, S. Conventional and photoinduced radioactive 137Cs removal by adsorption on FeFe, CoFe, and NiFe Prussian blue analogues. Chem. Eng. J. 2020, 405, 126568. [Google Scholar] [CrossRef]
- Delchet, C.; Tokarev, A.; Dumail, X.; Toquer, G.; Barré, Y.; Guari, Y.; Guerin, C.; Larionova, J.; Grandjean, A. Extraction of radioactive cesium using innovative functionalized porous materials. RSC Adv. 2012, 2, 5707–5716. [Google Scholar] [CrossRef]
- Kim, G.N.; Choi, W.K.; Lee, K.W. Decontamination of radioactive concrete using electrokinetic technology. J. Appl. Electrochem. 2010, 40, 1209–1216. [Google Scholar] [CrossRef]
- IAEA. Combined Methods for Liquid Radioactive Waste Treatment—Final Report of a Coordinated Research Project 1997–2001; IAEA-TECDOC-1336; IAEA: Vienna, Austria, 2003. [Google Scholar]
- Narbutt, J. Chapter 4—Fundamentals of Solvent Extraction of Metal Ions. In Handbooks in Separation Science-Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–155. [Google Scholar] [CrossRef]
- Pianese, E.; Mazzaro, M. Radiological decontamination procedures in emergency operations. ANPEQ Newsletter n17-72/May–December, 2005, Year XXV n 2–3. Available online: https://anpeq.it/index.php/117-notiziario-626541/notiziario/281-2005 (accessed on 6 July 2022).
- ENEA-DISP, Technical Guidance n26—Radioactive Waste Management, September 1987. Available online: https://www.depositonazionale.it/raccoltadocumenti/linee-guida/guida_tecnica_n26_gestione_rifiuti_radioattivi.pdf (accessed on 6 July 2022).
- Legislative Decree 101/2020, Implementation of Directive 2013/59/EURATOM, which establishes basic safety standards relating to protection against the dangers arising from exposure to ionizing radiation, and that repeals Directives 89/618/EURATOM, 90/641/ EURATOM, 96/29/ EURATOM, 97/43/ EURATOM and 2003/122/ EURATOM and reorganization of the sector legislation in implementation of Article 20, paragraph 1, letter a) of the Law n117 of October 4 2019. Gazzetta Ufficiale della Repubblica Italiana n.201, 31 July 2020.
- Marin, A. WOW Technology’s innovative treatment for HLW and ILW liquid Radwastes. In Proceedings of the Nuclear Decommissioning & Used Fuel Strategy Summit, Ritz-Carlton Hotel, Charlotte, NC, USA, 2–3 October 2017. [Google Scholar]
- Marin, A. Innovative radioactive liquid waste for Fukushima-Daiichi Remediation. In Proceedings of the Annual Meeting of the Spanish Nuclear Society at Coruña, Coruña, Spain, 23–25 September 2015. [Google Scholar]
- Marin, A. WOW Technology’s Innovative Radioactive Liquid Waste Treatment—16128; Report n° INIS-US--19-WM-16128; WM Symposia Inc.: Tempe, AZ, USA, 2016; Available online: https://archive.wmsym.org/2016/index.html (accessed on 1 July 2016).
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef]
- Remark, J.F.; Miller, A.D. Review of plant decontamination methods. In Decontamination and Decommissioning of Nuclear Facilities; Springer: Boston, MA, USA, 1989; pp. 33–45, EPRl NP-6169. [Google Scholar]
- Koutsoukos, P.G.; Kontoyannis, C.G. Precipitation of Calcium Carbonate in Aqueous Solutions. J. Chem. Soc. Faraday Trans. 1 1984, 80, 1181–1192. [Google Scholar] [CrossRef]
- Krauskopf, K.B. Dissolution and precipitation of silica at low temperatures. Geochim. Et Cosmochim. Acta 1956, 10, 1–26. [Google Scholar] [CrossRef]
- Yosifova, V.; Stoimenov, N.; Haralampieva, M. On-site research with a thermal camera on industrial heating. IOP Conf. Ser. Mater. Sci. Eng. 1031, 2021, 012082. [Google Scholar] [CrossRef]

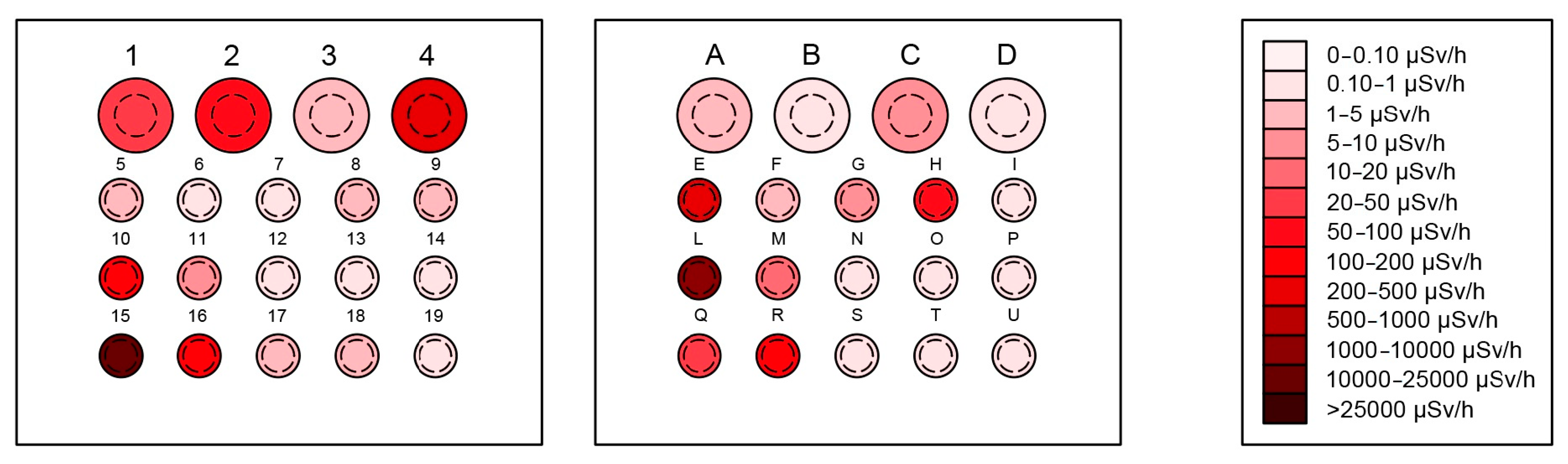
| Hole | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole-Weighted Average (Bq/cm2) | Effective Dose Rate (μSv/h) |
|---|---|---|---|---|
| A | 8.32 ± 0.82 | 1.76 ± 0.17 | 8.01 ± 0.79 | 2.5 ± 0.2 |
| B | 6.36 ± 0.64 | 0.61 ± 0.06 | 6.09 ± 0.61 | 0.5 ± 0.1 |
| C | 32.94 ± 3.12 | 94.24 ± 8.92 | 35.82 ± 3.01 | 8.5 ± 0.8 |
| D | 2.89 ± 0.29 | 1.15 ± 0.12 | 2.81 ± 0.28 | 0.3 ± 0.1 |
| E | 100.01 ± 3.01 | 10.52 ± 0.32 | 96.34 ± 2.88 | 300.0 ± 9.1 |
| F | 4.53 ± 0.44 | 2.91 ± 0.28 | 4.46 ± 0.43 | 4.0 ± 0.4 |
| G | 5.33 ± 0.49 | 2.90 ± 0.27 | 5.23 ± 0.48 | 10.0 ± 0.9 |
| H | 116.16 ± 4.963 | 38.24 ± 1.63 | 112.97 ± 4.76 | 82.0 ± 3.5 |
| I | 34.38 ± 3.44 | 2.09 ± 0.21 | 33.06 ± 3.29 | 0.6 ± 0.1 |
| L | 1250.10 ± 37.55 | 129.70 ± 3.89 | 1204.27 ± 35.97 | 2500.0 ± 75.0 |
| M | 1.59 ± 0.14 | 6.42 ± 0.58 | 1.79 ± 0.14 | 15.0 ± 1.4 |
| N | 3.34 ± 0.33 | 4.27 ± 0.43 | 3.38 ± 0.32 | 1.0 ± 0.1 |
| O | 3.21 ± 0.32 | 6.73 ± 0.67 | 3.34 ± 0.31 | 1.0 ± 0.1 |
| P | 3.66 ± 0.37 | 1.12 ± 0.11 | 3.56 ± 0.35 | 0.2 ± 0.1 |
| Q | 5.32 ± 0.44 | 1.52 ± 0.12 | 5.17 ± 0.42 | 27.0 ± 2.2 |
| R | 81.10 ± 2.43 | 110.01 ± 3.31 | 82.28 ± 2.34 | 154.0 ± 4.6 |
| S | 0.80 ± 0.08 | 6.02 ± 0.61 | 1.01 ± 0.08 | 1.0 ± 0.1 |
| T | 0.94 ± 0.09 | 1.61 ± 0.16 | 0.97 ± 0.09 | 0.10 ± 0.05 |
| U | 1.08 ± 0.11 | 0.16 ± 0.02 | 1.04 ± 0.11 | 0.11 ± 0.06 |
| 1 | 18.80 ± 1.53 | 36.15 ± 2.95 | 19.61 ± 1.47 | 27.0 ± 2.2 |
| 2 | 91.77 ± 5.67 | 44.18 ± 2.73 | 89.54 ± 5.41 | 55.0 ± 3.4 |
| 3 | 0.75 ± 0.08 | 0.62 ± 0.06 | 0.74 ± 0.07 | 1.0 ± 0.1 |
| 4 | 31.54 ± 0.95 | 168.27 ± 5.05 | 37.96 ± 0.93 | 250.0 ± 7.5 |
| 5 | 0.11 ± 0.01 | 0.85 ± 0.08 | 0.14 ± 0.01 | 3.0 ± 0.3 |
| 6 | 0.18 ± 0.02 | 2.69 ± 0.26 | 0.28 ± 0.02 | 0.6 ± 0.1 |
| 7 | 0.27 ± 0.03 | 1.17 ± 0.12 | 0.31 ± 0.03 | 0.3 ± 0.1 |
| 8 | 0.50 ± 0.05 | 2.13 ± 0.21 | 0.57 ± 0.05 | 1.0 ± 0.1 |
| 9 | 0.58 ± 0.06 | 2.27 ± 0.22 | 0.65 ± 0.06 | 2.0 ± 0.2 |
| 10 | 3.53 ± 0.11 | 56.01 ± 1.68 | 5.68 ± 0.12 | 171.0 ± 5.1 |
| 11 | 0.14 ± 0.01 | 2.52 ± 0.24 | 0.24 ± 0.02 | 8.0 ± 0.8 |
| 12 | 0.30 ± 0.03 | 0.45 ± 0.05 | 0.31 ± 0.03 | 0.5 ± 0.1 |
| 13 | 0.24 ± 0.02 | 1.06 ± 0.11 | 0.27 ± 0.02 | 0.2 ± 0.1 |
| 14 | 0.29 ± 0.03 | 0.55 ± 0.06 | 0.30 ± 0.03 | 0.4 ± 0.1 |
| 15 | 16,327.11 ± 489.81 | 10,556.36 ± 316.69 | 16,090.93 ± 469.95 | 10,000.0 ± 300.0 |
| 16 | 325.70 ± 9.77 | 11.33 ± 0.34 | 312.84 ± 9.37 | 128.0 ± 3.8 |
| 17 | 8.70 ± 0.87 | 0.39 ± 0.04 | 8.36 ± 0.83 | 1.5 ± 0.2 |
| 18 | 3.80 ± 0.37 | 5.73 ± 0.56 | 3.88 ± 0.36 | 4.0 ± 0.4 |
| 19 | 6.04 ± 0.60 | 0.73 ± 0.07 | 5.82 ± 0.58 | 0.2 ± 0.1 |
| Hole | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole-Weighted Average (Bq/cm2) | Effective Dose Rate (μSv/h) |
|---|---|---|---|---|
| A | 0.01 ± 0.005 | 0.04 ± 0.01 | 0.011 ± 0.001 | 2.4 ± 0.2 |
| B | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.032 ± 0.003 | 0.4 ± 0.1 |
| C | <MDA | 0.55 ± 0.06 | 0.026 ± 0.003 | 4.6 ± 0.5 |
| D | 0.09 ± 0.01 | 0.26 ± 0.03 | 0.098 ± 0.009 | 0.5 ± 0.1 |
| E | 0.14 ± 0.01 | 0.30 ± 0.03 | 0.147 ± 0.004 | 130.0 ± 3.9 |
| F | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.032 ± 0.003 | 2.7 ± 0.3 |
| G | 0.05 ± 0.01 | 1.15 ± 0.12 | 0.095 ± 0.007 | 2.2 ± 0.2 |
| H | 0.04 ± 0.01 | 0.15 ± 0.02 | 0.045 ± 0.003 | 22.4 ± 1.9 |
| I | 0.01 ± 0.005 | 0.31 ± 0.03 | 0.022 ± 0.002 | 0.4 ± 0.1 |
| L | 0.04 ± 0.01 | 0.45 ± 0.05 | 0.057 ± 0.001 | 600.0 ± 18.0 |
| M | 0.02 ± 0.01 | 0.21 ± 0.02 | 0.028 ± 0.002 | 8.6 ± 0.8 |
| N | 0.02 ± 0.01 | 0.23 ± 0.02 | 0.029 ± 0.002 | 0.7 ± 0.1 |
| O | 0.01 ± 0.005 | 0.11 ± 0.015 | 0.014 ± 0.001 | 0.7 ± 0.1 |
| P | 0.01 ± 0.005 | 0.18 ± 0.02 | 0.017 ± 0.001 | 0.7 ± 0.1 |
| Q | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.061 ± 0.005 | 25.0 ± 2.1 |
| R | <MDA | 0.20 ± 0.02 | 0.0008 ± 0.0001 | 81.0 ± 3.5 |
| S | <MDA | 0.30 ± 0.03 | 0.0010 ± 0.0005 | 0.7 ± 0.1 |
| T | <MDA | 0.42 ± 0.04 | 0.017 ± 0.002 | 0.3 ± 0.1 |
| U | <MDA | 0.15 ± 0.02 | 0.006 ± 0.001 | 0.3 ± 0.1 |
| 1 | 0.02 ± 0.01 | 0.22 ± 0.02 | 0.029 ± 0.002 | 21.0 ± 1.8 |
| 2 | 0.02 ± 0.01 | 0.28 ± 0.03 | 0.032 ± 0.002 | 24.0 ± 2.0 |
| 3 | 0.07 ± 0.01 | 0.11 ± 0.015 | 0.072 ± 0.007 | 1.4 ± 0.1 |
| 4 | 0.34 ± 0.03 | 0.34 ± 0.03 | 0.340 ± 0.010 | 110.0 ± 3.3 |
| 5 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.022 ± 0.002 | 2.6 ± 0.3 |
| 6 | 0.01 ± 0.005 | 0.41 ± 0.04 | 0.026 ± 0.002 | 0.7 ± 0.1 |
| 7 | 0.04 ± 0.01 | 0.18 ± 0.02 | 0.046 ± 0.004 | 0.4 ± 0.1 |
| 8 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.039 ± 0.004 | 0.9 ± 0.1 |
| 9 | 0.07 ± 0.01 | 0.03 ± 0.01 | 0.068 ± 0.007 | 3.0 ± 0.3 |
| 10 | 0.06 ± 0.01 | 2.10 ± 0.21 | 0.143 ± 0.003 | 170.0 ± 5.1 |
| 11 | 0.05 ± 0.01 | 0.50 ± 0.05 | 0.068 ± 0.005 | 10.0 ± 0.9 |
| 12 | 0.06 ± 0.01 | 0.43 ± 0.04 | 0.075 ± 0.006 | 1.2 ± 0.1 |
| 13 | 0.05 ± 0.01 | 1.22 ± 0.12 | 0.098 ± 0.007 | 0.4 ± 0.1 |
| 14 | 0.04 ± 0.01 | 0.40 ± 0.04 | 0.055 ± 0.004 | 0.6 ± 0.1 |
| 15 | 12.20 ± 1.22 | 125.00 ± 12.51 | 16.815 ± 0.383 | 1100.0 ± 33.0 |
| 16 | 0.78 ± 0.08 | 2.67 ± 0.27 | 0.857 ± 0.023 | 130.0 ± 3.9 |
| 17 | 0.03 ±0.01 | 0.37 ± 0.04 | 0.044 ± 0.003 | 1.7 ± 0.2 |
| 18 | 0.01 ± 0.005 | 0.04 ± 0.01 | 0.011 ± 0.001 | 2.8 ± 0.3 |
| 19 | <MDA | 0.16 ± 0.02 | 0.007 ± 0.001 | 0.4 ± 0.1 |
| Hole U–Waste Water | 137Cs (Bq/L) |
|---|---|
| First Wash | 657 ± 61 |
| Second Wash | 241 ± 23 |
| Hole U–Surface Contamination | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole–Weighted Average (Bq/cm2) |
|---|---|---|---|
| Initial Value | 1.08 ± 0.11 | 0.16 ± 0.02 | 1.04 ± 0.11 |
| After Decontaminating | <MDA | 0.50 ± 0.05 | 0.02 ± 0.01 |
| Hole R–Surface Contamination | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole–Weighted Average (Bq/cm2) |
|---|---|---|---|
| Initial Value | 81.10 ± 2.43 | 110.00 ± 3.30 | 82.28 ± 2.34 |
| After Decontaminating | 26.30 ± 0.79 | 157.90 ± 4.74 | 31.68 ± 0.95 |
| Hole U–Surface Contamination | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole–Weighted Average (Bq/cm2) |
|---|---|---|---|
| Initial Value | 1.08 ± 0.11 | 0.16 ± 0.02 | 1.04 ± 0.11 |
| After Decontaminating | <MDA | 0.50 ± 0.05 | 0.02 ± 0.01 |
| After Painting | <MDA | 0.15 ± 0.02 | 0.006 ± 0.001 |
| Hole R–Surface Contamination | Walls (Bq/cm2) | Bottom (Bq/cm2) | Hole–Weighted Average (Bq/cm2) |
|---|---|---|---|
| Initial Value | 81.10 ± 2.43 | 110.00 ± 3.30 | 82.28 ± 2.34 |
| After Decontaminating | 26.30 ± 0.79 | 157.90 ± 4.74 | 31.68 ± 0.95 |
| After Painting | <MDA | 0.20 ± 0.02 | 0.0008 ± 0.0001 |
| Drum | Cin (Bq/L) | Cout (Bq/L) | DF (-) | DE% (-) |
|---|---|---|---|---|
| 1 | 5430 ± 199 | 7.50 ± 0.75 | 724.00 ± 77.13 | 99.862% |
| 2 | 34,600 ± 1038 | 2.00 ± 0.20 | 17,300.00 ± 1806.17 | 99.994% |
| 3 | 68,600 ± 2058 | 60.40 ± 6.04 | 1135.76 ± 118.57 | 99.912% |
| 4 | 11,900 ± 357 | 69.70 ± 6.96 | 170.73 ± 17.81 | 99.414% |
| 5 | 43,300 ± 1299 | 78.80 ± 7.86 | 549.49 ± 57.25 | 99.818% |
| 6 | 6540 ± 196 | 80.70 ± 8.05 | 81.04 ± 8.44 | 98.766% |
| 7 | 84,700 ± 2541 | 24.90 ± 2.49 | 3401.61 ± 355.14 | 99.971% |
| 8 | 197,011 ± 5910 | 45.20 ± 4.52 | 4358.66 ± 455.06 | 99.977% |
| 9 | 106,000 ± 3180 | 15.20 ± 1.52 | 6973.68 ± 728.07 | 99.986% |
| 10 | 279,000 ± 8370 | 295.00 ± 28.68 | 945.76 ± 96.23 | 99.894% |
| 11 | 70,200 ± 2106 | 17.00 ± 1.70 | 4129.41 ± 431.12 | 99.976% |
| 12 | 171,000 ± 5130 | 21.00 ± 2.10 | 8142.86 ± 850.14 | 99.988% |
| 13 | 6210 ± 186 | 9.60 ± 0.96 | 646.88 ± 67.54 | 99.845% |
| 14 | 32,300 ± 969 | 16.20 ± 1.62 | 1993.83 ± 208.16 | 99.950% |
| 15 | 5520 ± 197 | 20.70 ± 2.07 | 266.67 ± 28.31 | 99.625% |
| 16 | 33,900 ± 1017 | 8.40 ± 0.84 | 4035.71 ± 421.34 | 99.975% |
| 17 | 160,047 ± 4801 | 17.00 ± 1.70 | 9414.53 ± 982.91 | 99.989% |
| 18 | 81,300 ± 2439 | 37.80 ± 3.78 | 2150.79 ± 224.55 | 99.954% |
| 19 | 7750 ± 233 | 16.60 ± 1.66 | 466.87 ± 48.74 | 99.786% |
| 20 | 4430 ± 215 | 69.90 ± 6.98 | 63.38 ± 7.04 | 98.422% |
| Mixture | pH (-) | Range of Concentration (v/v,%) | Operating Temperature (°C) |
|---|---|---|---|
| Carboxilide Acid and Zero Pi Universal Degreaser | 2.4 | 6–100 | 20–115 |
| Drum | Cin (Bq/L) | V(l) | A (Bq) |
|---|---|---|---|
| 1 | 5430 ± 199 | 104 | 564,720 ± 20,696 |
| 2 | 34,600 ± 1038 | 91 | 3,148,600 ± 94,458 |
| 3 | 68,600 ± 2058 | 97 | 6,654,200 ± 199,626 |
| 4 | 11,900 ± 357 | 115 | 1,368,500 ± 41,055 |
| 5 | 43,300 ± 1299 | 85 | 3,680,500 ± 110,415 |
| 6 | 6540 ± 196 | 120 | 784,800 ± 23,520 |
| 7 | 84,700 ± 2541 | 65 | 5,505,500 ± 165,165 |
| 8 | 197,011 ± 5910 | 109 | 21,473,000 ± 644,190 |
| 9 | 106,000 ± 3180 | 105 | 11,130,000 ± 333,900 |
| 10 | 279,000 ± 8370 | 35 | 4,865,000 ± 292,950 |
| 11 | 70,200 ± 2106 | 95 | 6,669,000 ± 200,070 |
| 12 | 171,000 ± 5130 | 115 | 19,665,000 ± 589 950 |
| 13 | 6210 ± 186 | 85 | 527,850 ± 15,810 |
| 14 | 32,300 ± 969 | 97 | 3,133,100 ± 93,993 |
| 15 | 5520 ± 197 | 104 | 574,080 ± 20,488 |
| 16 | 33,900 ± 1017 | 104 | 3,525,600 ± 105,768 |
| 17 | 160,047 ± 4801 | 90 | 14,400,000 ± 432,090 |
| 18 | 81,300 ± 2439 | 140 | 11,382,000 ± 341,460 |
| 19 | 7750 ± 233 | 90 | 697,500 ± 20,970 |
| 20 | 4430 ± 215 | 90 | 398,700 ± 19,350 |
| TOTAL | - | 1936 | 120,147,650 ± 1,189,988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonti, A.; Marin, A.; Rizzo, F.; Massaro, F.; Masiero, M.; Panizzolo, P.; Lesca, C.; Pratolongo, A.; Manzone, P.; Giannetti, F. Decontamination and Remediation of Underground Holes and Testing of Cleaning Techniques Based on the Use of Liquid Cold Decontaminant. Sustainability 2022, 14, 10565. https://doi.org/10.3390/su141710565
Tonti A, Marin A, Rizzo F, Massaro F, Masiero M, Panizzolo P, Lesca C, Pratolongo A, Manzone P, Giannetti F. Decontamination and Remediation of Underground Holes and Testing of Cleaning Techniques Based on the Use of Liquid Cold Decontaminant. Sustainability. 2022; 14(17):10565. https://doi.org/10.3390/su141710565
Chicago/Turabian StyleTonti, Andrea, Adriano Marin, Francesco Rizzo, Francesco Massaro, Mattia Masiero, Paolo Panizzolo, Claudio Lesca, Alessia Pratolongo, Paolo Manzone, and Fabio Giannetti. 2022. "Decontamination and Remediation of Underground Holes and Testing of Cleaning Techniques Based on the Use of Liquid Cold Decontaminant" Sustainability 14, no. 17: 10565. https://doi.org/10.3390/su141710565
APA StyleTonti, A., Marin, A., Rizzo, F., Massaro, F., Masiero, M., Panizzolo, P., Lesca, C., Pratolongo, A., Manzone, P., & Giannetti, F. (2022). Decontamination and Remediation of Underground Holes and Testing of Cleaning Techniques Based on the Use of Liquid Cold Decontaminant. Sustainability, 14(17), 10565. https://doi.org/10.3390/su141710565







