Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Collection and Preparation
2.3. Harvesting and Leachate Collection
3. Results
3.1. Plant Growth
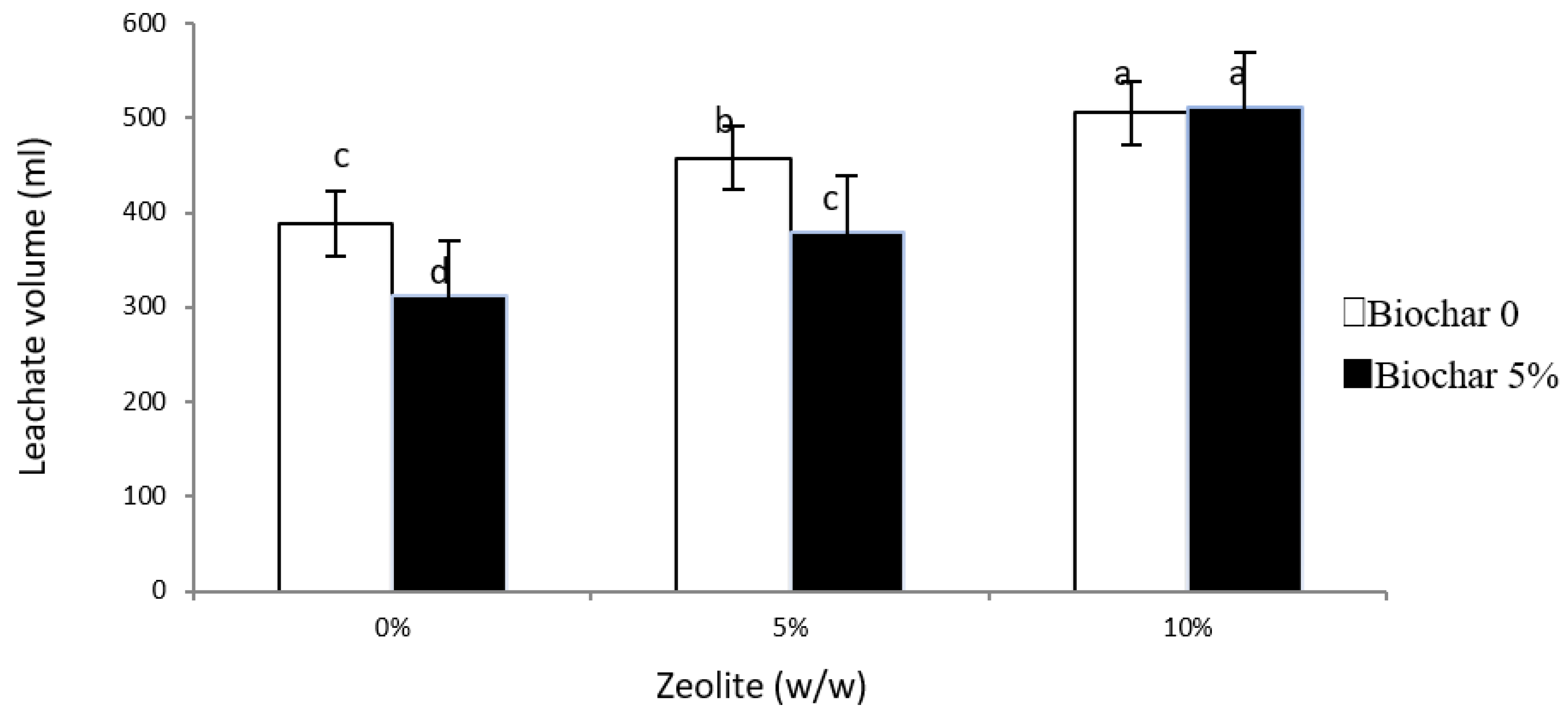
3.2. Leachate Volume
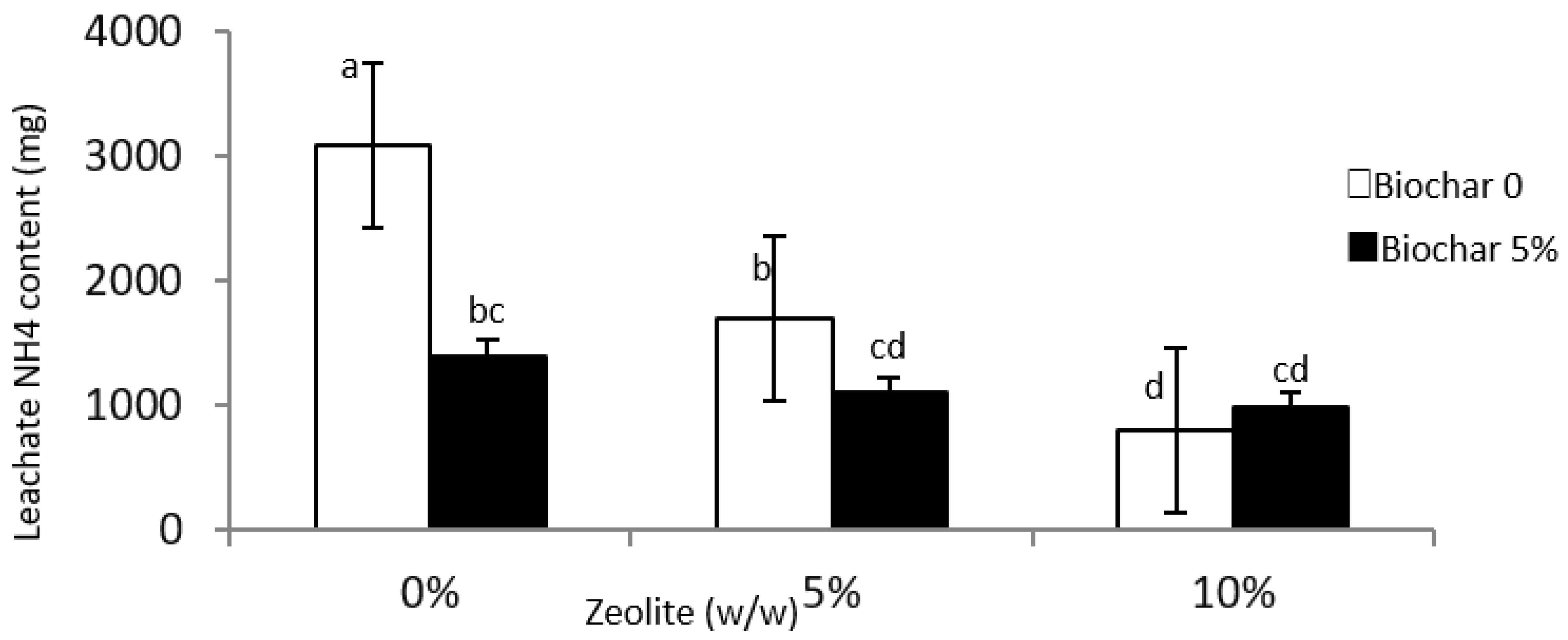
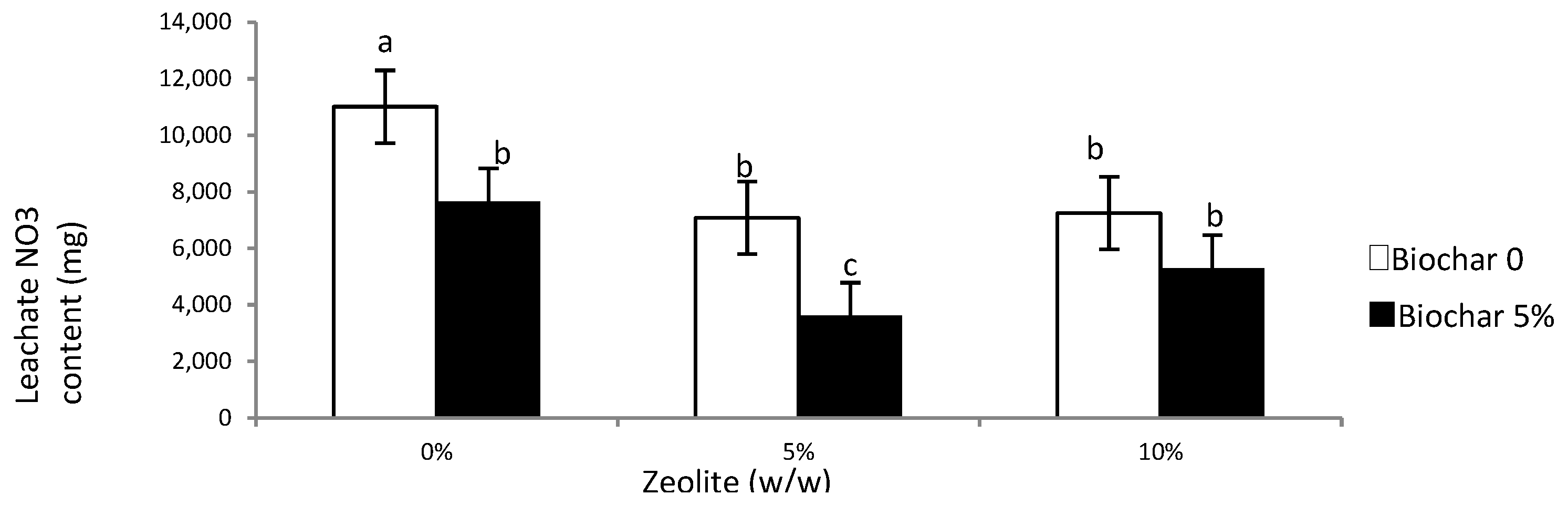
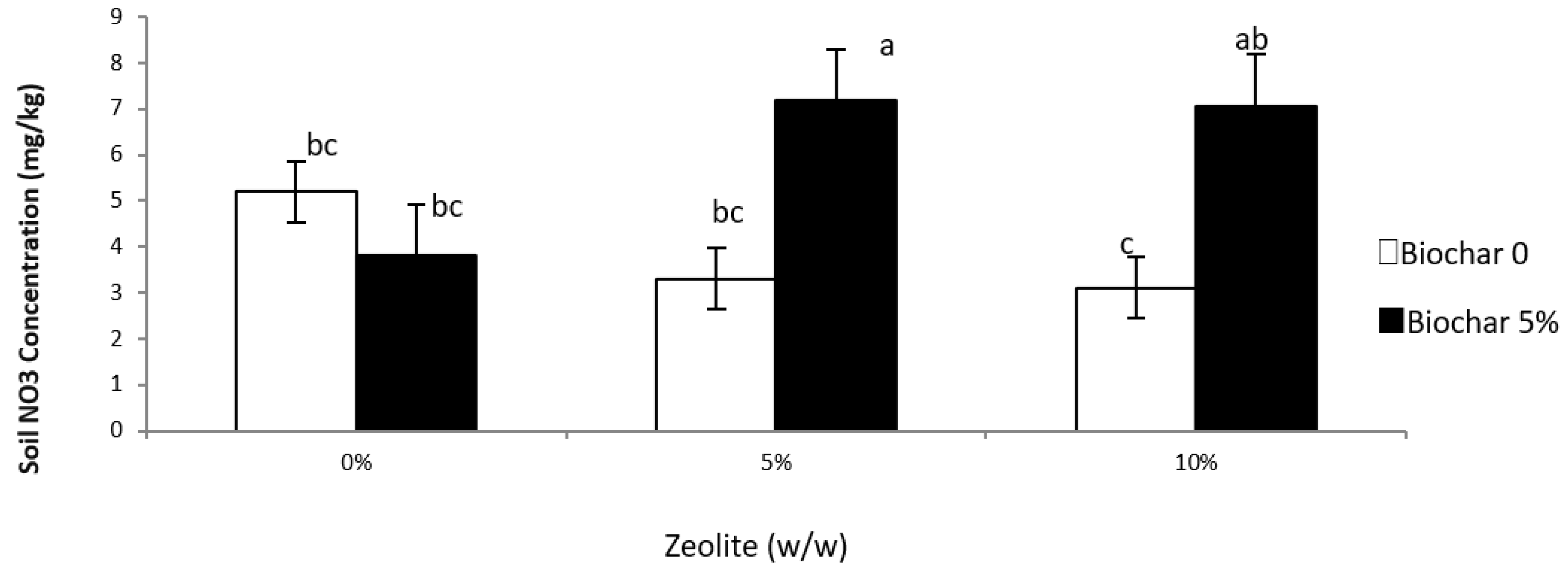
3.3. Leachate and Soil NO3 and NH4
3.4. Total and Fecal Coliforms in Leachate and Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| S.O.V | df | Total | Stem | Head | Grain |
|---|---|---|---|---|---|
| Replication | 3 | 1.35 ns | 0.067 ns | 2.245 ns | 0.453 ns |
| Biochar | 1 | 5.54 ns | 0.390 ns | 2.40 ns | 0.540 ns |
| Zeolite | 2 | 1.33 ns | 0.440 ns | 0.226 ns | 0.053 ns |
| Biochar × Zeolite | 2 | 1.02 ns | 0.063 ns | 0.762 ns | 0.608 ns |
| error | 15 | 1.79 | 0.248 | 0.973 | 0.243 |
| Total | 23 | 1.44 | |||
| CV (%) | - | 13.91 | 16.74 | 17.58 | 17.32 |

References
- FAO. The State of the worlds Land and Water Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; Earthscan: London, UK, 2011. [Google Scholar]
- Abedi-Koupai, J.; Mostafazadeh-Fard, B.; Afyuni, M.; Bagheri, M.R. Effect of treated wastewater on soil chemical and physical properties in an arid region. Plant Soil Environ. 2006, 52, 335. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, S.; Singh, J.S. Wastewater impact on human health and microorganism-mediated remediation and treatment through technologies. In Agro-Environmental Sustainability; Springer: Cham, Switzerland, 2017; pp. 235–250. [Google Scholar]
- Khalid, S.; Shahid, M.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [Green Version]
- Mateo-Sagasta, J.; Medlicott, K.; Qadir, M.; Raschid-Sally, L.; Drechsel, P. Proceedings of the UN-Water Project on the Safe Use of Wastewater in Agriculture; UN-Water Decade Programme on Capacity Development: Bonn, Germany, 2013. [Google Scholar]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Hube, S.; Wu, B. Mitigation of emerging pollutants and pathogens in decentralized wastewater treatment processes: A review. Sci. Total Environ. 2021, 779, 146545. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H. Removal of heavy metals from polluted solutions by zeolitic adsorbents: A review. Environ. Processes 2021, 8, 7–35. [Google Scholar] [CrossRef]
- Lu, Q.; Han, P.; Chen, F.; Liu, T.; Li, J.; Leng, L.; Zhou, W. A novel approach of using zeolite for ammonium toxicity mitigation and value-added Spirulina cultivation in wastewater. Bioresour. Technol. 2019, 280, 127–135. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Bitarafan, Z.; Asghari, H.R.; Hasanloo, T.; Gholami, A.; Moradi, F.; Khakimov, B.; Liu, F.; Andreasen, C. The effect of charcoal on medicinal compounds of seeds of fenugreek (Trigonella foenum-graecum L.) exposed to drought stress. Ind. Crops Prod. 2019, 131, 323–329. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Kulasekaran, R.; Reddy, D.D.; Biswas, A.K.; Rao, A.S. Chapter four-zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 215–236. [Google Scholar]
- De Rozari, P.; Greenway, M.; El Hanandeh, A. Phosphorus removal from secondary sewage and septage using sand media amended with biochar in constructed wetland mesocosms. Sci. Total Environ. 2016, 569, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Metes, A.; Kovačević, D.; Vujević, D.; Papić, S. The role of zeolites in wastewater treatment of printing inks. Water Res. 2004, 38, 3373–3381. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Asghari, H.R.; Cavagnaro, T.R. Arbuscular mycorrhizas enhance plant interception of leached nutrients. Funct. Plant Biol. 2011, 38, 219–226. [Google Scholar] [CrossRef]
- Asghari, H.R.; Cavagnaro, T.R. Arbuscular mycorrhizas reduce nitrogen loss via leaching. PLoS ONE 2012, 7, e29825. [Google Scholar] [CrossRef] [Green Version]
- Federation, W.E. APH Association Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Are, K.S. Biochar and soil physical health. In Biochar-An Imperative Amendment for Soil and the Environment; IntechOpen: London, UK, 2019; pp. 21–33. [Google Scholar]
- Mohawesh, O.; Durner, W. Effects of bentonite, hydrogel and biochar amendments on soil hydraulic properties from saturation to oven dryness. Pedosphere 2019, 29, 598–607. [Google Scholar] [CrossRef]
- Gondim, R.S.; Muniz, C.R.; Lima, C.E.P.; Santos, C.L.A.D. Explaining the water-holding capacity of biochar by scanning electron microscope images. Rev. Caatinga 2018, 31, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Batista, E.M.; Shultz, J.; Matos, T.T.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Sarkar, B.; Naidu, R. Nutrient and water use efficiency in soil: The influence of geological mineral amendments. In Nutrient Use Efficiency: From Basics to Advances; Springer: New Delhi, India, 2015; pp. 29–44. [Google Scholar]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 1–34. [Google Scholar] [CrossRef]
- Mirzaei, S.M.J.; Heidarpour, M.; Tabatabaei, S.H.; Najafi, P.; Hashemi, S.E. Immobilization of leachate’s heavy metals using soil-zeolite column. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Méndez Argüello, B.; Vera Reyes, I.; Cárdenas Flores, A.; Santos Villarreal, G.; Ibarra Jiménez, L.; Lira Saldivar, R.H. Water holding capacity of substrates containing zeolite and its effect on growth, biomass production and chlorophyll content of Solanum lycopersicum Mill. Nova Sci. 2018, 10, 45–60. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef]
- Troy, S.M.; Lawlor, P.G.; O’Flynn, C.J.; Healy, M.G. The impact of biochar addition on nutrient leaching and soil properties from tillage soil amended with pig manure. Water Air Soil Pollut. 2014, 225, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Sánchez, E.; Herrmann, C. Application of zeolites for biological treatment processes of solid wastes and wastewaters–A review. Bioresour. Technol. 2020, 301, 122808. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Malekian, R.; Abedi-Koupai, J.; Eslamian, S.S. Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J. Hazard. Mater. 2011, 185, 970–976. [Google Scholar] [CrossRef]
- Soudejani, H.T.; Kazemian, H.; Inglezakis, V.J.; Zorpas, A.A. Application of zeolites in organic waste composting: A review. Biocatal. Agric. Biotechnol. 2019, 22, 101396. [Google Scholar] [CrossRef]
- Gamze Turan, N.; Nuri Ergun, O. Ammonia uptake by natural zeolite in municipal solid waste compost. Environ. Prog. 2007, 26, 149–156. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Fuertes-Mendizábal, T.; Huérfano, X.; Vega-Mas, I.; Torralbo, F.; Menéndez, S.; Ippolito, J.A.; Kammann, C.; Wrage-Mönnig, N.; Cayuela, M.L.; Borchard, N.; et al. Biochar reduces the efficiency of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) mitigating N2O emissions. Sci. Rep. 2019, 9, 2346. [Google Scholar] [CrossRef]
- Ball, P.N.; MacKenzie, M.D.; DeLuca, T.H.; Montana, W.H. Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J. Environ. Qual. 2010, 39, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.F.; Chen, Q.H.; Yang, X.R.; Li, H.; Zheng, B.X.; Zhou, W.W.; Liu, X.X.; Dai, P.B.; Li, K.J.; Lin, X.Y. Effects of combined application of nitrogen fertilizer and biochar on the nitrification and ammonia oxidizers in an intensive vegetable soil. Amb Express 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, M.K.; Wang, M.; Pandey, A.; Chen, H.; Awasthi, S.K.; Wang, Q.; Ren, X.; Lahori, A.H.; Li, D.S.; Li, R.; et al. Heterogeneity of zeolite combined with biochar properties as a function of sewage sludge composting and production of nutrient-rich compost. Waste Manag. 2017, 68, 760–773. [Google Scholar] [CrossRef]
- Wu, X.; Ren, L.; Zhang, J.; Peng, H. Effects of zeolite and Biochar addition on ammonia-oxidizing bacteria and ammonia-oxidizing Archaea communities during agricultural waste composting. Sustainability 2020, 12, 6336. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Boehm, A.B. Escherichia coli removal in biochar-augmented biofilter: Effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ. Sci. Technol. 2014, 48, 11535–11542. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Cantrell, K.B.; Nelson, K.L.; Boehm, A.B. Efficacy of biochar to remove Escherichia coli from stormwater under steady and intermittent flow. Water Res. 2014, 61, 288–296. [Google Scholar] [CrossRef]
- Afrooz, A.N.; Boehm, A.B. Escherichia coli removal in biochar-modified biofilters: Effects of biofilm. PLoS ONE 2016, 11, e0167489. [Google Scholar] [CrossRef]
- Sasidharan, S.; Torkzaban, S.; Bradford, S.A.; Kookana, R.; Page, D.; Cook, P.G. Transport and retention of bacteria and viruses in biochar-amended sand. Sci. Total Environ. 2016, 548, 100–109. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S. Colloid interaction energies for physically and chemically heterogeneous porous media. Langmuir 2013, 29, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Torkzaban, S.; Bradford, S.A.; Vanderzalm, J.L.; Patterson, B.M.; Harris, B.; Prommer, H. Colloid release and clogging in porous media: Effects of solution ionic strength and flow velocity. J. Contam. Hydrol. 2015, 181, 161–171. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Unit | Value |
|---|---|---|
| pH | - | 7.5 |
| EC | dS/m | |
| SiO2 | % | 67.83 |
| Al2O3 | % | 11.64 |
| Fe2O3 | % | 0.54 |
| CaO | % | 0.84 |
| SO3 | % | 0.20 |
| Na2O | % | 4.5 |
| K2O | % | 4.32 |
| CEC | Cmol/kg | 2.64 |
| Specific surface area | m2/g | 47.2 |
| Parameter | Unit | Value |
|---|---|---|
| pH | - | 7.55 |
| EC | dS/m | 0.84 |
| Carbon | % | 69.63 |
| Nitrogen | % | 0.28 |
| Hydrogen | % | 3.3 |
| Oxygen | % | 19.51 |
| Ash | % | 5.5 |
| Bulk density | g/cm3 | 0.13 |
| Cation exchange capacity | Cmol(+)/kg | 36.3 |
| Specific surface area | m2/g | 160 |
| Parameter | Unit | Value |
|---|---|---|
| pH | - | 8.51 |
| EC | dS/m | 1.5 |
| BOD | mg/L O2 | 60 |
| COD | mg/L O2 | 174 |
| Ammonium | mg/L | 6.1 |
| Nitrate | mg/L | 42 |
| Total Dissolved Solid | mg/L | 1000 |
| Total Phosphorous | mg/L | 2 |
| Chloride | mg/L | 262 |
| Thermotolerant Coliform-MPN | cfu/mL | 1200 |
| Treatment | Leachate Volume mL | NO3 Concentration mg/L | NO3 Content mg | NH4 Concentration mg/L | NH4 Content mg | |
|---|---|---|---|---|---|---|
| Biochar | 0% | 425.9 ± 17 | 20.4 ± 1.5 | 8688 ± 157 | 4.7 ± 0.8 | 2002 ± 67 |
| 5% | 376.3 ± 23 | 15.9 ± 1.1 | 5983 ± 252 | 3.3 ± 0.5 | 1242 ± 55 | |
| Zeolite | 0% | 325.6 ± 12 | 28.5 ± 1.2 | 9335 ± 98 | 6.7 ± 1.2 | 2243 ± 124 |
| 5% | 394.3 ± 33 | 13.1 ± 0.8 | 5350 ± 111 | 3.6 ± 0.9 | 1404 ± 98 | |
| 10% | 483.5 ± 23 | 13.0 ± 1.5 | 6268 ± 322 | 1.9 ± 0.5 | 894 ± 304 | |
| Biochar | *** | * | ** | ** | *** | |
| Zeolite | *** | *** | ** | *** | *** | |
| Biochar × Zeolite | * | * | * | ** | *** |
| Treatment | Total Coliforms Concentration cfu/mL | Fecal Coliforms Concentration cfu/mL | |
|---|---|---|---|
| Biochar | 0% | 230 ± 10 | 27 ± 7 |
| 5% | 30 ± 4 | 0 ± 0 | |
| Zeolite | 0% | 230 ± 22 | 23 ± 6 |
| 5% | 230 ± 15 | 19 ± 4 | |
| 10% | 230 ± 25 | 19 ± 6 | |
| Biochar | *** | *** | |
| Zeolite | ns | ns | |
| Biochar × Zeolite | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghari, H.R.; Bochmann, G.; Tabari, Z.T. Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms. Sustainability 2022, 14, 8880. https://doi.org/10.3390/su14148880
Asghari HR, Bochmann G, Tabari ZT. Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms. Sustainability. 2022; 14(14):8880. https://doi.org/10.3390/su14148880
Chicago/Turabian StyleAsghari, Hamid Reza, Günther Bochmann, and Zahra Taghizadeh Tabari. 2022. "Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms" Sustainability 14, no. 14: 8880. https://doi.org/10.3390/su14148880
APA StyleAsghari, H. R., Bochmann, G., & Tabari, Z. T. (2022). Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms. Sustainability, 14(14), 8880. https://doi.org/10.3390/su14148880







