Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
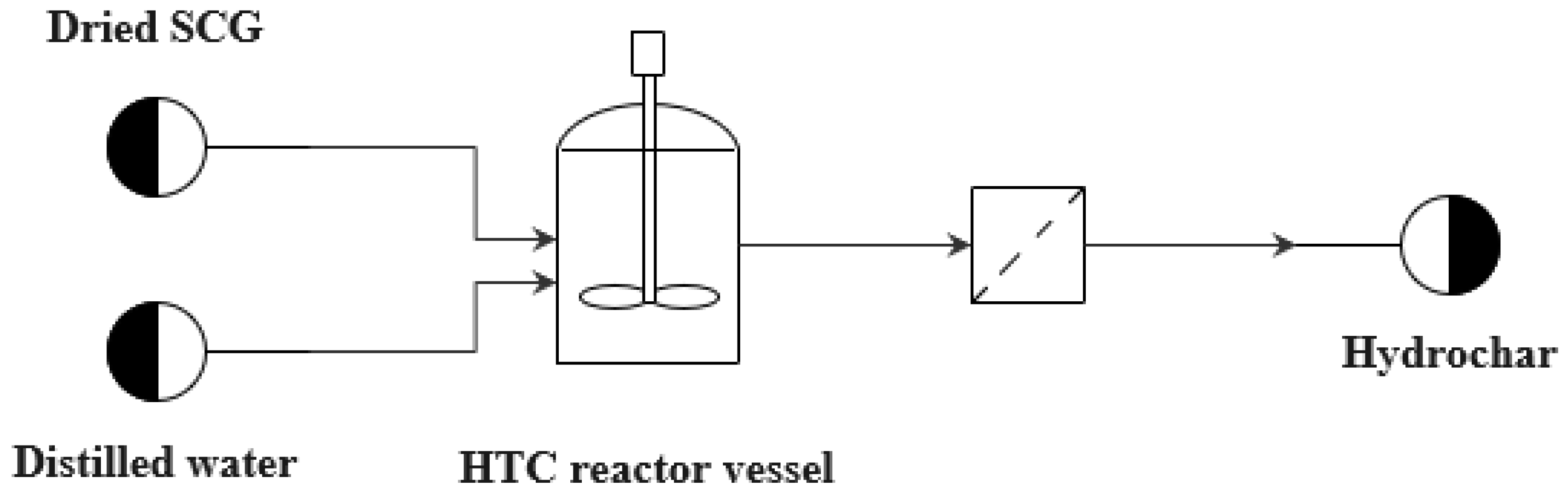
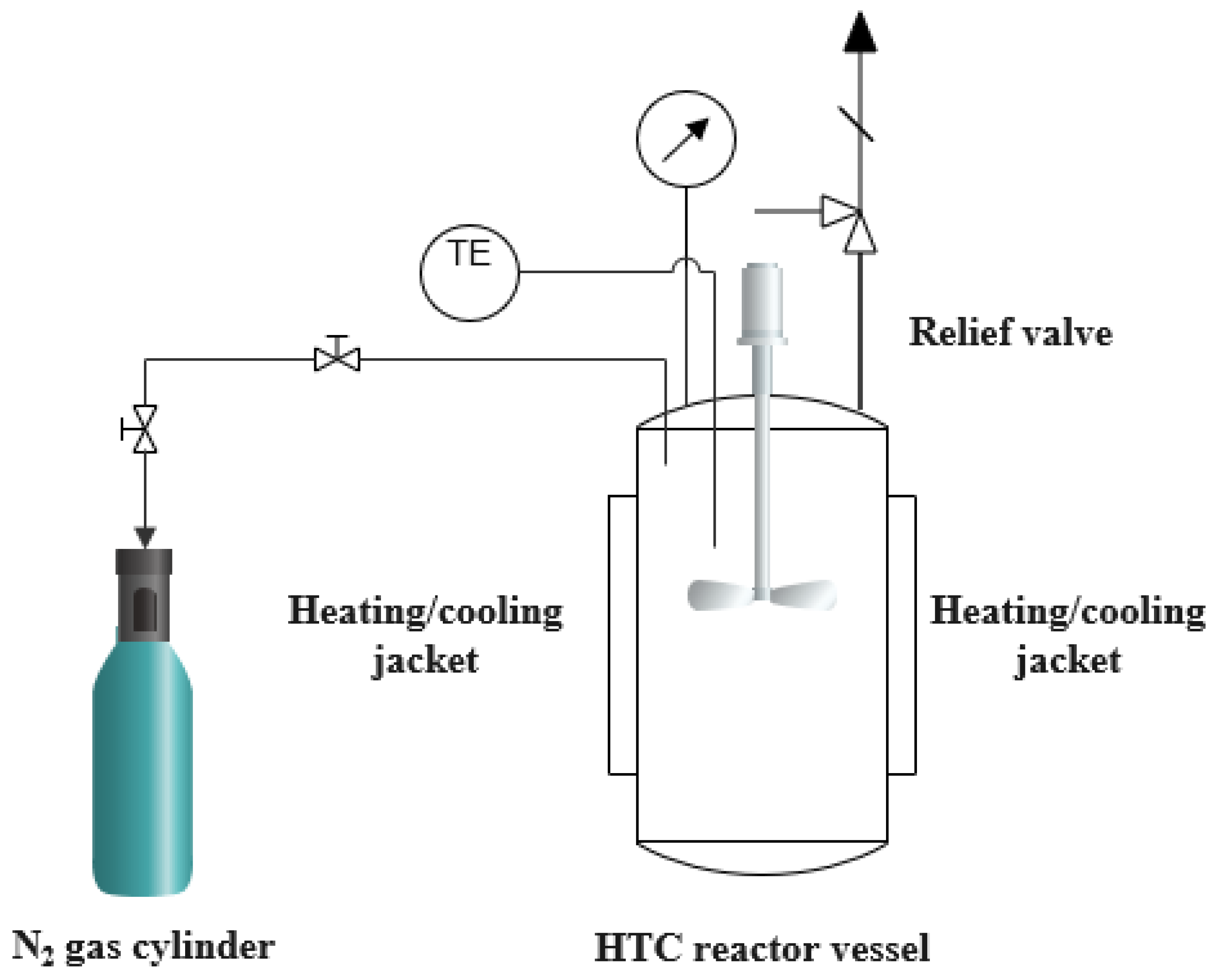
2.2. HTC Experiments
2.3. Characterizations of Feedstock and Hydrochar
3. Results and Discussion
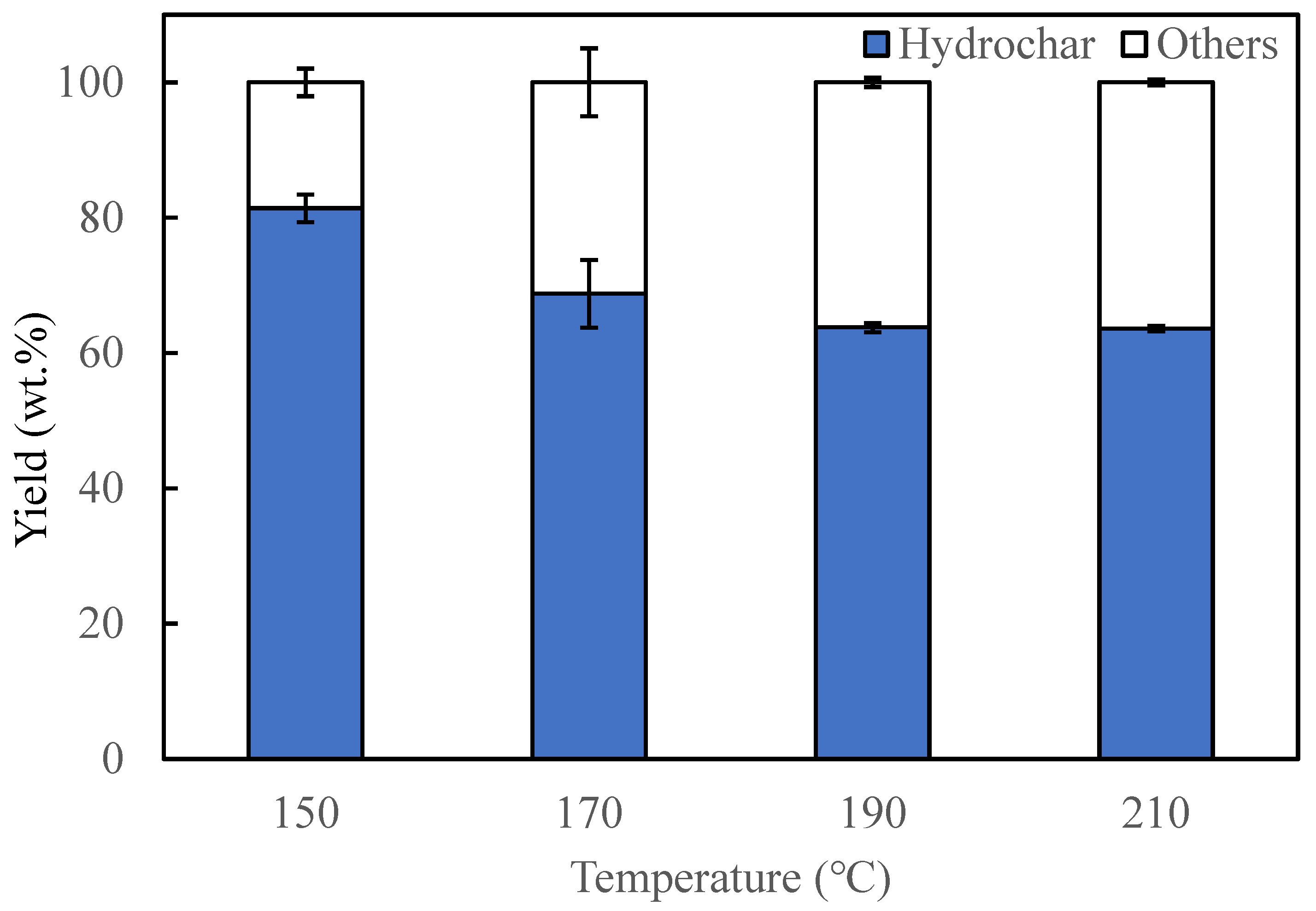
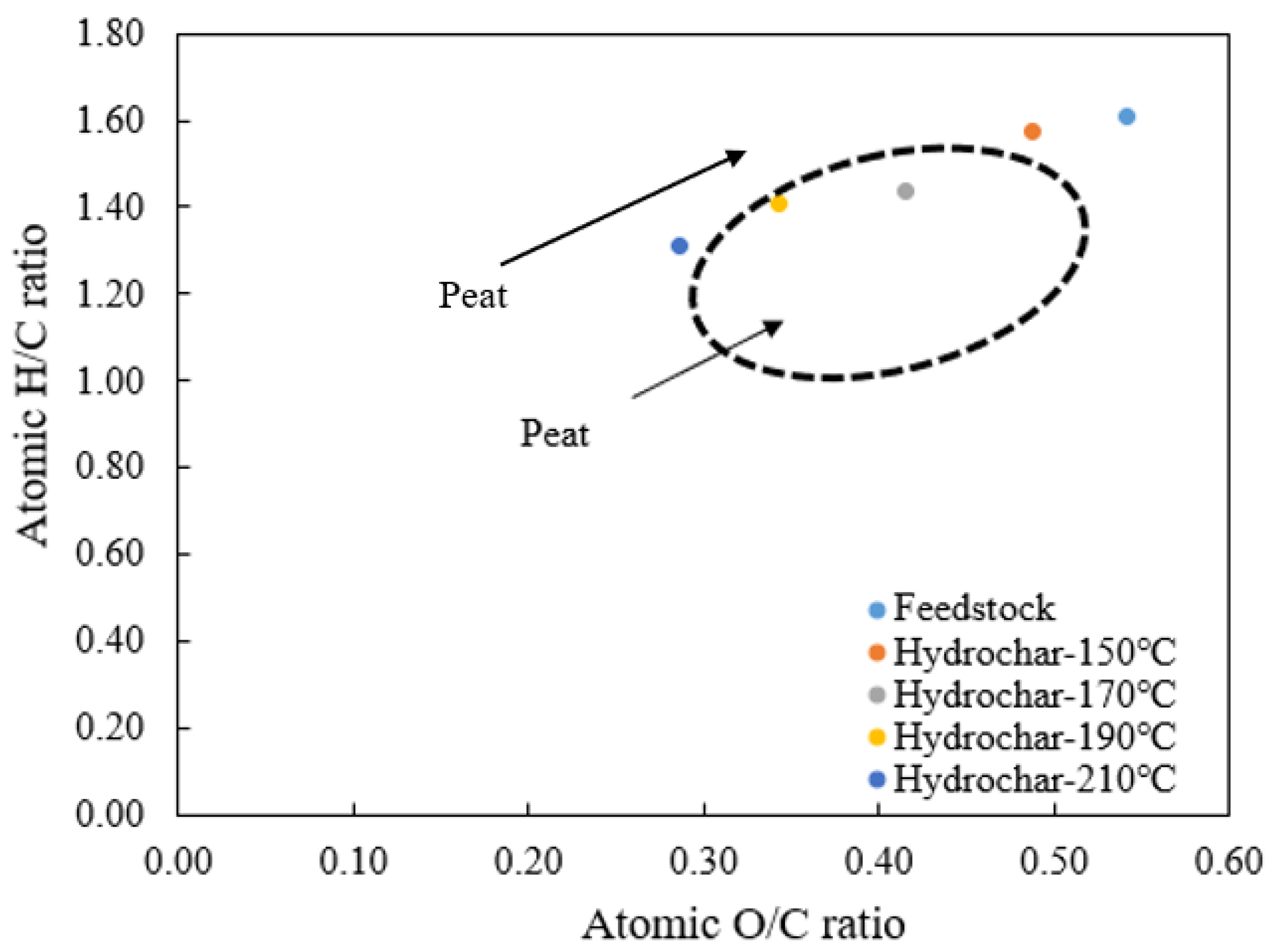
3.1. Effects of Temperature on Products Yield and Properties
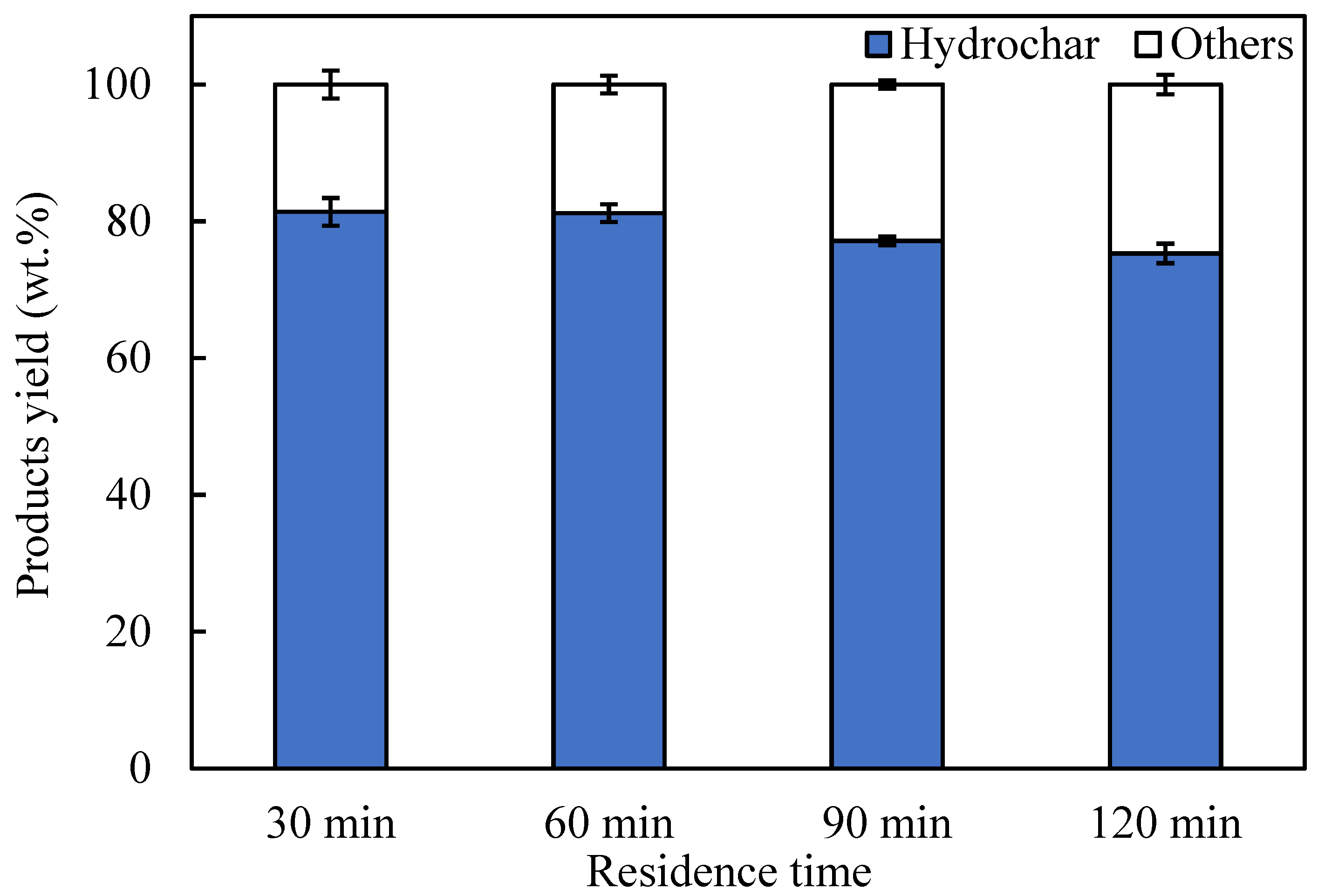
3.2. Effects of Residence Time on Products Yield and Properties
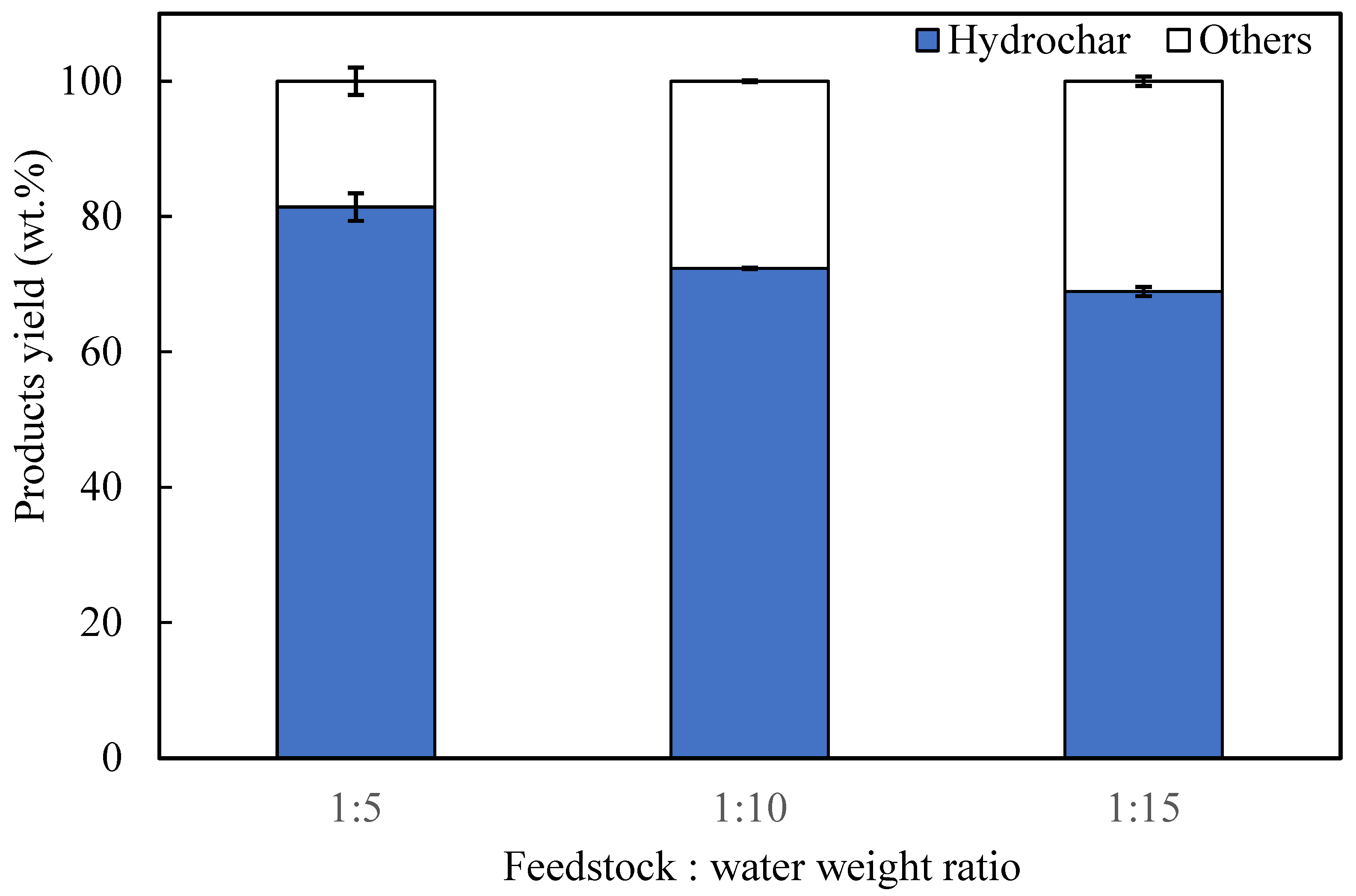
3.3. Effects of Feedstock to Water Weight Ratio on Products Yield and Properties
3.4. FTIR Analysis
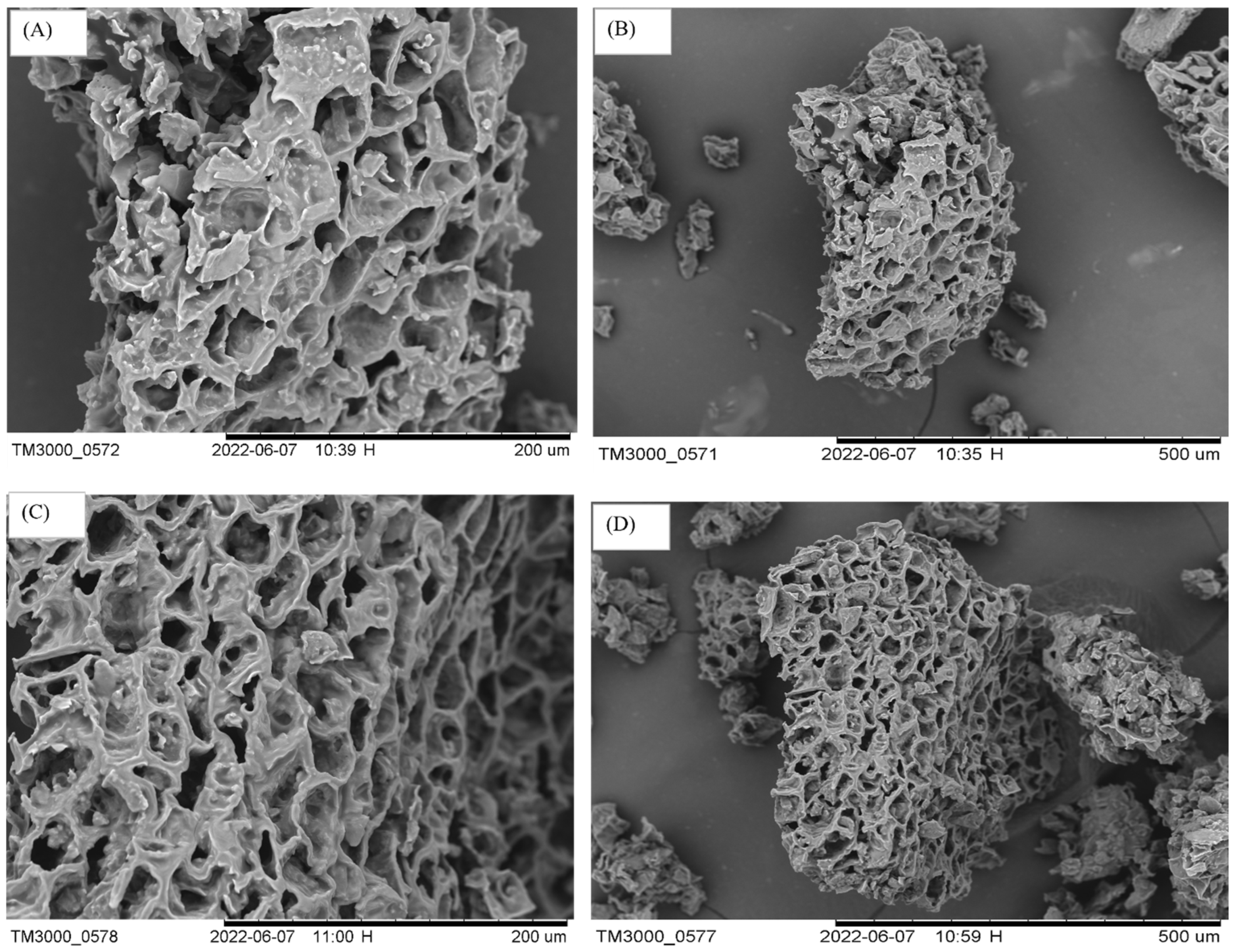
3.5. SEM Analysis
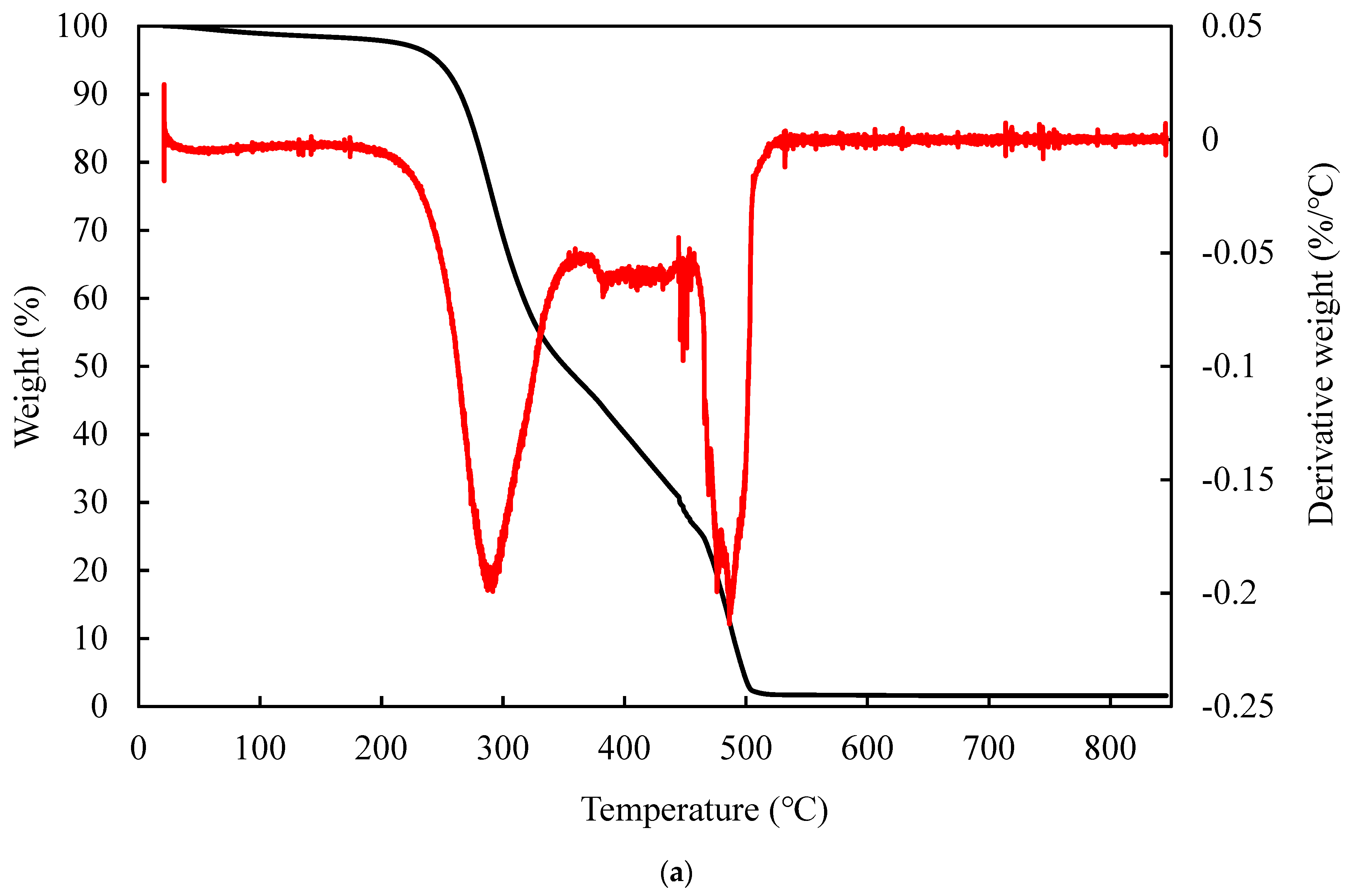
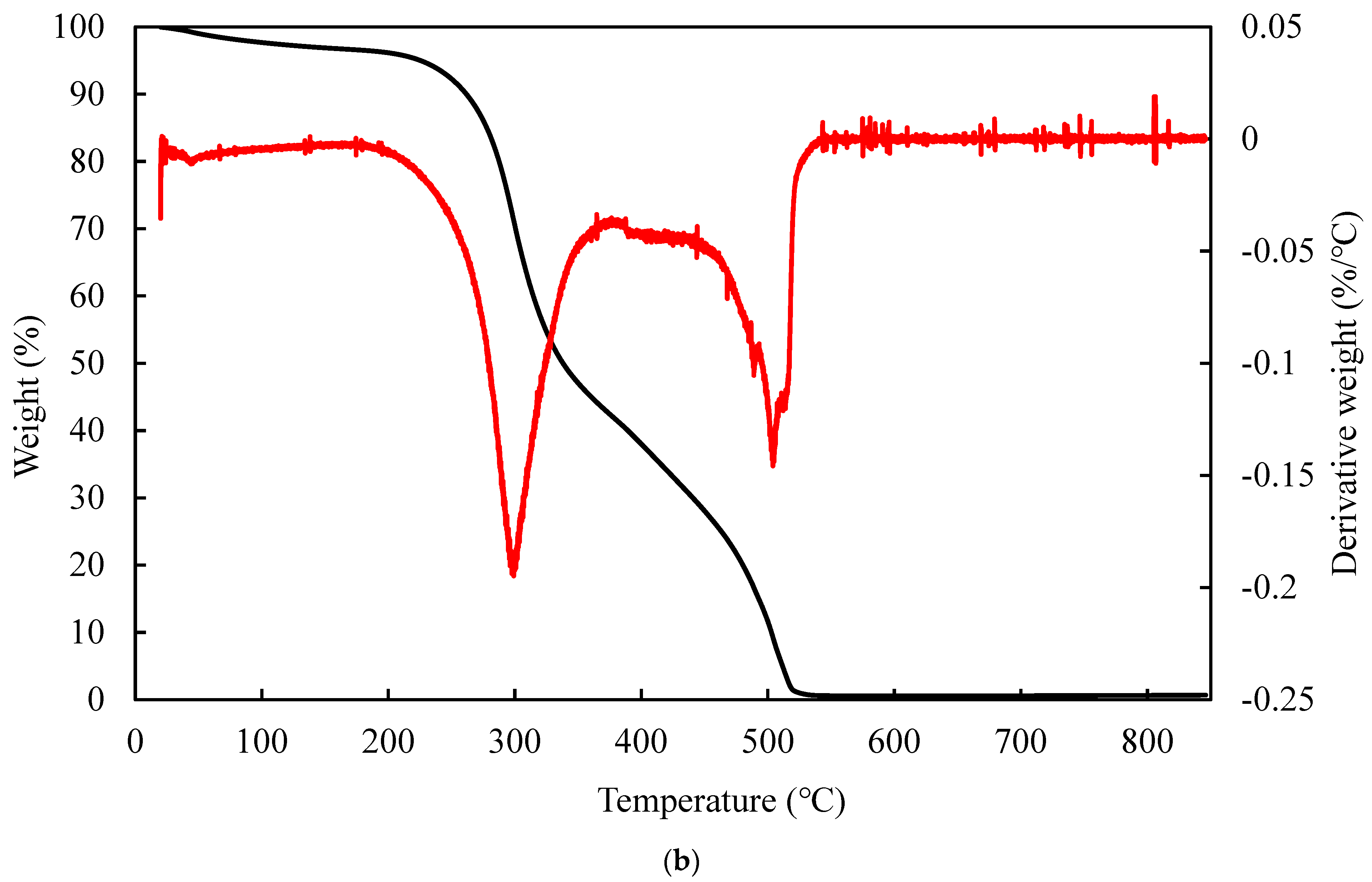
3.6. TGA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DTG | Derivative thermogravimetric |
| FTIR | Fourier-transform infrared spectroscopy |
| HTC | Hydrothermal carbonization |
| HHV | Higher heating value |
| PFD | Process flow diagram |
| SEM | Scanning electron microscope |
| SCG | Spent coffee ground |
| TGA | Thermogravimetric analysis |
| TG | Thermogravimetric |
References
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Sher, F. Energy, exergy, thermoeconomic and sustainability assessment of tire pyrolysis oil in common rail direct injection diesel engine. Fuel 2022, 311, 122622. [Google Scholar] [CrossRef]
- Murugesan, A.; Umarani, C.; Subramanian, R.; Nedunchezhian, N. Bio-diesel as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2009, 13, 653–662. [Google Scholar] [CrossRef]
- Rustamaji, H.; Prakoso, T.; Rizkiana, J.; Devianto, H.; Widiatmoko, P.; Guan, G. Synthesis and Characterization of Hydrochar and Bio-oil from Hydrothermal Carbonization of Sargassum sp. using Choline Chloride (ChCl) Catalyst. Int. J. Renew. Energy Dev. 2022, 11, 403–412. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Cardarelli, A.; Pinzi, S.; Barbanera, M. Effect of torrefaction temperature on spent coffee grounds thermal behaviour and kinetics. Renew. Energy 2022, 185, 704–716. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; He, Q.; Zhou, N. MW-assisted hydrothermal liquefaction of spent coffee grounds. Can. J. Chem. Eng. 2021, 100, 1729–1738. [Google Scholar] [CrossRef]
- Nepal, R.; Kim, H.J.; Poudel, J.; Oh, S.C. A study on torrefaction of spent coffee ground to improve its fuel properties. Fuel 2022, 318, 123643. [Google Scholar] [CrossRef]
- Barbanera, M.; Muguerza, I.F. Effect of the temperature on the spent coffee grounds torrefaction process in a continuous pilot-scale reactor. Fuel 2020, 262, 116493. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhang, Q.; Wang, C.; Zhan, H.; Ma, L. A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew. Sustain. Energy Rev. 2022, 154, 111877. [Google Scholar] [CrossRef]
- Tombarkiewicz, B.; Antonkiewicz, J.; Lis, M.W.; Pawlak, K.; Trela, M.; Witkowicz, R.; Gorczyca, O. Chemical properties of the coffee grounds and poultry eggshells mixture in terms of soil improver. Sci. Rep. 2022, 12, 2592. [Google Scholar] [CrossRef] [PubMed]
- Sangprasert, T.; Sattayarut, V.; Rajrujithong, C.; Khanchaitit, P.; Khemthong, P.; Chanthad, C.; Grisdanurak, N. Making use of the inherent nitrogen content of spent coffee grounds to create nanostructured activated carbon for supercapacitor and lithium-ion battery applications. Diam. Relat. Mater. 2022, 127, 109164. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Q.; Yang, G.; Li, X.; Du, W.; Leong, Y.K.; Chang, J.-S. Enhanced chlortetracycline removal by iron oxide modified spent coffee grounds biochar and persulfate system. Chemosphere 2022, 301, 134654. [Google Scholar] [CrossRef]
- Supang, W.; Ngamprasertsith, S.; Sakdasri, W.; Sawangkeaw, R. Ethyl acetate as extracting solvent and reactant for producing biodiesel from spent coffee grounds: A catalyst- and glycerol-free process. J. Supercrit. Fluids 2022, 186, 105586. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Niu, H.; Dalai, A.; Corscadden, K.; Zhou, N. Microwave-assisted hydrothermal liquefaction of biomass model components and comparison with conventional heating. Fuel 2020, 277, 118202. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Xu, R.; Ning, X.; Zhang, J.; Guo, X.; Ye, L.; Li, J.; Jiang, C.; Wang, P.; et al. Hydrothermal carbonization of forest waste into solid fuel: Mechanism and combustion behavior. Energy 2022, 246, 123343. [Google Scholar] [CrossRef]
- Shrestha, A.; Acharya, B.; Farooque, A.A. Study of hydrochar and process water from hydrothermal carbonization of sea lettuce. Renew. Energy 2021, 163, 589–598. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Yuan, Z.; Xu, C.; Bassi, A. Investigation of aqueous phase recycling for improving bio-crude oil yield in hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 239, 151–159. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Bassi, A.; Xu, C. Improvement in bio-crude yield and quality through co-liquefaction of algal biomass and sawdust in ethanol-water mixed solvent and recycling of the aqueous by-product as a reaction medium. Energy Convers. Manag. 2018, 171, 618–625. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C. Hydrothermal liquefaction of woody biomass in hot-compressed water: Catalyst screening and comprehensive characterization of bio-crude oils. Fuel 2015, 162, 74–83. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. Heating values of wood pellets from different species. Biomass Bioenergy 2011, 35, 2634–2639. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Farru, G.; Asquer, C.; Cappai, G.; De Gioannis, G.; Melis, E.; Milia, S.; Muntoni, A.; Piredda, M.; Scano, E.A. Hydrothermal carbonization of hemp digestate: Influence of operating parameters. Biomass Convers. Biorefinery 2022, 70. [Google Scholar] [CrossRef]
- Ipiales, R.P.; Mohedano, A.F.; Diaz, E.; de la Rubia, M.A. Energy recovery from garden and park waste by hydrothermal carbonisation and anaerobic digestion. Waste Manag. 2022, 140, 100–109. [Google Scholar] [CrossRef]
- Cheng, C.; He, Q.; Ismail, T.M.; Mosqueda, A.; Ding, L.; Yu, J.; Yu, G. Hydrothermal carbonization of rape straw: Effect of reaction parameters on hydrochar and migration of AAEMs. Chemosphere 2022, 291, 132785. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Chen, X.; Chen, A.; Xiao, R.; Chen, X. Hydrothermal conversion of Chinese cabbage residue for sustainable agriculture: Influence of process parameters on hydrochar and hydrolysate. Sci. Total Environ. 2022, 812, 152478. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.L. Optimisation and characterisation of hydrochar production from spent coffee grounds by hydrothermal carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Cheng, C.; Ding, L.; Guo, Q.; He, Q.; Gong, Y.; Alexander, K.N.; Yu, G. Process analysis and kinetic modeling of coconut shell hydrothermal carbonization. Appl. Energy 2022, 315, 118981. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Xu, Z.R.; Zhang, H.W.; Yang, H.P. Influence of sludge properties on the direct gasification of dewatered sewage sludge in supercritical water. Renew. Energy 2014, 66, 605–611. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of sweet potato waste to solid fuel via hydrothermal carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef]
- Li, C.; Cai, R. Preparation of solid organic fertilizer by co-hydrothermal carbonization of peanut residue and corn cob: A study on nutrient conversion. Sci. Total Environ. 2022, 838, 155867. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.-M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]

| C (%) | H (%) | O (%) | N (%) | S (%) | HHV (MJ/kg) | Energy Densification | Energy Recovery (%) | |
|---|---|---|---|---|---|---|---|---|
| Feedstock | 52.77 ± 1.25 | 7.08 ± 0.12 | 38.14 ± 1.37 | 2.01 ± 0.01 | n.d. * | 22.83 ± 0 | / | / |
| Hydrochar | ||||||||
| 150 °C | 55.12 ± 0.01 | 7.23 ± 0.11 | 35.87 ± 0.42 | 1.78 ± 0.32 | n.d. * | 23.54 ± 0.08 | 1.03 | 83.93 |
| 170 °C | 58.50 ± 3.93 | 7.01 ± 0.30 | 32.44 ± 4.01 | 2.05 ± 0.33 | n.d. * | 26.55 ± 2.46 | 1.16 | 79.95 |
| 190 °C | 62.05 ± 0.33 | 7.29 ± 0.03 | 28.40 ± 0.18 | 2.26 ± 0.12 | n.d. * | 26.44 ± 0.40 | 1.16 | 73.88 |
| 210 °C | 65.59 ± 0.90 | 7.15 ± 0.03 | 25.08 ± 0.79 | 2.18 ± 0.09 | n.d. * | 27.65 ± 0.11 | 1.21 | 77.05 |
| HHV (MJ/kg) | Energy Densification | Energy Recovery (%) | |
|---|---|---|---|
| Feedstock | 22.83 ± 0 | / | / |
| Hydrochar | |||
| 30 min | 23.54 ± 0.08 | 1.03 | 83.93 |
| 60 min | 23.44 ± 0.10 | 1.03 | 83.37 |
| 90 min | 24.38 ± 0.14 | 1.07 | 82.36 |
| 120 min | 24.62 ± 0.24 | 1.08 | 81.21 |
| Residence Time (min) | C (%) | H (%) | O (%) | N (%) | S (%) | Atomic O/C Ratio | Atomic H/C Ratio |
|---|---|---|---|---|---|---|---|
| Feedstock | 52.77 ± 1.25 | 7.08 ± 0.12 | 38.14 ± 1.37 | 2.01 ± 0.01 | n.d. * | 0.54 | 1.61 |
| Hydrochar | |||||||
| 30 | 55.12 ± 0.01 | 7.23 ± 0.11 | 35.87 ± 0.42 | 1.78 ± 0.32 | n.d. * | 0.49 | 1.57 |
| 60 | 53.39 ± 2.85 | 6.90 ± 0.22 | 38.04 ± 3.14 | 1.67 ± 0.06 | n.d. * | 0.53 | 1.55 |
| 90 | 56.98 ± 0.69 | 7.41 ± 0.25 | 34.05 ± 0.93 | 1.56 ± 0.26 | n.d. * | 0.45 | 1.56 |
| 120 | 57.32 ± 0.07 | 7.52 ± 0.08 | 33.66 ± 0.16 | 1.50 ± 0.17 | n.d. * | 0.44 | 1.57 |
| Feedstock to Water Weight Ratio | HHV (MJ/kg) | Energy Densification | Energy Recovery (%) |
|---|---|---|---|
| Feedstock | 22.83 ± 0 | / | / |
| Hydrochar | |||
| 1:5 | 23.54 ± 0.08 | 1.03 | 83.93 |
| 1:10 | 23.85 ± 0 | 1.04 | 75.59 |
| 1:15 | 23.98 ± 0.06 | 1.05 | 72.39 |
| Feedstock to Water Weight Ratio | C (%) | H (%) | O (%) | N (%) | S (%) | Atomic O/C Ratio | Atomic H/C Ratio |
|---|---|---|---|---|---|---|---|
| Feedstock | 52.77 ± 1.25 | 7.08 ± 0.12 | 38.14 ± 1.37 | 2.01 ± 0.01 | n.d.* | 0.54 | 1.61 |
| Hydrochar | |||||||
| 1:5 | 55.12 ± 0.01 | 7.23 ± 0.11 | 35.87 ± 0.42 | 1.78 ± 0.32 | n.d.* | 0.49 | 1.57 |
| 1:10 | 55.99 ± 0.81 | 7.30 ± 0.08 | 34.88 ± 1.01 | 1.83 ± 0.11 | n.d.* | 0.47 | 1.57 |
| 1:15 | 55.62 ± 0.40 | 7.39 ± 0.21 | 35.05 ± 0.41 | 1.93 ± 0.22 | n.d.* | 0.47 | 1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Gallant, R.; Salaudeen, S.; Farooque, A.A.; He, S. Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel. Sustainability 2022, 14, 8818. https://doi.org/10.3390/su14148818
Hu Y, Gallant R, Salaudeen S, Farooque AA, He S. Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel. Sustainability. 2022; 14(14):8818. https://doi.org/10.3390/su14148818
Chicago/Turabian StyleHu, Yulin, Rhea Gallant, Shakirudeen Salaudeen, Aitazaz A. Farooque, and Sophia He. 2022. "Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel" Sustainability 14, no. 14: 8818. https://doi.org/10.3390/su14148818
APA StyleHu, Y., Gallant, R., Salaudeen, S., Farooque, A. A., & He, S. (2022). Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel. Sustainability, 14(14), 8818. https://doi.org/10.3390/su14148818










