Response of Antioxidant Enzyme Activities of the Green Microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Microalgal Species
2.2. Experimental Setup
2.3. Extraction and Detection of MDA, SOD, CAT and POD
2.4. Statistical Analyses
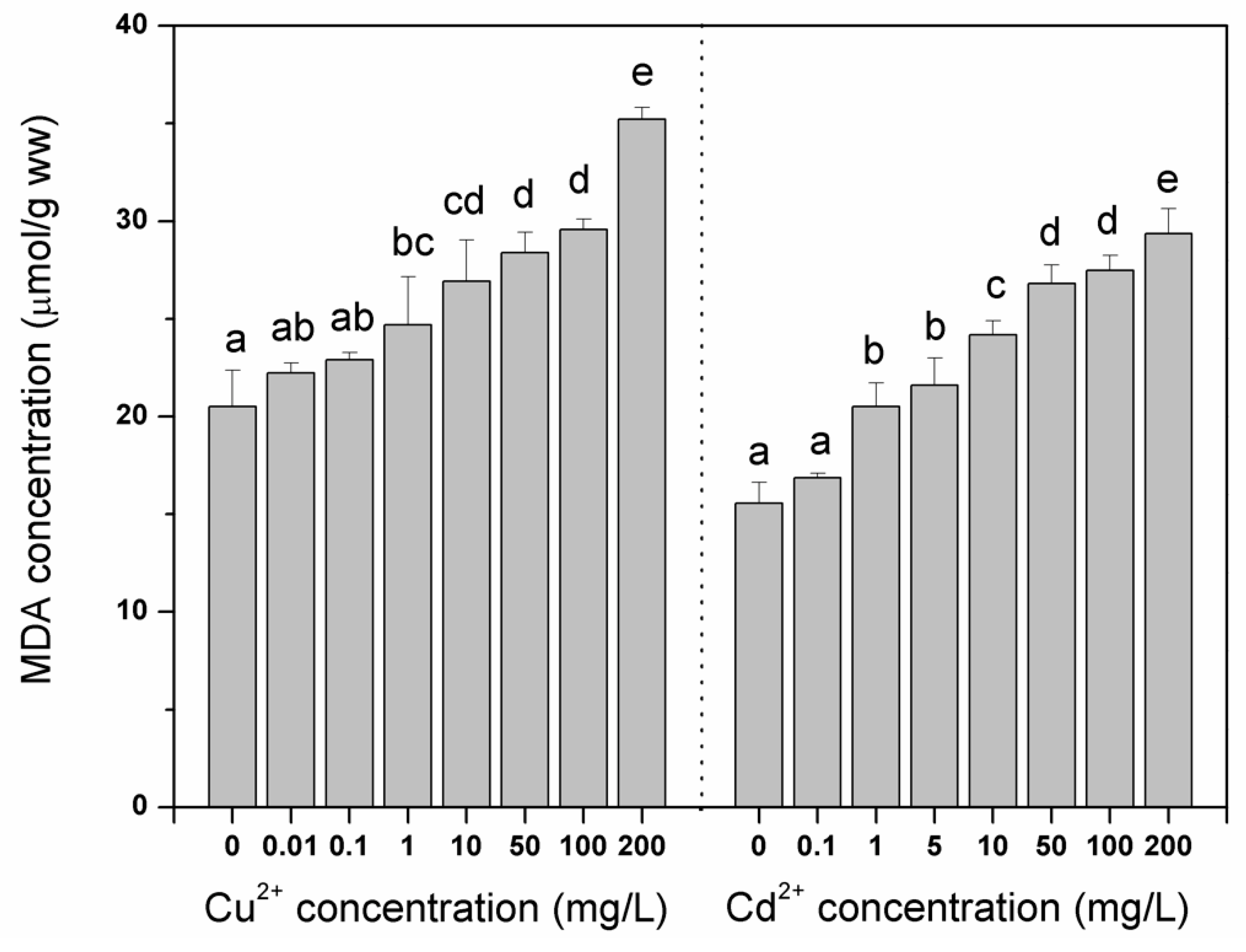
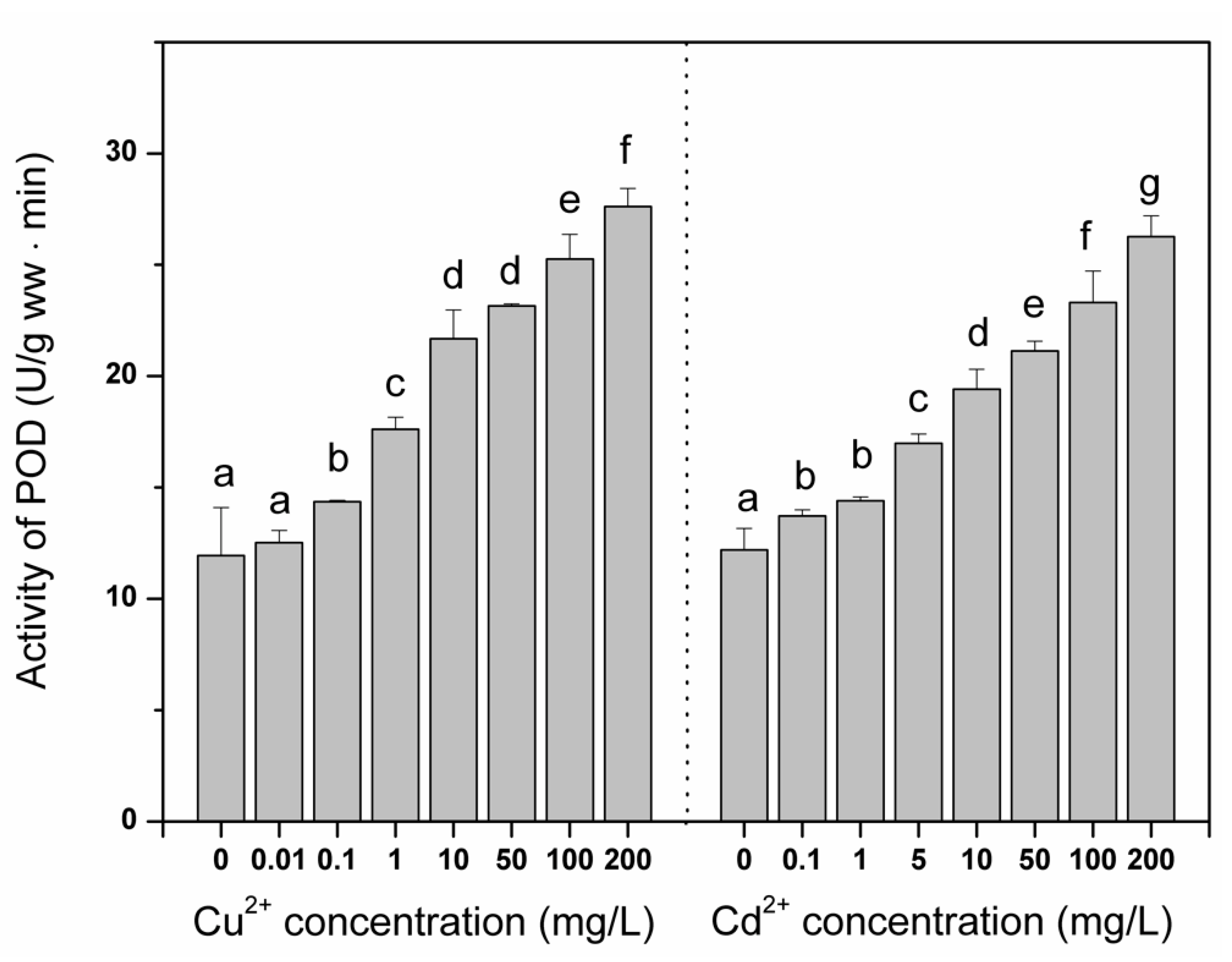
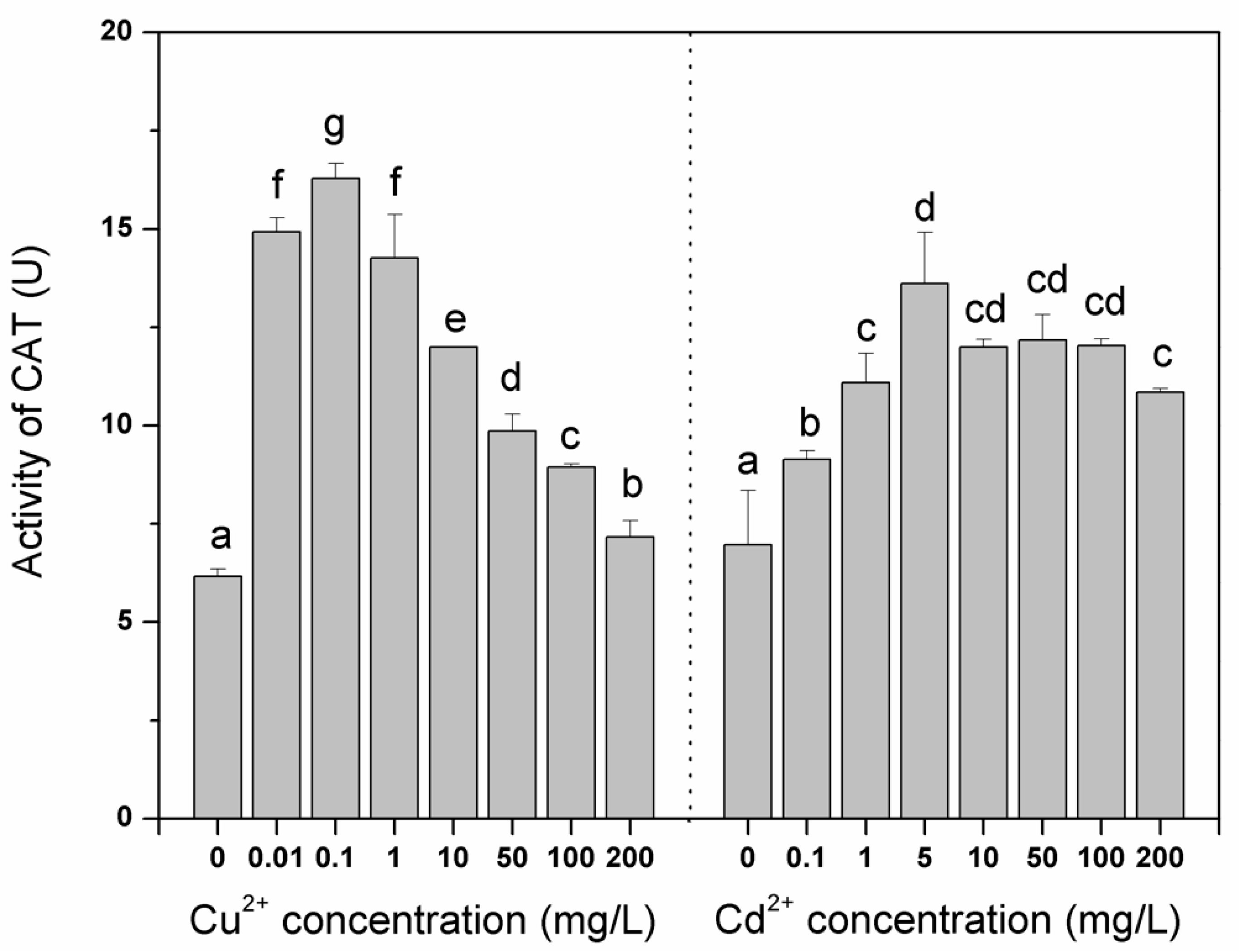
3. Results and Discussion
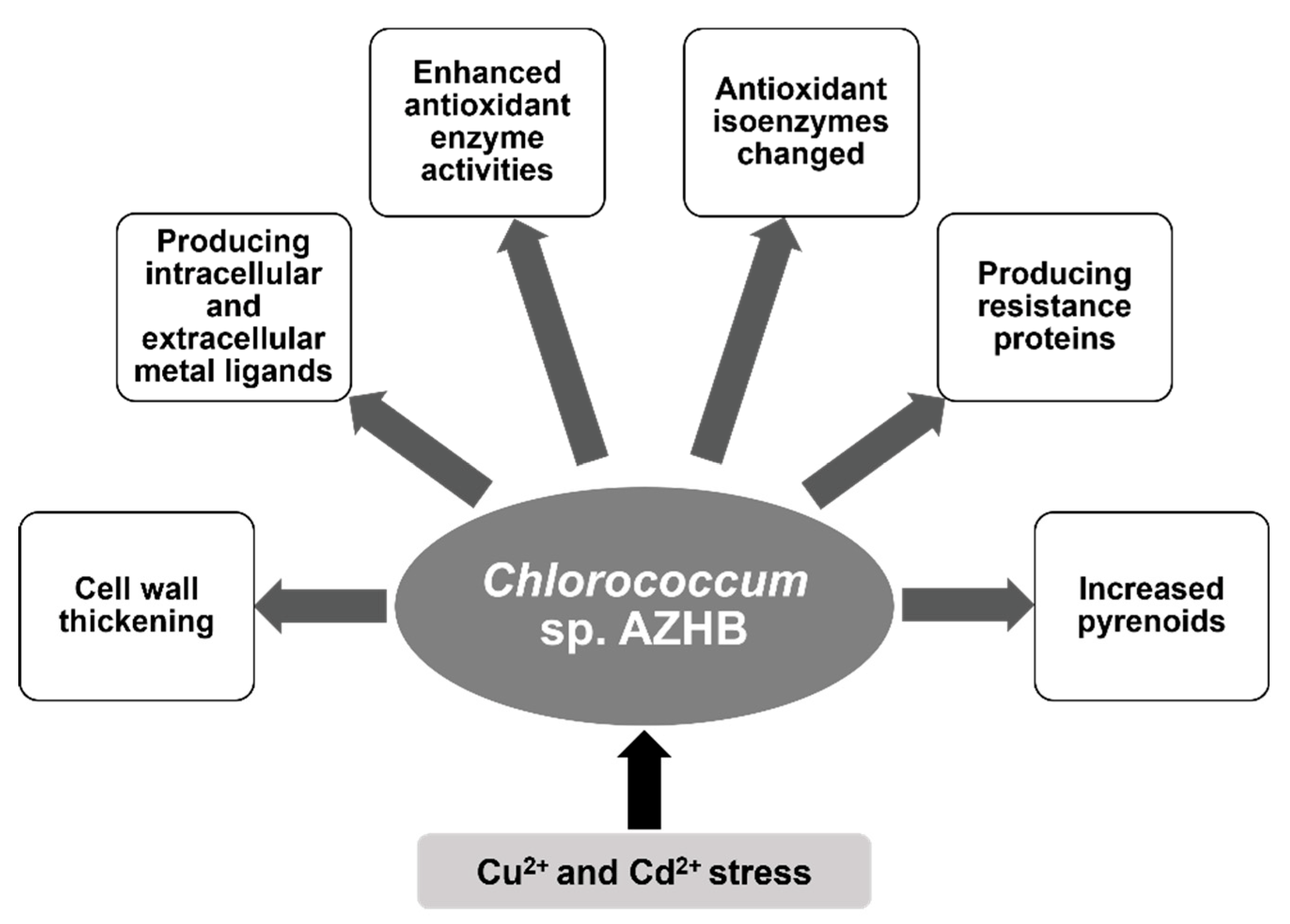
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Nafees, M.; Ge, L.; Khan, M.H.; Bilal, M.; Chan, W.P.; Lisak, G. Assessment of industrial wastewater for potentially toxic elements, human health (dermal) risks, and pollution sources: A case study of Gadoon Amazai industrial estate, Swabi, Pakistan. J. Hazard Mater. 2021, 419, 126450. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. CheBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotox. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Zhou, G.J.; Peng, F.Q.; Zhang, L.J.; Ying, G.G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2012, 19, 2918–2929. [Google Scholar] [CrossRef]
- Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Nirmalakhandan, N. Algal pathway for nutrient recovery from urban sewage. Algal Res. 2020, 51, 102023. [Google Scholar] [CrossRef]
- Zhou, G.J.; Ying, G.G.; Liu, S.; Zhou, L.J.; Chen, Z.F.; Peng, F.Q. Simultaneous removal of inorganic and organic compounds in wastewater by freshwater green microalgae. Environ. Sci. Process. Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B.M. Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Xia, L.; Yin, H.; Meza, J.V.G.; Ju, W. Enhanced Pb(II) removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem. Eng. J. 2019, 361, 167–179. [Google Scholar] [CrossRef]
- Qiu, C.E.; Kuang, Q.J.; Bi, Y.H.; Liu, G.X.; Hu, Z.Y. Response of Chlorococcum sp. AZHB to copper and cadmium stress. Bull. Environ. Contam. Toxicol. 2006, 77, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotox. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef]
- Aksmann, A.; Pokora, W.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Wielgomas, B.; Dziadziuszko, M.; Tukaj, Z. Time-dependent changes in antioxidative enzyme expression and photosynthetic activity of Chlamydomonas reinhardtii cells under acute exposure to cadmium and anthracene. Ecotox. Environ. Saf. 2014, 110, 31–40. [Google Scholar] [CrossRef]
- Ken, C.F.; Hsiung, T.M.; Huang, Z.X.; Juang, R.H.; Lin, C.T. Characterization of the Fe/Mn-superoxide dismutase from diatom (Thallassiosira weissflogii): Cloning, expression, and property. J. Agric. Food Chem. 2005, 53, 1470–1474. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Amar, A.; Gaur, J.P. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef]
- Lozano, P.; Trombini, C.; Crespo, E.; Blasco, J.; Moreno-Garrido, I. ROI-scavenging enzyme activities as toxicity biomarkers in three species of marine microalgae exposed to model contaminants (copper, Irgarol and atrazine). Ecotox. Environ. Saf. 2014, 104, 294–301. [Google Scholar] [CrossRef]
- Tang, Z.C. The Experimental Guide of Modern Plant Physiology; Academic Press: Beijing, China, 1999; pp. 95, 305, 314. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; pp. 164–165. [Google Scholar]
- Yan, H.; Pan, G. Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere 2002, 49, 471–476. [Google Scholar] [CrossRef]
- Travieso, L.; Cañizares, R.O.; Borja, R.; Benítez, F.; Domínguez, A.R.; Dupeyrón, R.; Valiente, V. Heavy metal removal by microalgae. Bull. Environ. Contam. Toxicol. 1999, 62, 144–151. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. SpringerPlus 2016, 5, 1290. [Google Scholar] [CrossRef]
- Chen, S.Y. Injury of membrane lipid peroxidation to plant cell. Plant Physiol. Commun. 1999, 27, 84–90. [Google Scholar] [CrossRef]
- Soto, P.; Gaete, H.; Hidalgo, M.E. Assessment of catalase activity, lipid peroxidation, chlorophyll-a, and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat. Am. J. Aquat. Res. 2011, 39, 280–285. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef]
- Zutshi, S.; Choudhary, M.; Bharat, N.; Abdin, M.Z.; Fatma, T. Evaluation of antioxidant defense response to lead stress in Hapalosiphon fontinalis-339. J. Phycol. 2008, 44, 889–896. [Google Scholar] [CrossRef]
- Sáez, C.A.; Roncarati, F.; Moenne, A.; Moody, A.J.; Brown, M.T. Copper-induced intra-specific oxidative damage and antioxidant responses in strains of the brown alga Ectocarpus siliculosus with different pollution histories. Aquat. Toxicol. 2015, 159, 81–89. [Google Scholar] [CrossRef]
- Hörnström, E. Toxicity test with algae—A discussion on the batch method. Ecotox. Environ. Saf. 1990, 20, 343–353. [Google Scholar] [CrossRef]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Lee, M.Y.; Shin, H.W. Cadmium-induced changes in antioxidant enzymes from the marine alga Nannochloropsis oculata. J. Appl. Phycol. 2003, 15, 13–19. [Google Scholar] [CrossRef]
- Dai, L.; Gao, H.; Xia, J. Tolerance to heavy metal Cd and detoxification of Synechococcus cedrorum. Chin. J. Appl. Environ. Biol. 1998, 4, 192–195. [Google Scholar] [CrossRef]
- Pistocchi, R.; Mormile, A.M.; Guerrini, F.; Isani, G.; Boni, L. Increased production of extra-and intracellular metal-ligands in phytoplankton exposed to copper and cadmium. J. Appl. Phycol. 2000, 12, 469–477. [Google Scholar] [CrossRef]

| Cu2+ Concentrations | MDA | SOD | POD | CAT | |
|---|---|---|---|---|---|
| Cu2+ concentrations | 1.000 | 0.923 ** | 0.780 * | 0.821 * | −0.576 |
| MDA | 1.000 | 0.949 ** | 0.962 ** | −0.431 | |
| SOD | 1.000 | 0.992 ** | −0.396 | ||
| POD | 1.000 | −0.436 | |||
| CAT | 1.000 |
| Cd2+ Concentrations | MDA | SOD | POD | CAT | |
|---|---|---|---|---|---|
| Cd2+ concentrations | 1.000 | 0.789 * | 0.763 * | 0.879 ** | 0.154 |
| MDA | 1.000 | 0.960 ** | 0.974 ** | 0.640 | |
| SOD | 1.000 | 0.950 ** | 0.652 | ||
| POD | 1.000 | 0.523 | |||
| CAT | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Wang, W.; Zhang, Y.; Zhou, G.-J.; Bi, Y. Response of Antioxidant Enzyme Activities of the Green Microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ Stress. Sustainability 2022, 14, 10320. https://doi.org/10.3390/su141610320
Qiu C, Wang W, Zhang Y, Zhou G-J, Bi Y. Response of Antioxidant Enzyme Activities of the Green Microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ Stress. Sustainability. 2022; 14(16):10320. https://doi.org/10.3390/su141610320
Chicago/Turabian StyleQiu, Changen, Weidong Wang, Yuheng Zhang, Guang-Jie Zhou, and Yonghong Bi. 2022. "Response of Antioxidant Enzyme Activities of the Green Microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ Stress" Sustainability 14, no. 16: 10320. https://doi.org/10.3390/su141610320
APA StyleQiu, C., Wang, W., Zhang, Y., Zhou, G.-J., & Bi, Y. (2022). Response of Antioxidant Enzyme Activities of the Green Microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ Stress. Sustainability, 14(16), 10320. https://doi.org/10.3390/su141610320







