Research on the Removal of Sodium from Vanadium Tailings by Calcification Roasting and NaOH Leaching
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Thermodynamic Calculation
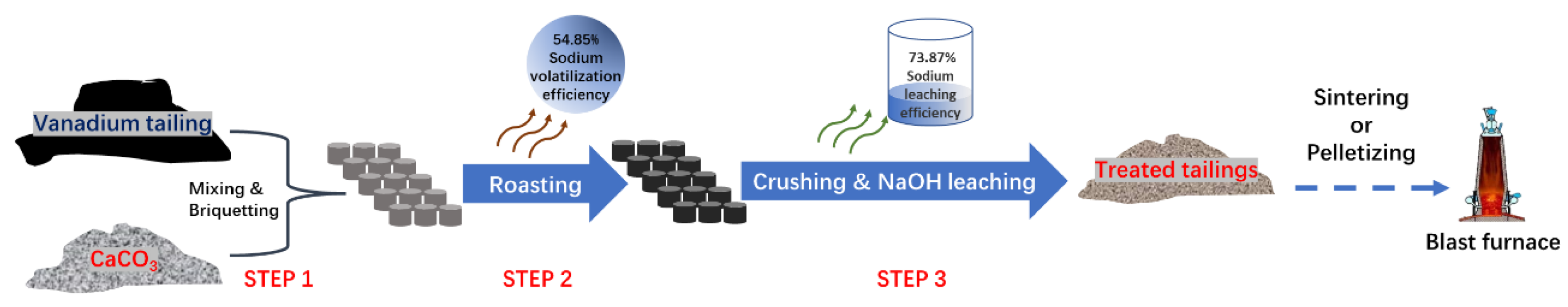
2.3. Experimental Procedure
2.3.1. Calcification Roasting
2.3.2. NaOH Leaching
2.4. Definition of Parameters
2.5. Analysis and Characterization
3. Results and Discussion
3.1. Vanadium Tailings
3.2. Thermodynamic Calculation
3.2.1. Mass ratio of CaCO3 to Tailings
3.2.2. Reaction Temperature
3.3. Calcification Roasting of Vanadium Tailings
3.3.1. Effect of Calcification Roasting on the NaOH Leaching of Sodium in Vanadium Tailings
3.3.2. Volatilization Behavior of Sodium during Calcification Roasting
Mass Ratio of CaCO3
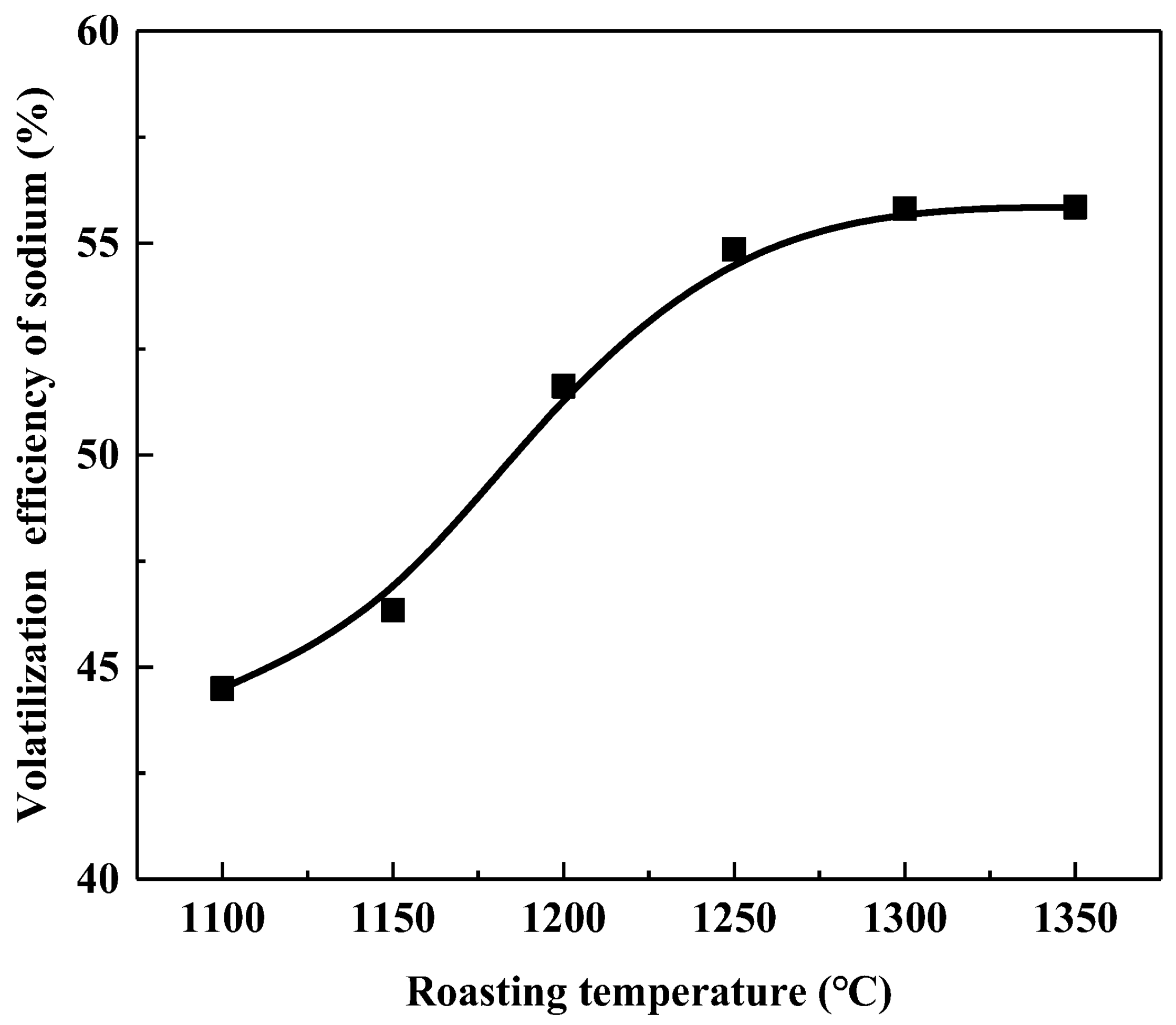
Roasting Temperature
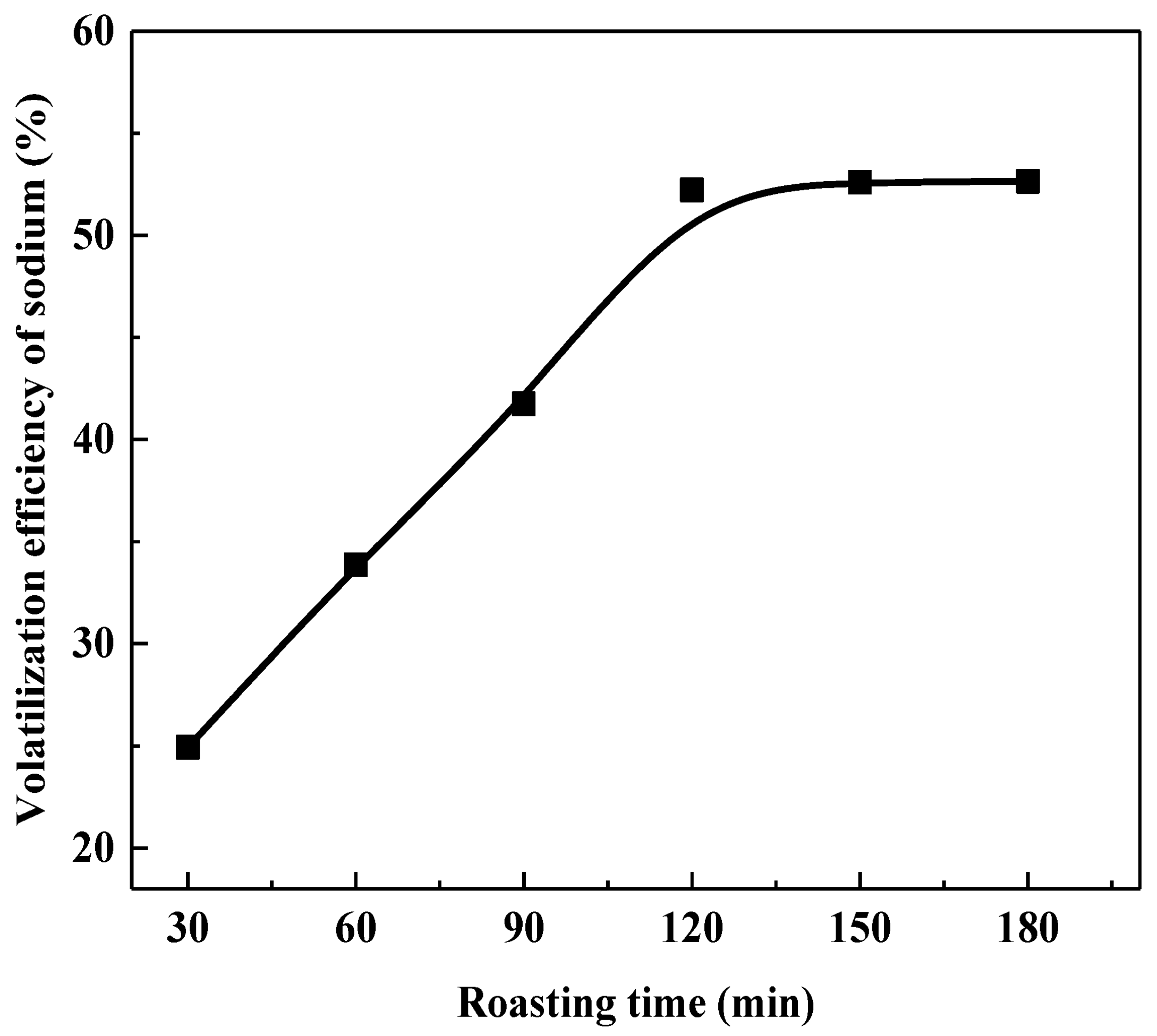
Roasting Time
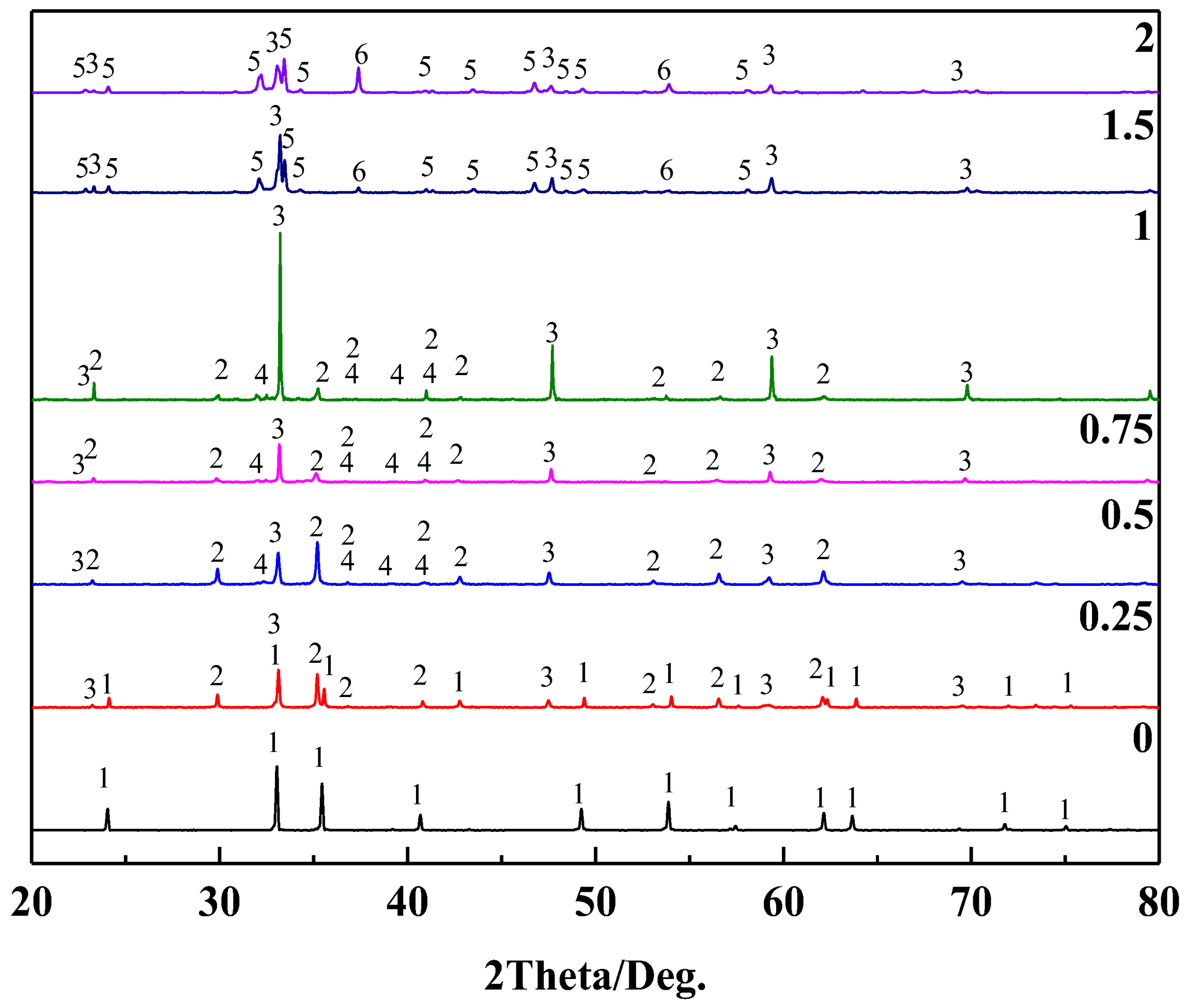
3.3.3. Mineral Phase Transformation of Sodium during Calcification Roasting
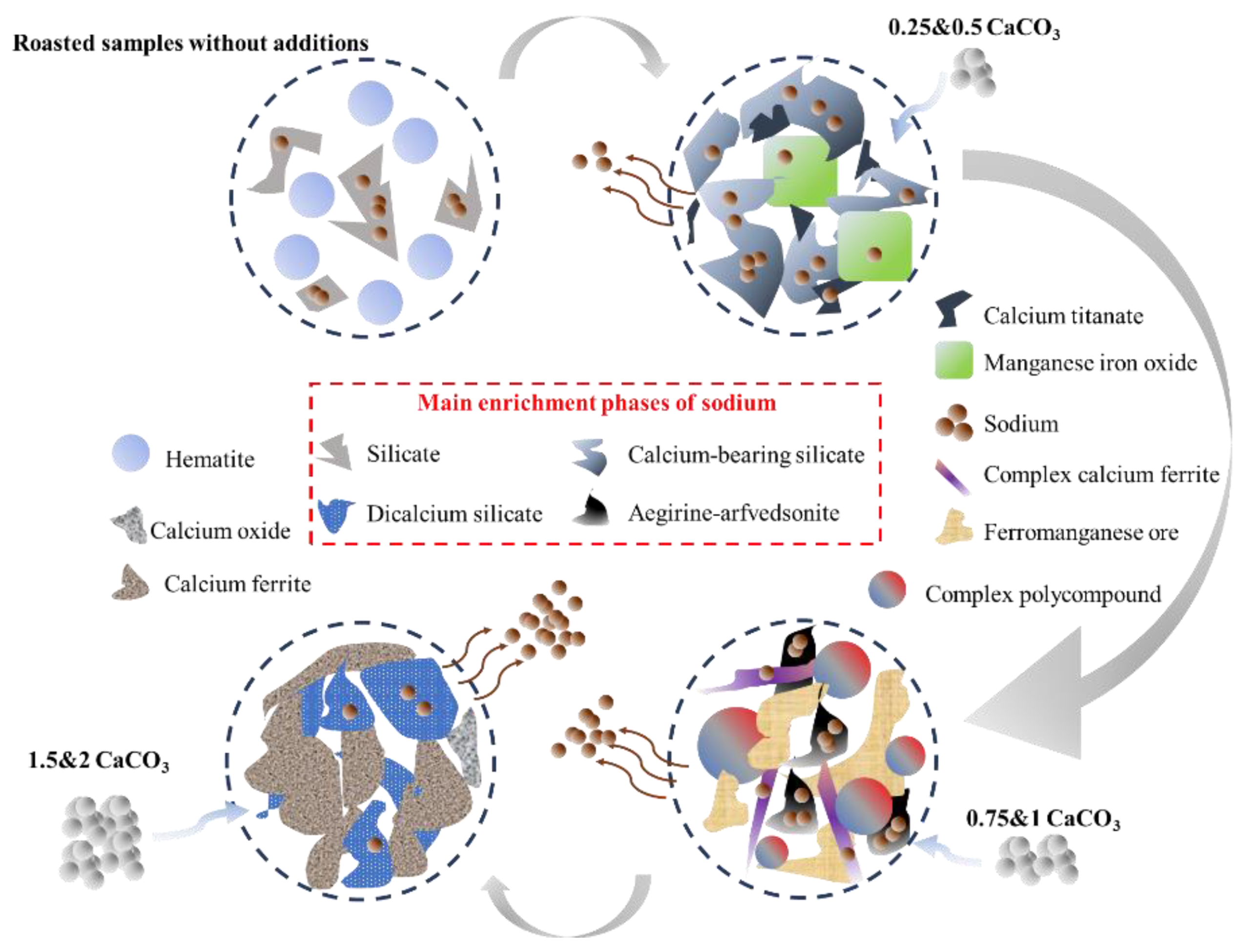
3.3.4. Microstructural Change of Sodium during Calcification Roasting
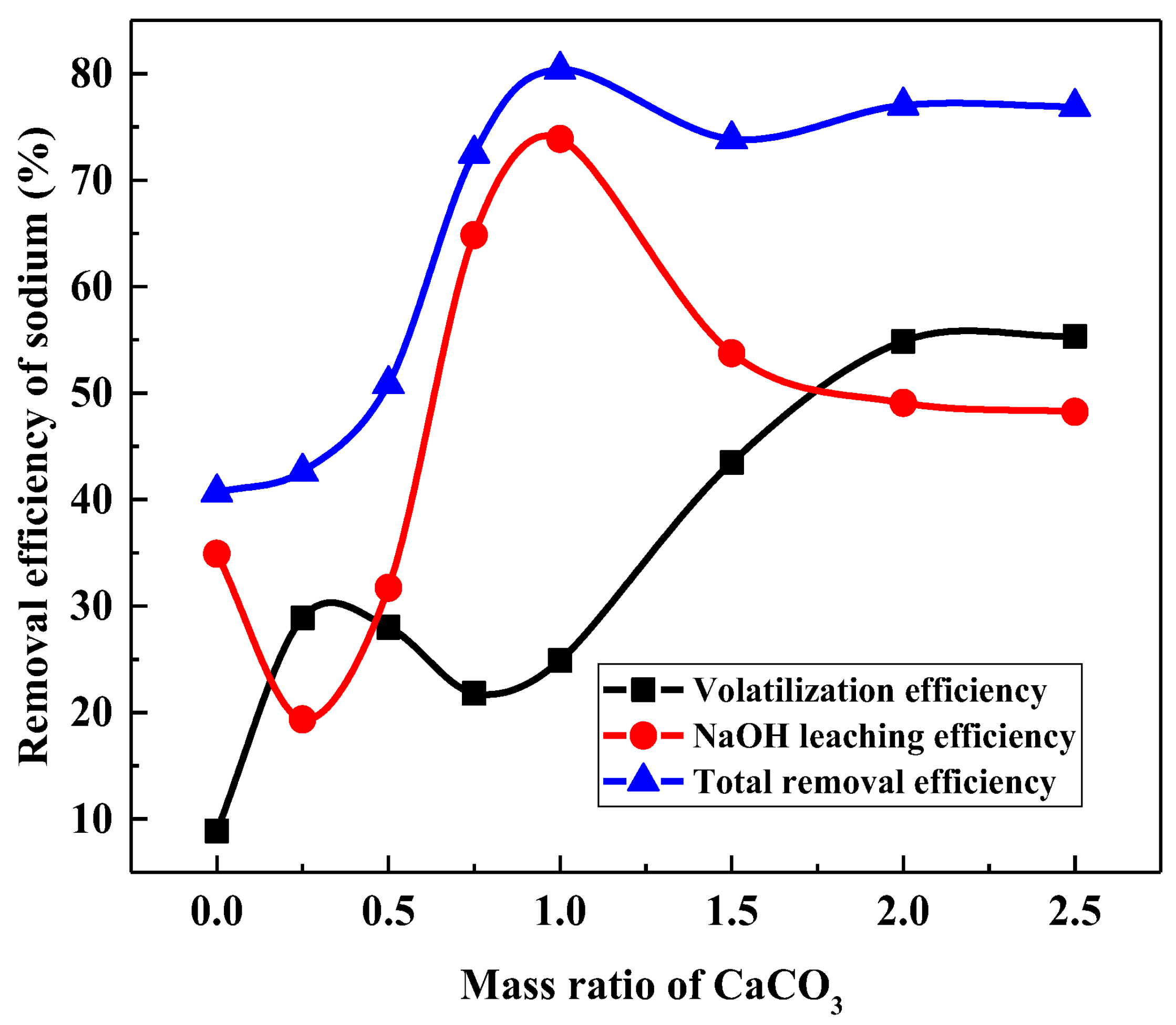
3.4. Optimalization of Total Removal of Sodium in the Whole Process
3.5. Final Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, S.; He, X.; Wang, Y.; Wang, L. Cleaner and effective extraction and separation of iron from vanadium slag by carbothermic reduction-chlorination-molten salt electrolysis. J. Clean. Prod. 2020, 284, 124674. [Google Scholar] [CrossRef]
- Zheng, F.Q.; Liu, X.; Guo, Y.F.; Wang, S.; Chen, F.; Yang, L.Z.; Jiang, T.; Qiu, G.Z. Transformation and separation of metallic iron in reduced ilmenite during corrosion process. J. Iron Steel Res. Int. 2020, 27, 1372–1381. [Google Scholar] [CrossRef]
- Manuel, A.; Nadiia, I.G.; Giuseppe, S.; Eugenio, G.; Craig, C.M.; Annette, R.; Debbie, C.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 45, 214344. [Google Scholar]
- Manuel, A.; Nadiia, I.G.; Giuseppe, S.; Eugenio, G.; Annette, R.; Debbie, C.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar]
- Agnieszka, Ś.; Ewa, W.; Dorota, G. Wild animals in studies on vanadium bioaccumulation—Potential animal models of environmental vanadium contamination: A comprehensive overview with a Polish accent. Sci. Total Environ. 2021, 785, 147205. [Google Scholar]
- Yang, J.; Tang, Y.; Yang, K.; Rouff, A.A.; Elzinga, E.J.; Huang, J. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef]
- Qu, S.; Guo, Y.; Ma, Z.; Chen, W.; Liu, J.; Liu, G.; Wang, Y.; Xu, M. Implications of China’s foreign waste ban on the global circular economy. Resour. Conserv. Recycl. 2019, 144, 252–255. [Google Scholar] [CrossRef]
- Deng, R.; Xiao, H.; Xie, Z.; Liu, Z.; Yu, Q.; Chen, G.; Tao, C. A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag. Chin. J. Chem. Eng. 2020, 28, 2208–2213. [Google Scholar] [CrossRef]
- Wu, G.; Pan, P.; Fan, H.; Wang, R.; Xu, Z. A review on the research status and development trend of vanadium extraction from vanadium slag. Jiangxi Metal. 2020, 40, 19–27. [Google Scholar]
- Xiang, J.Y.; Huang, Q.Y.; Lv, W.; Pei, G.S.; Lv, X.W.; Bai, C.G. Recovery of tailings from the vanadium extraction process by carbothermic reduction method: Thermodynamic, experimental and hazardous potential assessment. J. Hazard. Mater. 2018, 357, 128–137. [Google Scholar] [CrossRef]
- Yang, H.F.; Jing, L.L.; Zhang, B.G. Recovery of iron from vanadium tailings with coal-based direct reduction followed by magnetic separation. J. Hazard. Mater. 2021, 185, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Xiu, D.P.; Wang, Q.C.; Yang, Y.G.; Gu, S.L.; Sun, Q.Z. Techniques of making vanadium and titanium black porcelain and its application in modern industry. Chin. Ceram. 2008, 44, 41–43. [Google Scholar]
- Yang, S.L.; Ma, L.; Liu, J.F.; Lei, Z.J. Titanium, T. Prospect of Researching and Developing Vanadium Functional Materials Using Vanadium Resources in Panxi Area. Iron Steel Vanadium Titan. 2016, 37, 84–91. [Google Scholar]
- Li, L.J.; Chen, D.H.; Bai, R.G.; Zheng, S.L.; Zhang, Y. Leaching of vanadium from vanadium-containing residue by NaOH sub-molten salt. Chin. J. Process Eng. 2011, 11, 747–754. [Google Scholar]
- Fan, G.; Wei, C.; Ge, W.H.; Li, M.T.; Deng, Z.G.; Li, C.X. Vanadium Recovery from Extracted Vanadium Residue by Atmospheric Pressure Acid Leaching. Nonferrous Met. 2010, 62, 65–68. [Google Scholar]
- Wang, Q.; Jiang, M.F.; Wang, M.; Zhang, J. Leaching and extraction of vanadium and chromium from vanadium tailings. Nonferrous Met. 2016, 9, 13–16. [Google Scholar]
- Mazurek, K. Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy 2013, 134, 26–31. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Z.; Chen, L.; Liu, Y.; Zhang, T. Effect of microwave heating on the pressure leaching of vanadium from converter slag. Hydrometallurgy 2019, 184, 45–54. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Lü, G.; Zhang, Y.; Liu, Y.; Xie, G. Extraction of vanadium from LD converter slag by pressure leaching process with titanium white waste acid. Rare Met. Mater. Eng. 2015, 44, 1894–1898. [Google Scholar]
- Du, W.; Chen, H. Resource utilization of vanadium tailings back into the steel process. North V-Ti-Bear. 2011, 1, 22–24. [Google Scholar]
- Wang, X.; Xiang, J.W.; Ling, J.W.; Huang, Q.Y.; Lv, X.W. Comprehensive utilization of vanadium extraction tailings: A brief review. Miner. Met. Mater. Soc. 2020, 327–334. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, Q.; Zhao, Y.; Jiang, Q.; He, Z. Spheroidization of eutectic crystals in wear-resistant manganese steel. ACTA Metall. Sci. 1995, 31, 518–522. [Google Scholar]
- Luo, G.; Wu, S.; Zhang, G.; Wang, Y. Effects of Compound Silicate Gangue on Formation of Complex Calcium Ferrite During Sintering Process. J. Iron Steel Res. 2013, 3, 21–26. [Google Scholar] [CrossRef]
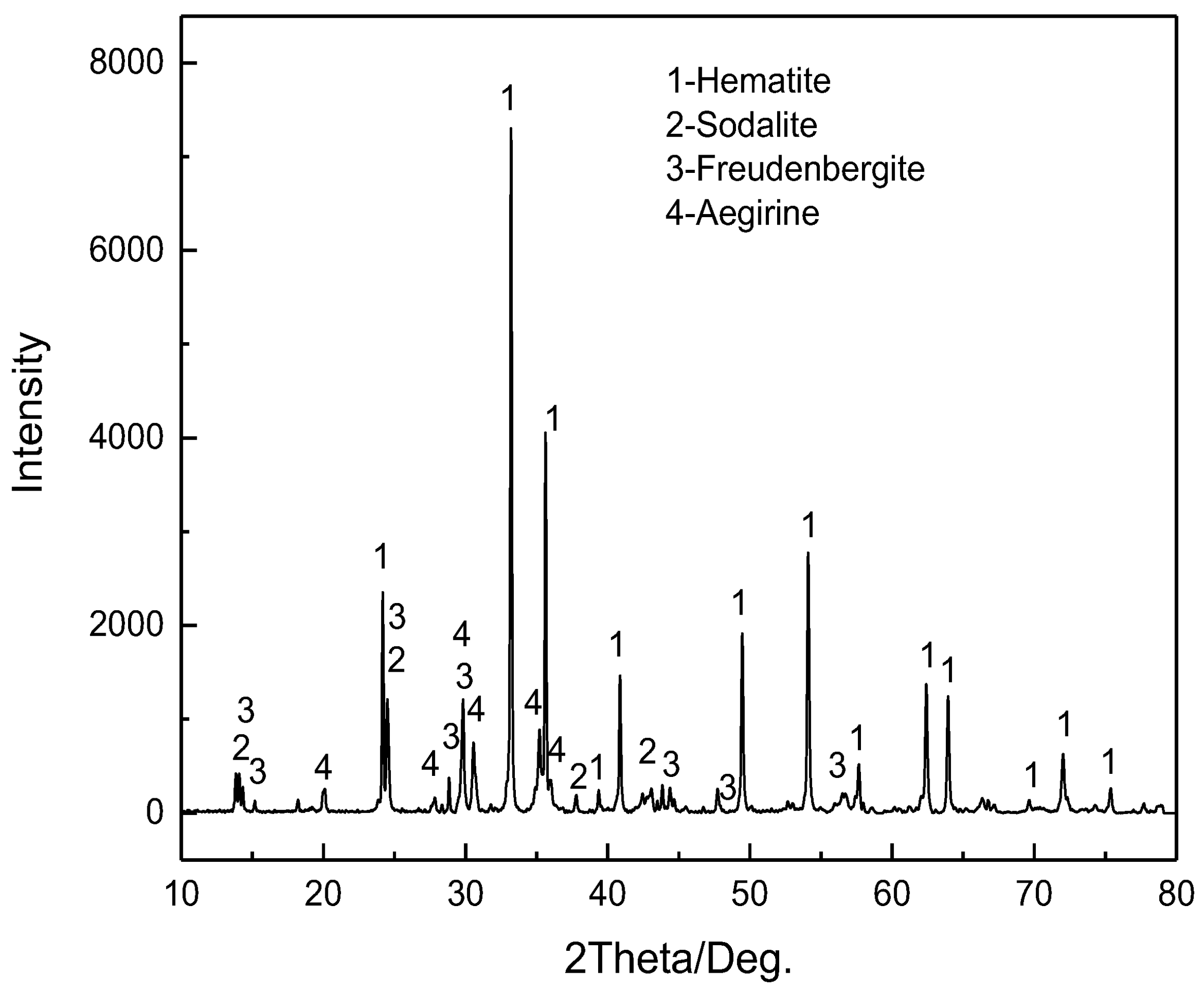
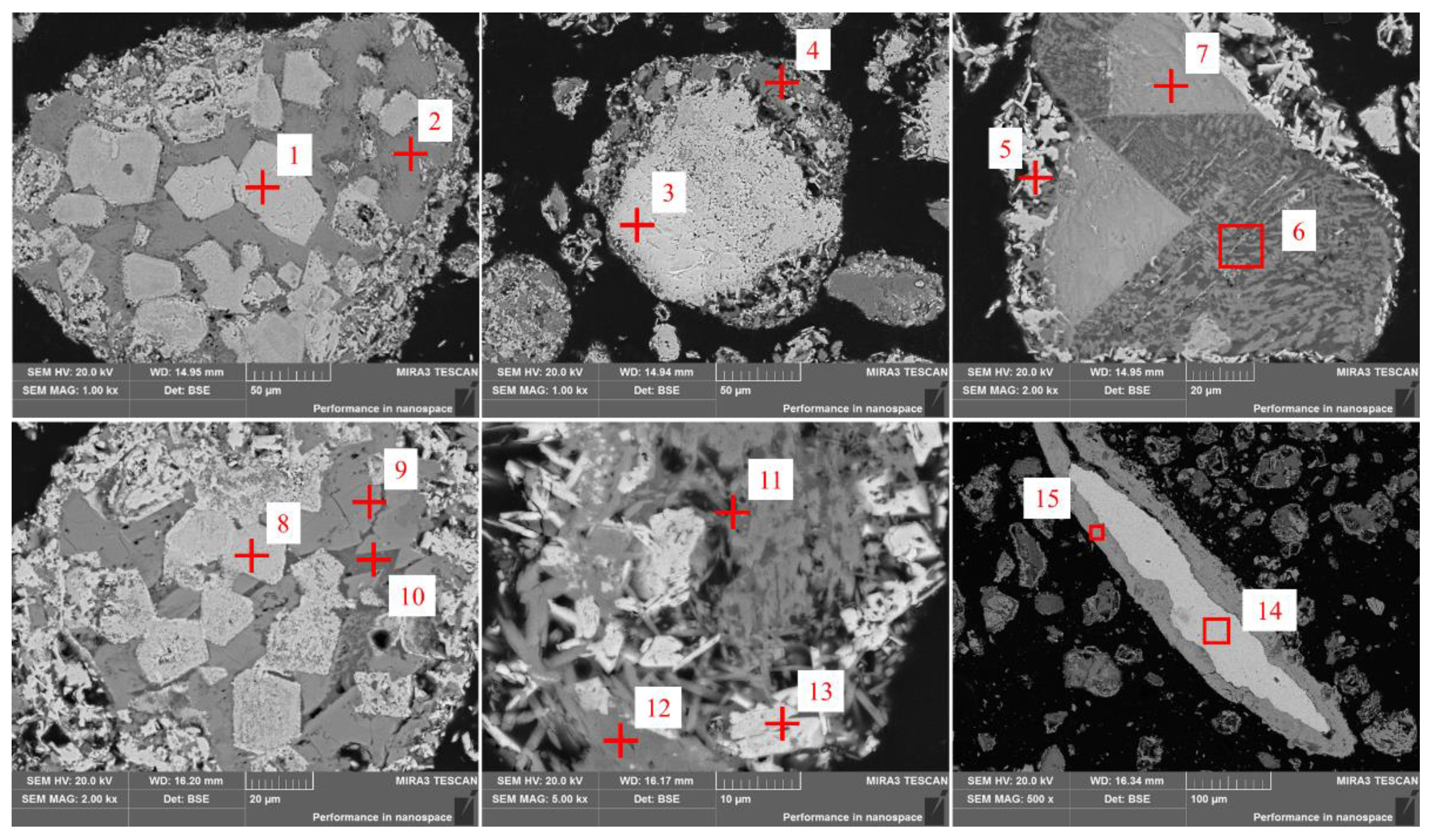
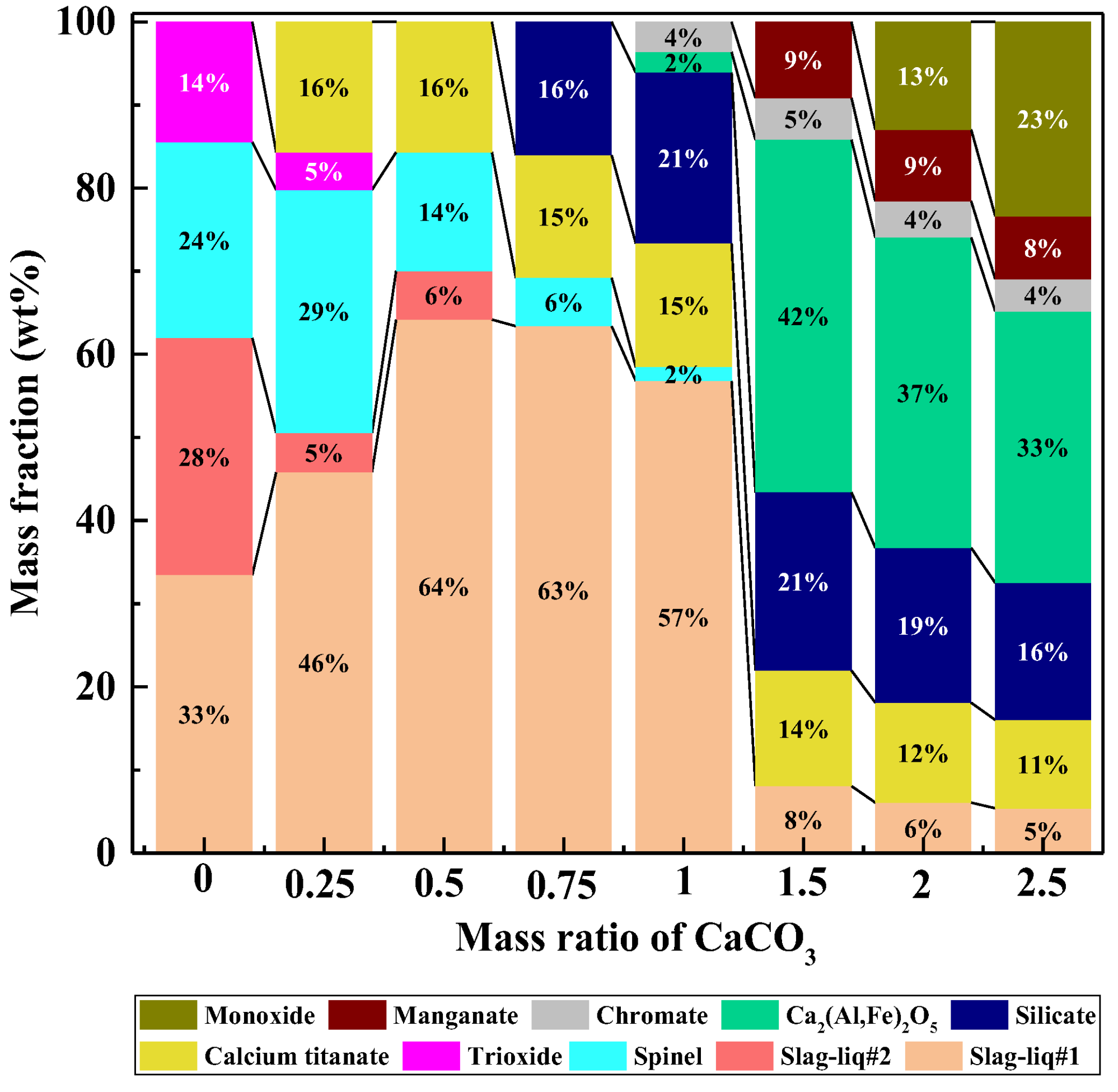


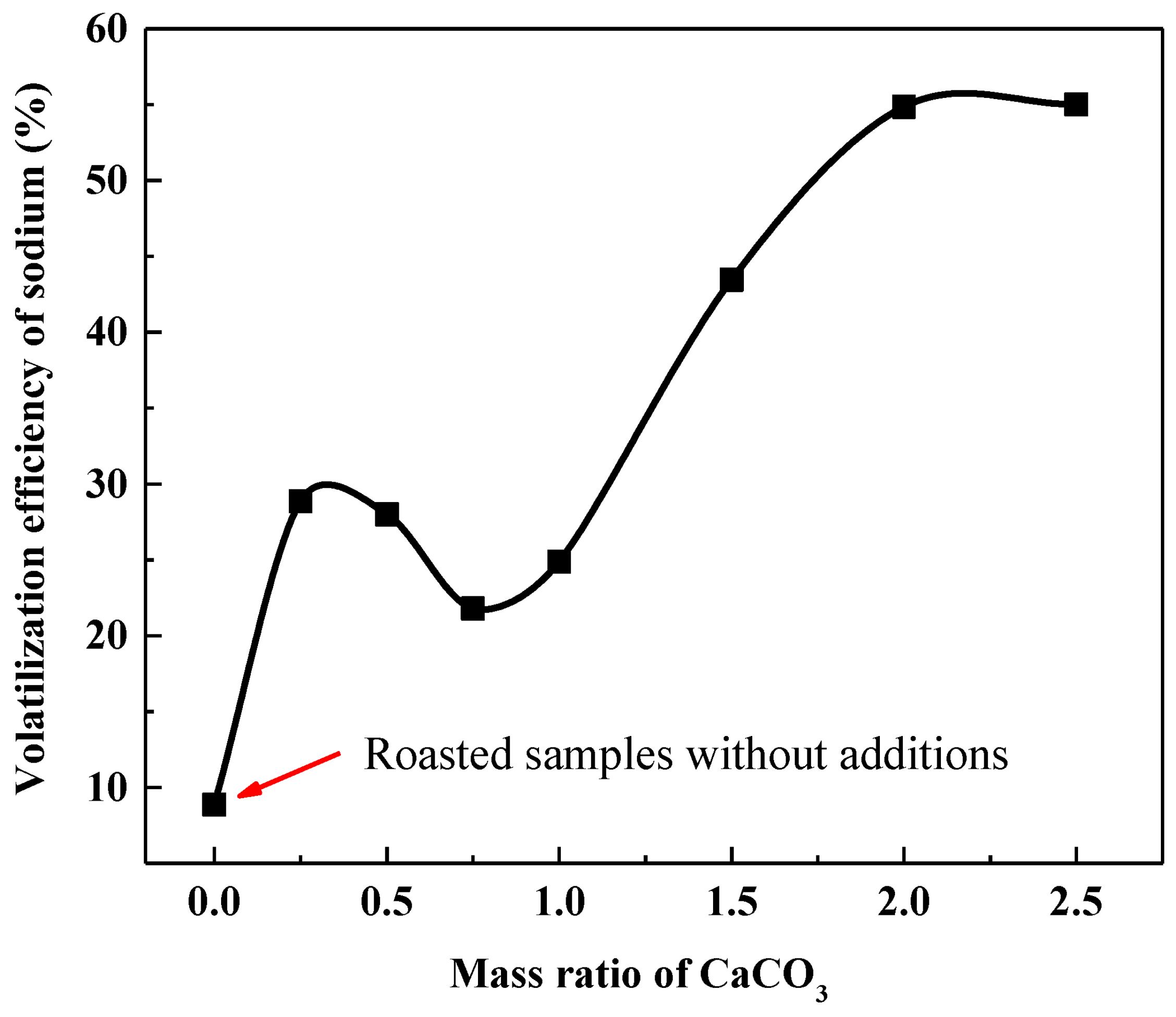

| Component | Na2O | K2O | Total Fe | SiO2 | TiO2 | MnO | Cr2O3 | Al2O3 | CaO | MgO | V2O5 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 4.45 | 0.02 | 32.32 | 13.64 | 11.92 | 6.33 | 4.18 | 2.78 | 1.88 | 1.69 | 1.34 | 0.47 |
| Point/ Area | Atom/mol% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Ti | Ca | Cr | Mn | Fe | Al | Si | Na | |
| 1 | 46.71 | 8.33 | - | 7.9 | 2.84 | 16.6 | - | - | - |
| 2 | 56.31 | - | 2.53 | - | 3.1 | 9.92 | 2.71 | 21.84 | - |
| 3 | 50.96 | - | - | - | 1.57 | 47.07 | - | - | - |
| 4 | 53.21 | 1.63 | 2.29 | - | - | 6.25 | 3.37 | 20.07 | 9.45 |
| 5 | 53.53 | 2.72 | - | 5.73 | - | 33.68 | 1.25 | - | - |
| 6 | 57.91 | 1.14 | 4.42 | - | - | 4.90 | 5.24 | 23.17 | 2.14 |
| 7 | 55.96 | - | - | - | 3.08 | 19.31 | - | 17.83 | - |
| 8 | 61.28 | 9.98 | - | 4.85 | - | 16.19 | - | 1.00 | 1.77 |
| 9 | 63.28 | - | - | - | 2.95 | 7.29 | - | 18.86 | - |
| 10 | 65.20 | - | - | - | - | 3.07 | 5.20 | 19.17 | 4.59 |
| 11 | 63.17 | 1.19 | 1.27 | - | - | 2.67 | 4.81 | 12.58 | 10.30 |
| 12 | 61.70 | 1.16 | 4.88 | - | - | 4.37 | - | 18.69 | 3.76 |
| 13 | 65.59 | 2.89 | - | 2.15 | 2.86 | 25.10 | - | - | - |
| 14 | - | - | - | - | - | 100 | - | - | - |
| 15 | 59.51 | - | - | - | - | 38.50 | - | - | - |
| Point/ Area | Atoms (mol%) | Main Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Ti | Ca | Cr | Mn | Fe | Al | Si | Na | ||
| A | 53.74 | 3.30 | 2.45 | - | 1.12 | 5.61 | 2.45 | 22.15 | 6.95 | Silicate |
| B | 56.98 | 4.26 | - | 2.93 | 2.18 | 32.59 | - | - | - | Hematite |
| C | 58.47 | 0.46 | 0.62 | 4.08 | 6.95 | 24.47 | 0.66 | - | - | Manganese iron oxide |
| D | 56.59 | 1.45 | 9.80 | - | 0.60 | 4.74 | 1.98 | 16.06 | 7.43 | Calcium-bearing silicate |
| E | 62.80 | 17.81 | 16.70 | - | 0.39 | 1.02 | - | 0.45 | 0.83 | Calcium titanate |
| F | 57.26 | - | 1.24 | 4.06 | 5.49 | 26.51 | 0.68 | - | 0.98 | Manganese iron oxide |
| G | 62.83 | 17.24 | 16.60 | - | 0.23 | 1.12 | - | 0.52 | 1.46 | Calcium titanate |
| H | 58.64 | 0.58 | 13.70 | - | - | 4.34 | 2.21 | 13.05 | 6.45 | Calcium-bearing silicate |
| I | 54.20 | - | 2.01 | 3.31 | 5.68 | 27.25 | 1.21 | - | - | Ferromanganese ore |
| J | 58.33 | 7.49 | 20.12 | 0.78 | 1.44 | 8.27 | - | 3.57 | - | Complex polycompound |
| K | 58.33 | 0.48 | 4.59 | - | - | 9.75 | 5.84 | 9.32 | 11.69 | Aegirine-arfvedsonite syenite |
| L | 63.10 | 1.34 | 8.98 | 0.62 | 1.79 | 19.75 | 1.13 | 1.51 | 1.07 | Complex calcium ferrite |
| M | 54.14 | 0.23 | 4.71 | 0.70 | - | 14.18 | 5.31 | 5.54 | 15.19 | Aegirine-arfvedsonite |
| N | 57.02 | 4.43 | 20.99 | 0.40 | 2.20 | 10.47 | 0.54 | 3.95 | - | Complex polycompound |
| O | 54.96 | 0.22 | 1.92 | 1.77 | 3.93 | 24.57 | 1.46 | - | - | Ferromanganese ore |
| P | 54.86 | - | 10.76 | 0.40 | 1.63 | 29.70 | 1.17 | - | 1.48 | Complex calcium ferrite |
| Q | 61.59 | 4.98 | 19.06 | - | 2.26 | 9.24 | 0.41 | 2.46 | - | Calcium ferrite |
| R | 57.53 | - | 25.87 | - | - | - | - | 12.90 | 3.23 | Dicalcium silicate |
| S | 60.27 | 1.31 | 19.04 | 0.29 | 0.85 | 15.49 | 0.36 | 0.75 | 1.21 | Calcium ferrite |
| T | 59.68 | 0.54 | 22.94 | 0.28 | - | - | - | 11.56 | 2.31 | Dicalcium silicate |
| U | 69.34 | - | 27.22 | 0.87 | 0.41 | - | - | 1.10 | 0.60 | Calcium oxide |
| CaCO3 Ratio | Na2O | Fe2O3 | SiO2 | TiO2 | MnO | Cr2O3 | Al2O3 | CaO | MgO | V2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.60 | 32.78 | 9.15 | 7.47 | 4.13 | 1.36 | 1.31 | 35.28 | 1.35 | 0.81 |
| 2 | 0.52 | 22.18 | 6.87 | 4.94 | 2.72 | 1.09 | 2.04 | 44.25 | 0.88 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, C.; Wang, S.; Chen, F.; Wang, X.; An, Z.; Yang, L. Research on the Removal of Sodium from Vanadium Tailings by Calcification Roasting and NaOH Leaching. Sustainability 2022, 14, 9051. https://doi.org/10.3390/su14159051
Guo Y, Wang C, Wang S, Chen F, Wang X, An Z, Yang L. Research on the Removal of Sodium from Vanadium Tailings by Calcification Roasting and NaOH Leaching. Sustainability. 2022; 14(15):9051. https://doi.org/10.3390/su14159051
Chicago/Turabian StyleGuo, Yufeng, Chao Wang, Shuai Wang, Feng Chen, Xueyuan Wang, Zhiwei An, and Lingzhi Yang. 2022. "Research on the Removal of Sodium from Vanadium Tailings by Calcification Roasting and NaOH Leaching" Sustainability 14, no. 15: 9051. https://doi.org/10.3390/su14159051
APA StyleGuo, Y., Wang, C., Wang, S., Chen, F., Wang, X., An, Z., & Yang, L. (2022). Research on the Removal of Sodium from Vanadium Tailings by Calcification Roasting and NaOH Leaching. Sustainability, 14(15), 9051. https://doi.org/10.3390/su14159051










