Abstract
Sustainable phosphorus (P) recovery from sewage sludge is crucial to reconciling the simultaneous shortage and excess of P. In this study, magnetic biochar (MBC) was synthesized and innovatively applied to enhance P recovery as vivianite. The effects of anaerobic digestion (AD) time, hydrothermal (HT) pretreatment temperature and MBC dose on vivianite formation were investigated using batch experiments and a modified sequential P extraction protocol. The P fractionation results showed that the concentration of pure vivianite-bound P (Fe(II)-P) reached a maximum on the 10th day of AD treatment, and then declined sharply due to vivianite oxidation and P limitation. HT pretreatment operated at relatively high temperatures (135 and 185 °C) reduced vivianite formation; this negative effect of HT pretreatment was partially compensated by MBC supplementation. The proportion of Fe(II)-P in the solid phase of sludge was substantially raised up to 57.1% from 8.3~17.4% with an increasing dose of MBC from 0 to 12.5 g/L, indicating that MBC had a markedly enhanced effect on vivianite formation; this could be attributed to the MBC-improved Fe(II) production, as evidenced by the elevated proportion of Fe(II) in Fe2p XPS spectra and the increased ratio of Fe(II)-P to oxidized vivianite-bound P (Fe(III)-P) in the sludge after MBC supplementation. MBC addition also decreased the proportion of water-extractable P by sorption and promoted organic P decomposition, which further facilitated vivianite production. These findings reveal a new strategy for enhancing P recovery from HT-pretreated AD sludge.
1. Introduction
To feed the Green Revolution, phosphorus (P) is extracted from phosphate rocks and then undergoes a largely one-way flow from farmland soil to surface and groundwater, which creates P shortage and aquatic eutrophication. Today, food could not be produced at current global levels if there is phosphorus deficiency in soils [1]. In addition, to prevent eutrophication in receiving waters, about 1.3 million tons of P are removed from municipal wastewater per year globally and concentrated in sewage sludge [2]. If P can be completely recovered from sewage sludge, it could meet 15–20% of the global P demand [3]; however, current sludge P recovery technologies, such as struvite precipitation, land application, and sludge incineration, are limited by low recovery efficiency, poor applicability, and/or high environmental risk [4]. Thus, there is still a need for efficient, economical, environment-friendly, and universal P recovery approaches from sewage sludge.
Recently, the ferrous Fe (Fe(II)) phosphate mineral vivianite (Fe3(PO4)2·8H2O) has been found in anaerobic digestion (AD) of sewage sludge [5]. It is probably the most thermodynamically stable phosphate form in the AD of sludge [6] with relatively simple forming conditions and predictable economic value as a raw material for phosphate fertilizer production and lithium battery synthesis [5,7]. The chemical formation mechanism of vivianite in sludge treatment is listed below:
Organic P→PO43−
Fe (OH)3 + 3H+ + e−→Fe2+ + 3H2O
3Fe2+ + 2PO43− + 8H2O→Fe3 (PO4)2•8H2O
In a reducing environment rich in organic matter (OM), Fe(III) is reduced to Fe(II) by dissimilatory Fe-reducing (DIR) bacteria, while organic P can be converted to phosphate [8,9], and then free Fe(II) combines with phosphate to form vivianite. At present, vivianite formation in the AD of sludge is hindered by the low molar ratio of Fe/P in sludge, the constrained availability of sludge P due to its association with organic matter (OM), and the limited Fe(III) reduction [8]. To promote the formation of vivianite, exogenous Fe can be added to the sludge treatment process. Furthermore, due to the difficulty in vivianite quantitation, little is known about the kinetics of vivianite formation during the AD process. As the AD proceeded, the oxidative and reductive potential (ORP) of the sludge became more negative [10], indicating that AD time likely has an influence on the formation of vivianite. Therefore, the most suitable AD time needs to be explored to obtain the maximum production of vivianite. On the other hand, AD with a prestage hydrothermal (HT) treatment (i.e., HT-AD) is an emerging strategy to improve sewage sludge biodegradability and dewatering rate [11]; however, HT pretreatment has a negative effect on vivianite formation. The abundance of vivianite considerably decreased from 55.8% in the AD-alone sludge to 51.0%, 39.1% and 16.0% in the AD sludge after HT pretreatment at 90, 135, and 185 °C, respectively [12]. Less formation of vivianite in HT-AD solids can be attributed to the inhibited Fe(III) reduction [12].
Moreover, the optimization of sludge treatment conditions to enhance vivianite production is strongly obstructed by the quantification of pure vivianite. Due to the replacement of Fe(II) by metal ions like Ca(II) or Mg(II) in vivianite and/or their partial oxidation during sample processing, the vivianite particles in sludge always contain impurities [13], which cannot be differentiated by Powder X-ray diffraction (XRD) and common sequential P. In addition, X-ray absorption spectroscopy (XAS) and Mössbauer require specialized instrumentation and are not available on demand to most researchers [14]. More recently, the quantification of pure vivianite-bound P (Fe(II)-P) as an independent fraction has been achieved by inserting an additional extraction step (0.2 wt% 2,2′-bipyridine + 0.1 M KCl, hereafter abbreviated as “Bipy solution”) to the conventional P extraction protocol. Bipy solution demonstrates a high selectivity towards Fe(II) dissolved from vivianite with a recovery rate of 89–100% [15]; this quantitative method can be utilized to examine the correlations of sludge treatment conditions (e.g., AD time) to vivianite production, which still remain largely unknown.
This study loaded Fe(III) on biochar (BC) to prepare magnetic carbon (MBC) material, which was added into the AD of sludge as an external Fe source. In addition, it has been revealed that biochar, containing redox-active components (e.g., aromatic carbon and quinone structures), can act as an electron shuttle to enhance the dissimilatory bioreduction of Fe(III) oxides [16] and the decomposition of organic compounds [17]; thus, MBC addition may have the ability to improve Fe(III) reduction and P release from the complex OM in sludge, which in turn promotes vivianite formation; moreover, MBC may increase the contact between phosphate and Fe(II) by adsorption, which may further enhance P recovery during AD of sludge. Specifically, by the combination of scanning electron microscopy (SEM), XRD, X-ray photoelectron spectroscopy (XPS), and the modified P sequential extraction protocol that can quantify vivianite-bound P as an independent fraction, this study aims (1) to explore the effect of AD time and HT pretreatment temperature on vivianite production; (2) to study the effect of MBC addition on P transformation in the HT-pretreated AD sludge; and (3) to reveal the mechanism and pathway of P conversion in the HT-AD sludge with MBC supplementation.
2. Materials and Methods
2.1. Chemicals and Solutions
Ascorbic acid (≥99%) and 2-2′-bipyridine (99%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Potassium antimony tartrate (C8H4K2O12Sb2, 99%), ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, 99%), and sodium hydroxide (NaOH) were purchased from Tianjin Fu Chen Chemical Reagent Company. Concentrated sulfuric acid (H2SO4), concentrated nitric acid (HNO3), hydrochloric acid (HCl (aq)), and hydrogen carbonate (NaHCO3) were all purchased from Tianjin Deng Feng Chemical Reagent Factory. The water used in the laboratory was deionized water (the resistivity is 18.25 mΩ/cm at 25 °C).
2.2. Preparation of MBC and BC
In this study, the rice straw used was collected from the countryside of Jining, Shandong Province, China. Using the chemical co-precipitation method [18], 50 g of the rice straw was added to a 1000 mL solution containing ferrous sulfate and ferric chloride (molar ratio = 1:1). Under intense magnetic stirring, 4 mol/L NaOH was dropped into the mixture until pH = 10. After the mixture was stirred for 0.5 h, it was centrifuged at 3000 rpm for 10 min. The solid in the lower layer after centrifugation was heated in the muffle furnace at 100 °C for 2 h. Next, the pyrolysis temperature was raised to 300 °C at a rate of 10 °C/min and maintained for 4 h. The obtained solid was ground to powder and passed through a 100 mesh sieve for standby. The final product is Fe3O4-loaded biochar (MBC) as evidenced below (Section 3.1). In addition, the rice straw was placed in the muffle furnace to produce pure BC, and the operation method was the same as that for preparing MBC. Detailed information regarding the characterization and phosphate sorption of BC and MBC are provided in the Supplementary Information.
2.3. The HT-AD Experiments
The AD process often adopts the mode of mixed co-digestion of raw sludge and inoculated digestion sludge. The primary sludge used in this experiment was the actual municipal sewage sludge, which was collected from Beijing Xiaojiahe WWTP. The sewage treatment adopted the modified A2/O process. The inoculated digested sludge was taken from the Environmental Laboratory of Beijing Forestry University.
In order to explore the effect of HT temperature on the conversion of Fe and P in the subsequent AD processes, HT pretreatment was carried out at three temperatures (i.e., 90, 135, and 185 °C) in this study. After adding to an 80-mL hydrothermal reactor, the raw sludge mixture was pretreated under HT conditions at each temperature. The reactor was then sealed and heated in an oven at the target temperature for 4 h [12]. Subsequent AD processes were carried out in 500 mL glass reactors. Specifically, 400 mL of a mixture of HT-treated sewage sludge slurries and inoculated digested sludge (V:V = 8:2) were added to the AD reactor [19]. Next, based on the elemental compositions (Fe and P) in MBC and the sludge samples (Tables S1 and S2), calculated amounts (0, 2.5, 6.25, or 12.5 g/L) of MBC were weighed into the reactor to achieve a final molar Fe:P ratio of 0.62, 0.92, 1.35, or 2.07 (Table S3). It is reported that higher molar Fe:P ratios (Fe:P = 1.5~3) favoured the production of vivianite in sludge [20]. Before the AD process, the initial pH of the mixture was adjusted to 7.2, and nitrogen (N2) was fed into the system for 40 min to ensure an anaerobic environment. After being capped with rubber stoppers with a hole sealed with parafilm for subsequent sampling, the AD reactors were placed in a shaking incubator (ZHPL-200, China) and anaerobically digested for 15 days at a constant temperature of 38 °C and a shaking speed of 220 rpm. On the 2nd, 5th, 10th, and 15th day of the AD process, 0.5 mL of sludge mixture was extracted from the reactor with a syringe for P extraction without further drying to avoid the oxidation of vivianite. All HT-AD experiments were performed in duplicate. The processing conditions and sample labels are shown in Table 1. Detailed information regarding the characterization of the HT-AD sludge is provided in the Supplementary Information.

Table 1.
Conditions and sample labels for HT-AD-treated samples.
2.4. Sequential Chemical Extraction of Phosphorus
The fresh sludge sampled from the AD reactors was immediately used for P extraction with no further treatment to minimise the oxidation of vivianite. A modified sequential chemical extraction protocol of P with an additional extraction step (Bipy solution) for vivianite-bound P quantification [14,15] was applied to analyze the formation of vivianite in sludge and evaluate the phase distribution of P. Briefly, P was extracted successively using H2O, Bipy solution, NH4F, NaOH, Na3C3H6O7 + Na2S2O4 + NaHCO3, HCl, and HCl+ HNO3. The extracted P fractions were exchangeable P (Loosely-P), pure vivianite (Fe(II)-P), Al-P, oxidized vivianite (Fe(III)-P), reductant-soluble P (Reductant-P), Ca-P, and Organic-P, respectively. The method of extracting specific P components in the modified sequential chemical extraction protocol is listed in Table S4. The centrifuged supernatants extracted in each step were passed through a 0.45 μm membrane and the P concentration was determined by the molybdenum blue method. The samples were left at room temperature for 15 min and absorbance was measured at 700 nm by using an ultraviolet-visible (UV-VIS) spectrophotometer (10-mm optical path) [21].
During the extraction of vivianite-bound P, [Fe(Bipy)3]2+ complex with a marked red colour will form, which would critically obstruct the quantitative analysis of vivianite-bound P. To remove the red colour of the Bipy-extract for subsequent determination, sulfuric acid (H2SO4) was added to the extraction solution and the reaction lasted for 12 h to fully degrade the [Fe(Bipy)3]2+ complex [14]. When extracting Al-P, 3 mL of 0.8 mol/L H3BO3 was added before colour development to eliminate the reduction interference of NH4F on phosphomolybdic heteropoly acids [22].
2.5. Statistical Analyses
One-way analysis of variance (ANOVA) was used to analyze the results obtained from different treatments undertaken in the current study (anaerobic digestion (AD) time, hydrothermal (HT) pretreatment temperature, and MBC dose). Statistical analysis was applied with the use of IBM SPSS software (version 24.0).
3. Results and Discussion
3.1. Properties of BC and MBC
The content of Fe increased remarkably from 0.726% in the pure rice straw BC to 8.359% in the Fe-loaded MBC, while the P content slightly decreased from 0.084% to 0.063% (Table S1). Based on the P content in MBC, we can calculate that up to 3.19 mg of MBC-derived P would be introduced into the AD system at the highest dose level of MBC (i.e., MBC = 12.5 g/L). Compared with the high total P content of 155.77 mg in the sludge sample (original sewage sludge + digested sludge) used in the HT-AD experiment (Table S2), the MBC-derived P was almost negligible. Therefore, MBC-derived P is expected to exhibit an insignificant effect on the solubility and phase transformation of P in the sludge mixture during the AD process and thus was not taken into consideration in this study. In addition, the surface area, total pore volume, and average pore diameter of MBC were 44.75 m2/g, 0.1494 cm3/g, and 13.358 nm, respectively, which were significantly larger than those of BC (i.e., 13.07 m2/g, 0.0219 cm3/g, and 6.719 nm, respectively) (Table S1). The increase in surface area and pore volume have also been found after the introduction of Fe to sawdust biochar [23]. The iron oxide formed during pyrolysis and its surface structure may increase the effective biochar adsorption area. MBC with a large surface area and abundant pores may have a better adsorption capacity for phosphate [20] and provide more growth sites for microbes (e.g., Fe(III)-reducing bacteria), both of which would facilitate the combination of phosphate with Fe(II) to form vivianite through chemical precipitation.
FTIR, XRD, and XPS analyses of BC and MBC were carried out to verify the successful loading of Fe(III) on MBC. The FTIR spectra of MBC and BC from 400 cm−1 to 4000 cm−1 in the near-infrared region are presented in Figure S1a. Upon chemical coprecipitation treatment, sharp adsorption bands at 613 cm−1 and 880 cm−1 were newly added in the MBC spectrum, which could be assigned to Fe-O and Fe-OH vibration peaks [24], respectively, indicating the successful loading of Fe(III) on biochar. The peaks at 1122 cm−1 ascribed to C-O ether stretching vibration weakened in MBC spectra, suggesting the participation of C-O in Fe(III) loading to biochar. Fe-O and Fe-OH were found to be responsible for phosphate sorption by Fe(III)-loaded biochars [23]. Another interesting finding is that the aromatic C=O and C=C stretching vibrations (~1600 cm−1) were enhanced after modification; this may be attributed to the presence of Fe minerals that have the ability to catalyze condensation and polymerization reactions during biomass pyrolysis [25]. It has been documented that the quinoid C=O and condensed aromatic structures (i.e., conjugated π-electron systems) of biochars are major contributors to the redox properties [26]. Therefore, phosphate sorption and Fe(III) reduction in the HT-AD sludge are expected to be strongly impacted by MBC supplementation.
The XRD patterns of BC and MBC show that MBC had seven main diffraction peaks at 30.09°, 35.42°, 43.05°, 56.94°, 53.39°, 62.51° and 73.95°, which could be indexed to Fe3O4 cubic crystal (Figure S1b). The XRD result clearly confirmed the successful generation of magnetic biochar. In addition, the reflections from KCl and NaCl observed in the XRD spectra of BC and MBC were due to the high contents of inorganic elements in the raw material, which were transformed into crystalline compounds during pyrolysis.
As the XPS spectra of Fe and O for MBC shows (Figure S2), the Fe2p spectrum exhibited two contributions, i.e., Fe2+ at 710.1 (27.27%) and Fe3+ (53.54%) at 712 eV [27]. The ratio of Fe2+ to Fe3+ was 0.27:0.54, which was comparable to that in Fe3O4 (0.33:0.67) [28], suggesting the loading of Fe3O4 on MBC. During the pyrolysis process, FeCl3 was decomposed into FeCl2 and Cl (g) [29]. FeCl3 and the produced FeCl2 were further heated to produce Fe3O4 and FeO. The spectrum of O1s could be best-fitted with three separate peaks at 530.12, 531.5 and 532.8 eV, which could be attributed to Fe−O (9.27%), Fe−OH/−C=O− (65.27%), and −C−O−/H2O (25.46%), respectively [30]. The O−containing functional group is beneficial to the adsorption of organic substances, while Fe oxides can concentrate Fe(III)-reducing microbes that are involved in the decomposition of OM through DIR [17,31]. On the other hand, as high as 25% of all phosphate in sewage sludge was reported to exist in the form of organics [32], remarkably limiting the formation of vivianite. It is therefore expected that the introduction of MBC may promote the breakdown of the organics in sludge and lead to the release of free PO43−, which may contribute to vivianite formation.
3.2. Characterization of the HT-AD Sludge Samples
3.2.1. SEM
Figure 1 shows the SEM images of M2T1, M3T1 and M4T1 sampled on the 10th day of the AD process. SEM micrographs showed that MBC had a bumpy surface owing to the aggregation of carbon structures, induced by the binding effect of Fe [33]. The enlarged images in the white circles (Figure 1a,b) clearly showed that the morphology of vivianite was sheet crystal with spherical or irregular nodular, which was consistent with the vivianite morphology reported in the literature [14]. According to SEM-EDS elemental mapping of M4T1 (Figure S3), it could be observed that flower-shaped vivianite crystals appeared in the zones with high contents of Fe and P. Vivianite was generated as evidenced by the dominated Fe and P elements within the same zones with a strong overlapped area of Fe and P. With the increased dosage of MBC, lamellar crystals increased considerably and became more densely distributed. As aforementioned, MBC in an AD reactor can act as a carrier to provide growth sites for DIR bacteria and binding sites for Fe/P, which in turn promote the formation of vivianite. Notably, larger gaps between the particles and an enhanced degree of porous agglomeration occurred in the digested sludge amended with a higher amount of MBC. With the reduction of MBC-derived Fe3O4 by DIR bacteria, part of the sludge OM was decomposed under the action of the magnetic field, which made the sludge granulated [34].
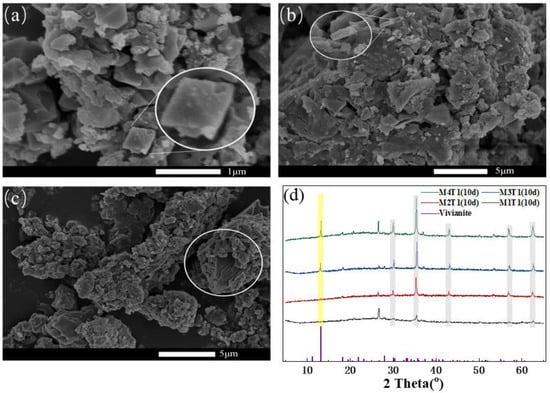
Figure 1.
SEM analysis of the HT-AD sludge (10 d) with 2.5 g/L (a), 6.25 g/L (b), and 12.5 g/L (c) of MBC addition; XRD patterns of M1T1, M2T1, M3T1, and M4T1 (10 d) (d). Gray and yellow vertical bars indicate XRD peak positions for magnetite (PDF No.19-0629) and vivianite (PDF no.30-0662), respectively.
3.2.2. XRD
The mineralogy of the HT-AD sludge samples was determined by XRD analysis. The distinct reflection at 2θ = 13.1° can be well indexed to vivianite (PDF no.30-0662), while the five obvious reflections at 2θ = 30.1°, 35.4°, 43.1°, 57.0°, and 62.5° are characteristics of magnetite (Fe3O4, PDF no.19-0629) (Figure 1d). With the increased dose of MBC, the diffraction peaks of vivianite were sharper and more intense as expected, indicating that more vivianite with a highly crystalline nature was generated. For M1T1 and M2T1 samples, no distinct characteristic diffraction peaks of vivianite were observed; this result indicated that less vivianite was produced in M1T1 and M2T1 samples, likely due to their low Fe:P ratios (0.62 for M1T1 and 0.92 for M2T1). In addition, the vivianite crystallization would be more dispersed and unstable in M1T1 and M2T1. As a result, the formed vivianite can be easily and quickly oxidized before XRD spectra were obtained. It was reported that vivianite contained impurities that were nearly completely oxidized within 48 h after exposure to air [35]. The characteristic peak of Fe3O4 in M1T1 without MBC addition was derived from the reduction of FeOOH during the HT process of sludge [36], while that in other samples mainly came from Fe3O4 on MBC. The existence of Fe3O4 clearly indicated that the incomplete reduction of Fe(III) occurred under anaerobic conditions for the sludge samples. Similar results have been found in a previous study [19]. The Fe3O4 presented in the sludge may adsorb phosphate by Equations (4) and (5), which may partly explain the incomplete Fe(III) reduction.
3.2.3. XPS
To further explain the mechanism of vivianite precipitation induced by Fe(III) reduction, XPS analysis was performed on the M1T1, M2T1, M3T1, and M4T1 samples. The XPS spectra of P and Fe are illustrated in Figure 2, and the specific fitting parameters are listed in Table S5. There were three peaks in the P2p fine spectrum (Figure 2a), namely O=P=O (134 eV), O-P=O (133.8 eV), and P-OH (132.66 eV) single peaks. By integrating the peak area of the M1T1 sample, the constitutive ratios of P-OH, O-P=O, and O=P=O reached 52.39%, 37.50%, and 10.11%, respectively. With the increased content of MBC, the relative proportion of O=P=O gradually decreased to close to 0 (M4T1), while those of P-OH and O-P=O continuously increased, implying the conversion of organophosphorus to orthophosphate. Microorganisms attached to MBC grew and multiplied to form abundant embryonic particles. MBC-derived Fe3+ could neutralize the negative charge on the surface of microorganisms, reduce their electrostatic repulsion, and combine with extracellular polymer (EPS) to act as the growth skeleton of microorganisms [34,37]. Along with the enrichment of microorganisms, the efficiency of microbial decomposition of OM in the sludge system was improved, and part of organic P was converted to inorganic forms with the breakdown of O=P=O.
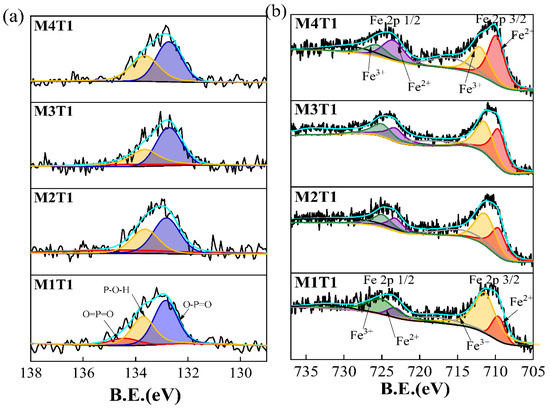
Figure 2.
P2p (a) and Fe2p (b) XPS spectra of M1T1, M2T1, M3T1, and M4T1.
In order to determine the chemical bonding and valence state of Fe in the HT-AD sludge, the Fe2p spectra of M1T1, M2T1, M3T1, and M4T1 were analyzed (Figure 2b). The Fe2p spectrum clearly evidences that Fe atoms have two chemical environments (i.e., Fe2p 3/2 and Fe2p 1/2). The spectrum was fitted by considering two resolved doublets (with a spin-orbit splitting of~13.6 eV between 2p3/2 and 2p1/2) [28]. The peaks at 709.6 eV and 711.3 eV were represented as Fe2+ and Fe3+ in the Fe2p 3/2 fine map, respectively. With the increased dose of MBC from 0 to 12.5 g/L, the proportion of Fe2+ increased continuously from 16.38% to 44.76%, while that of Fe3+ decreased from 64.80% to 31.48%. These results clearly verified that MBC could effectively promote the reduction of Fe(III) to Fe(II) in the HT-AD sludge, so as to promote the combination of Fe(II) and free phosphate ions to form vivianite, consistent with the results of SEM and XRD.
3.3. P Classification Extraction
The quantification of vivianite was achieved by a modified P extraction method proposed by Gu et al. [15] and Wang et al. [14], which could separate pure vivianite-bound-P (Fe(II)-P) from the oxidized fraction of vivianite (Fe(III)-P) by inserting a Bipy-extraction step targeting ideal vivianite-bound P. It was found that vivianite was detected in the raw sludge by powder XRD analysis but was not observed after Bipy-extraction, suggesting that the Bipy step could effectively and completely extract vivianite [14]. Therefore, using the modified P extraction method, we can quantify vivianite formed in the HT-AD sludge as well as examine the correlations between vivianite production and the HT-AD processing conditions (e.g., digestion time in AD, HT temperature, and MBC addition), facilitating optimized vivianite production. Additionally, the relative abundance of each P species measured by the modified P extraction method (Table S6) will provide information on the P transformation mechanism during the AD process.
3.3.1. Effect of Digestion Time in AD
The existence of vivianite in the AD process of sludge has been demonstrated in a number of studies [5,38]. Little is known, however, about the kinetics of vivianite formation due to the difficulty in vivianite quantification. In this study, the modified sequential extraction method was used to examine the effect of digestion time on the formation and oxidation of vivianite by fractionizing the sludge sampled at different intervals within 15 days of AD, and the result is shown in Figure 3a. The vivianite formation in M1T1 (without MBC supplementation) demonstrated little change during the AD process. In contrast, the content of pure vivianite-bound P (Fe(II)-P) in the MBC-added sludge rose significantly from the 2nd (4.56~8.61 mg/g) to the 10th (6.49~13.18 mg/g) day of digestion. After that, contrary to expectation, a substantial reduction in the content of pure vivianite was observed, which could be attributed to the oxidation of vivianite as evidenced by the fact that the concentrations of oxidized vivianite (Fe(III)-P) in the MBC-added samples were remarkably elevated from the 10th (3.55~5.24 mg/g) to 15th (5.21~7.02 mg/g) day of digestion. It has previously been reported that as the AD proceeded, the ORP of the sludge became more negative in the first 4–6 days of digestion, and then the trend reversed and the potential increased and reached a plateau after 10 days of digestion [10]. In the meantime, the growth of the anaerobic consortium reached a stable level on the 10th day of AD [10]. Both the less reductive environment and the stagnant microbial growth would induce less vivianite formation. The further production of vivianite may be also limited by phosphate availability since it is unlikely that all phosphate is associated with vivianite. Phosphate may be presented in various forms via adsorption, coagulation, biological uptake, and co-precipitation with metals like Ca, Al, and Mg (Figure 3b), which are unavailable for Fe(II) complexation. Our research extends the knowledge into the kinetics of vivianite formation in the AD of sludge with HT pretreatment. As far as we know, the formation of vivianite in sludge reached a maximum just a few days after AD has not been reported before. Previous studies usually examined the production of vivianite in sludge after anaerobic incubation for a long time ranging from 20 to 46 days [5,39]. According to the results of this study, a short-term anaerobic incubation for a few days is sufficient if the objective is P recovery as vivianite from HT-AD sludge. It must also be mentioned that long-term anaerobic incubation may facilitate the growth of vivianite crystal to a larger size, which would be beneficial for the separation of vivianite from sludge flocs [40].
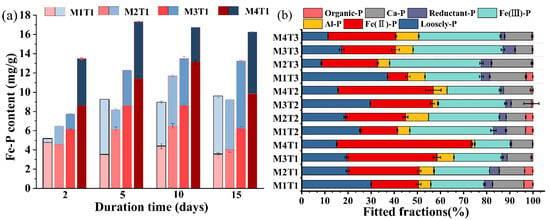
Figure 3.
The content of Fe-P in the AD sludge after HT pretreatment at 90 °C (Fe(II)-P is pure vivianite-bound P (red series), Fe(III)-P is oxidized vivianite-bound P (blue series), and Fe-P is total vivianite (Fe(II)-P + Fe(III)-P)) (a). The relative percentage of P species is determined by the modified P extraction protocol (b).
P release during the AD process is an important concern, so the concentration of dissolved P in the supernatant during the AD process was also analyzed (Figure S4). The release of soluble phosphate peaked on day 5 of AD, independent of MBC addition. The lysis of microbial cells such as DIR/sulfur-reducing bacteria and the release of P metabolized by P accumulating bacteria lead to the increase of P concentration in the supernatant [41]. After that, the content of soluble phosphate decreased and reached its lowest value on day 10, which can be attributed to the combination of phosphate with free metal ions, such as Fe2+, Ca2+, Al3+, etc. to form phosphate precipitates. On day 15, soluble phosphate slightly increased, suggesting that phosphates were partly decomposed into HT-AD solution owing to their unstable properties.
3.3.2. Effect of HT Temperature
As mentioned above, the amount of pure vivianite reached its highest value on the 10th day of AD, so the subsequent fractional extraction was executed on the sludge sampled on the 10th day. The relative abundance of contributing P species is clearly illustrated in Figure 3b, and the P concentrations are listed in Table S6. At a specific dose of MBC, the relative abundance of Fe(II)-P decreased while that of Fe(III)-P increased with the increase of HT temperature. Therefore, HT operated at relatively low temperatures was conducive to Fe(III) reduction and vivianite formation, which was consistent with the conclusion drawn by Wang et al. [12]. During HT, cellulose is hydrolyzed to produce glucose. The further hydrolysis of glucose will produce active furfural derivatives (e.g., 5-hydroxymethylfurfural (5-HMF)), which can reduce Fe(III) to Fe(II) [42]. At high HT temperature, these intermediates can further form hydrochar-like materials with low reduction ability for Fe(III) [38]. As a result, less vivianite was formed in the AD solids after HT pretreatment at 135 and 185 °C than that at 90 °C. It is noteworthy that in the HT-pretreated AD sludge at 135 and 185 °C, MBC addition significantly increased the relative abundance of total vivianite from 39.35% to 53.28% and from 31.45% to 50.24%, respectively, much higher than that in M1T1. These results highlighted that MBC supplementation can compensate for the negative effect of HT pretreatment at high temperatures on vivianite formation in the subsequent AD process.
3.3.3. Effect of MBC Addition
Figure 4 illustrates the effect of MBC addition on vivianite formation in the sludge that has been anaerobically digested for 10 days. We can see that Fe(II)-P only occupied 8.3~17.4% of total P in the sludge without MBC supplementation. With an increasing dose of MBC at a specific HT temperature, the proportions of the pure and total vivianite-bound P (i.e., Fe(II)-P + Fe(III)-P) were greatly elevated. Specifically, under the addition of 12.5 g/L of MBC (molar Fe:P = 2.07), the relative abundance of pure and total vivianite-bound P reached their peak values of 57.1% and 72.11%, respectively, in M4T1 (Figures S3b and S5a). These results were consistent with a previous finding [13] that an apparent increase of P bound in vivianite was achieved by adding Fe into the sludge during the AD process. As aforementioned, the Fe(III) on MBC can be reduced to Fe(II) by DIR in an anaerobic environment and then combine with phosphate to form vivianite crystals. With the increase in MBC dosage, the Fe:P ratio of the sludge also increased and reached as high as 2.07 at 12.5 g/L of MBC addition (Table S3), higher than the stoichiometry of vivianite (1.5). Overdose of Fe is necessary for increasing P recovery efficiency because the Fe present in sludge is partly unavailable for vivianite formation. Fe(II) in sludge may combine with the hydroxyl groups of the organic substances to form Fe(OH)2, react with sulphide to form FeSx, or be oxidized to Fe(III) again [13,43]. The vivianite content in this study was comparable to the result of Wang et al. [14] that 52% of total P was bound in vivianite (Bipy-P) in the digested sludge collected from a concentration tank. Wilfert et al. [13] obtained a higher proportion (70–90%) of vivianite in sludge likely because they used a higher molar Fe:P ratio (2.5); moreover, they may over quantify vivianite bound phosphate by using XRD and Mössbauer spectroscopy, given that the impurities existed in vivianite and the partial oxidation of vivianite during sample handling cannot be distinguished by these methods [13,14].
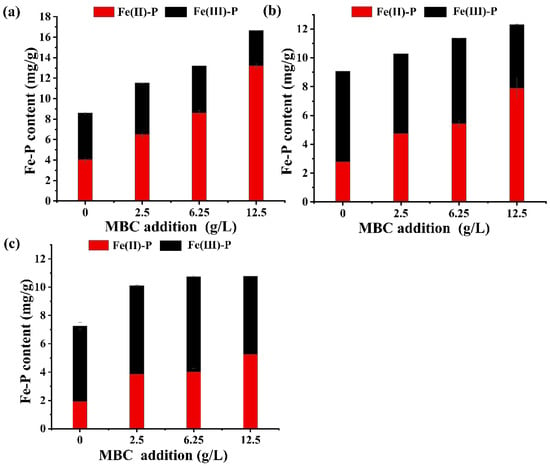
Figure 4.
The content of Fe(II)-P and Fe(III)-P in the AD sludge after HT pretreatment at 90 (a), 135 (b), and 185 °C (c).
Contrary to Fe(II)-P, the relative abundance of Fe(III)-P obviously decreased from 19.74~27.25% to 15.01~19.08% with an increasing dose of MBC from 0 to 12.5 g/L. To examine the formation/transformation of Fe(II)-P and Fe(III)-P in detail, the ratio of Fe(II)-P to Fe(III)-P was calculated (Table S6). It was found that the ratio of Fe(II)-P to Fe(III)-P was substantially elevated from 0.88 in M1T1 to 1.28, 1.87, and 3.80 in M2T1, M3T1, and M4T1, respectively; moreover, as shown in Figure 3a, from day 2 to day 15, Fe(III)-P content of M1T1 (without MBC addition) was increased by 13.4 times, while that of M4T1 (at a dose of 12.5 g/L of MBC) was only increased by 0.3 times. These results clearly demonstrated that more Fe(III)-P was converted to Fe(II)-P and/or the re-oxidation of vivianite to Fe(III)-P was inhibited with MBC addition. A possible explanation is that MBC may enhance the growth of DIR bacteria and facilitate the microbial reduction of Fe(III) through electron shuttling [44]. The magnetic field, loaded Fe3O4 particles, and porous structure of MBC were beneficial for the enriching of Fe(III)-reducing microorganisms on its surface; moreover, with abundant quinone moieties, pyrogenic materials (MBC in this study) are well known for their critical roles in mediating the electron transport between bacterial and Fe(III) oxides [44].
The added MBC can also affect the transformation of other P forms. As Figure 3b shows, for the sludge without MBC addition, 25.6~35.3% of P was water extractable (Loosely-P). The addition of MBC dramatically decreased the relative abundance of Loosely-P to as low as 8.9~14.8%. The promotion effect of MBC on the transformation of exchangeable P to less soluble forms can also be evidenced by the alteration in P concentration in the liquid phase of AD sludge. As depicted in Figure S4, on the 10th day of AD, with the increased dose of MBC, concentrations of dissolved P in the supernatant decreased from 0.32 (M1T1) to 0.17 (M2T1), 0.16 (M3T1), and 0.11 (M4T1) mg/g. MBC facilitated the transportation of phosphate from the liquid digestate to the solid phase mainly via the following mechanisms: (1) the effective sorption of phosphate from aqueous solution to MBC; and (2) providing Fe source for the formation of phosphate precipitation, mainly in the form of vivianite as analyzed above. The maximum adsorption amount of P by MBC was 9.5 mg-P/g (Figure S5b), higher than that of the reported magnetic iron oxide nanoparticles (5.03 mg-P/g) [45]. The enhanced P adsorption by MBC would promote the combination of P and Fe on its surface.
Organic-P is also an inhibitory factor for vivianite formation. The combination of P and OM will reduce its combination with Fe(II). As shown in Figure 3b, the increase of MBC addition led to a sharp decrease in the relative abundance of Organic-P, which was almost negligible in M4T1. The XPS analysis of P (Figure 2a) showed that O=P=O was converted to P-OH, indicating that organic P was converted to inorganic orthophosphate in the case of MBC addition. The reducing microorganisms, adsorbed on the surface of MBC, participated in the decomposition of complex OM through the DIR method [17], which promoted the transformation of organic P to inorganic form. In addition, as inhibiting factors of vivianite formation, Al3+ and Ca2+ could compete with Fe2+ for PO43− to inhibit vivianite formation [46]. In this study, the formation of Al-P and Ca-P was basically not affected by MBC addition, suggesting that the vivianite formed in the MBC-amended sludge was unlikely derived from the transformation of Al-P and Ca-P; moreover, the enhanced vivianite formation caused by MBC supplementation may account for the decrease of amorphous Fe-associated P (Reductant-P).
Based on above analysis, we can conclude that MBC played multiple roles in the formation of vivianite in the HT-AD sludge: (1) The Fe(III) contained in MBC can be reduced to Fe(II) during the AD process, which may further combine with favorable phosphates to form vivianite [8]; (2) MBC can promote Fe(III) reduction by increasing the biomass of Fe(III) reducing microorganisms (such as DIR bacteria); (3) MBC produced from the pyrolysis of rice straw is expected to contain abundant aromatic functional groups and quinone moieties [44]; this unique structural feature enables MBC to facilitate electron transport during redox reactions as an electron shuttle between bacteria and Fe(III) minerals, promoting Fe(III) reduction and inhibiting the re-oxidation of vivianite to Fe(III)-P [44,47]; (4) The adsorption of phosphate by MBC provides more opportunities for the combination of Fe(II) and P; (5) MBC promotes the conversion of organic P to inorganic orthophosphate via enhancing the decomposition of complex OM through DIR [17].
4. Conclusions
This study demonstrated the enhanced effect of the environmentally friendly synthetic MBC on vivianite formation in the HT-AD sewage sludge using a modified P fractionation method for vivianite-bound P quantification. The concentration of pure vivianite reached its peak value on the 10th day of AD. Characterization and P fractionation indicated that MBC addition remarkably promoted vivianite formation and inhibited its re-oxidation during AD of sludge. HT pretreatment had an adverse effect on vivianite formation in the subsequent AD process, which, however, was compensated by MBC addition; this study has important implications for P recovery from the AD of sludge after HT pretreatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14148690/s1, Figure S1. FTIR spectra (a) and XRD intensity patterns (b) for BC and MBC. Gray, blue and yellow vertical bars indicate XRD peak positions for NaCl (PDF no. 99-0059), quartz (PDF no. 46-1045), and KCl (PDF no. 99-0101), respectively. Figure S2. O 1s (a) and Fe 2p (b) XPS spectra of MBC. Figure S3. EDS-mapping of M4T1 sample on the 10th day of AD. Figure S4. The concentration of Loosely-P during AD of M1T1 (a), M2T1 (b), M3T1 (c), and M4T1 (d). Figure S5. Three-dimensional diagram of pure vivianite formation (i.e., Fe (II)-P) (a). Comparison of adsorption capacities of BC and MBC (b). Table S1. Elemental compositions (Fe and P), BET specific surface area (SA), total pore volume (TPV), and average pore diameter (APD) of the rice straw biochar (BC) and magnetic biochar (MBC). Table S2. Sample information and elemental analysis (based on fresh weight) of the raw sewage and digested sludge samples. Table S3. Molar ratio and mass ratio of Fe and P in the AD sludge with different dosages of MBC. Table S4. Operational speciation of P in each step of the modified P extraction protocol. Table S5. The chemical compositions of Fe and P for M1T1, M2T1, M3T1, and M4T1 analyzed by XPS. Table S6. Phosphorus distribution in the sludge after AD for 10 days and the ratio of Fe(Ⅱ)-P to Fe(Ⅲ)-P. E-supplementary data for this work can be found in the online version of the paper [48].
Author Contributions
Y.L.: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Investigation, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Project administration, Funding acquisition. J.J.: Resources, Investigation, Writing—Review and Editing, Data Curation. J.L.: Software, Investigation, Supervision. Z.Z.: Software, Validation, Formal analysis. R.L.: Resources, Investigation, Data Curation. J.S.: Supervision, Resources, Visualization. J.X.: Supervision, Resources, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 42177204, 41703097 and U2067215.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Van Drecht, G.; Bouwman, A.; Harrison, J.; Knoop, J. Global nitrogen and phosphate in urban wastewater for the period 1970 to 2050. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, D.; Li, J.; Guo, G.; Tang, S. Phosphate recovery from swine wastewater using plant ash in chemical crystallization. J. Clean. Prod. 2017, 168, 338–345. [Google Scholar] [CrossRef]
- Wilfert, P.; Mandalidis, A.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Temmink, H.; Witkamp, G.J.; Van Loosdrecht, M.C.M. Vivianite as an important iron phosphate precipitate in sewage treatment plants. Water Res. 2016, 104, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Nriagu, J.O. Stability of vivianite and ion-pair formation in the system fe3 (PO4)2-H3PO4H3PO4-H2O. Geochim. Cosmochim. Acta 1972, 36, 459–470. [Google Scholar] [CrossRef]
- Jowett, C.; Solntseva, I.; Wu, L.; James, C.; Glasauer, S. Removal of sewage phosphorus by adsorption and mineral precipitation, with recovery as a fertilizing soil amendment. Water Sci. Technol. 2018, 77, 1967–1978. [Google Scholar] [CrossRef]
- Rothe, M.; Kleeberg, A.; Hupfer, M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth Sci. Rev. 2016, 158, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Phillips, E.J.; Lonergan, D.J. Enzymic versus nonenzymic mechanisms for iron (III) reduction in aquatic sediments. Environ. Sci. Technol. 1991, 25, 1062–1067. [Google Scholar] [CrossRef]
- Chu, C.; Lee, D.; Chang, B.-V.; You, C.; Liao, C.; Tay, J. Anaerobic digestion of polyelectrolyte flocculated waste activated sludge. Chemosphere 2003, 53, 757–764. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Li, J.; Li, Z.; Yu, G.; Wang, Y. A process combining hydrothermal pretreatment, anaerobic digestion and pyrolysis for sewage sludge dewatering and co-production of biogas and biochar: Pilot-scale verification. Bioresour. Technol. 2018, 254, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Patel, D.; Jung, H.; Liu, P.; Wan, B.; Pavlostathis, S.G.; Tang, Y. Coevolution of iron, phosphorus, and sulfur speciation during anaerobic digestion with hydrothermal pretreatment of sewage sludge. Environ. Sci. Technol. 2020, 54, 8362–8372. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, P.; Dugulan, A.; Goubitz, K.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kim, T.-H.; Reitzel, K.; Almind-Jørgensen, N.; Nielsen, U.G. Quantitative determination of vivianite in sewage sludge by a phosphate extraction protocol validated by PXRD, SEM-EDS, and 31P NMR spectroscopy towards efficient vivianite recovery. Water Res. 2021, 202, 117411. [Google Scholar] [CrossRef]
- Gu, S.; Qian, Y.; Jiao, Y.; Li, Q.; Pinay, G.; Gruau, G. An innovative approach for sequential extraction of phosphorus in sediments: Ferrous iron P as an independent P fraction. Water Res. 2016, 103, 352–361. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi) conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef]
- Saleh, S.; Kamarudin, K.B.; Ghani, W.A.W.A.K.; Kheang, L.S. Removal of organic contaminant from aqueous solution using magnetic biochar. Procedia Eng. 2016, 148, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhao, Z.; Chen, N.; Feng, C.; Lei, Z.; Zhang, Z. Insight into efficient phosphorus removal/recovery from enhanced methane production of waste activated sludge with Chitosan-Fe supplementation. Water Res. 2020, 187, 116427. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Xie, C.; Xu, J.; Tang, J.; Baig, S.A.; Xu, X. Comparison of phosphorus determination methods by ion chromatography and molybdenum blue methods. Commun. Soil Sci. Plant Anal. 2013, 44, 2535–2545. [Google Scholar] [CrossRef]
- Williams, J.; Syers, J.; Harris, R.; Armstrong, D. Fractionation of inorganic phosphate in calcareous lake sediments. Soil Sci. Soc. Am. J. 1971, 35, 250–255. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, Y.; Kasiulienė, A.; Huang, C.; Ai, P. Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol. Environ. Policy 2017, 19, 761–774. [Google Scholar] [CrossRef]
- Rawal, A.; Joseph, S.D.; Hook, J.M.; Chia, C.H.; Munroe, P.R.; Donne, S.; Lin, Y.; Phelan, D.; Mitchell, D.R.; Pace, B. Mineral–biochar composites: Molecular structure and porosity. Environ. Sci. Technol. 2016, 50, 7706–7714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, X.; Cao, L.; Ok, Y.S.; Cao, X. Characterization and quantification of electron donating capacity and its structure dependence in biochar derived from three waste biomasses. Chemosphere 2018, 211, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, J.; Wang, Y.; Liu, Y.; Cai, C.; Davarpanah, A. Study on the Removal Efficiency and Mechanism of Tetracycline in Water Using Biochar and Magnetic Biochar. Coatings 2021, 11, 1354. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Larson, R.A.; Weber, E.J. Reaction Mechanisms in Environmental Organic Chemistry; Routledge: London, UK, 2018. [Google Scholar]
- Zhou, K.; Wu, B.; Su, L.; Xin, W.; Chai, X. Enhanced phosphate removal using nanostructured hydrated ferric-zirconium binary oxide confined in a polymeric anion exchanger. Chem. Eng. J. 2018, 345, 640–647. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.J.; Cha, I.T.; Min, D.; Kim, J.S.; Chung, W.H.; Chae, J.C.; Jeon, C.O.; Rhee, S.K. Metabolic versatility of toluene-degrading, iron-reducing bacteria in tidal flat sediment, characterized by stable isotope probing-based metagenomic analysis. Environ. Microbiol. 2014, 16, 189–204. [Google Scholar] [CrossRef]
- Uhlmann, D.; Röske, I.; Hupfer, M.; Ohms, G. A simple method to distinguish between polyphosphate and other phosphate fractions of activated sludge. Water Res. 1990, 24, 1355–1360. [Google Scholar] [CrossRef]
- Peng, L.; Ren, Y.; Gu, J.; Qin, P.; Zeng, Q.; Shao, J.; Lei, M.; Chai, L. Iron improving bio-char derived from microalgae on removal of tetracycline from aqueous system. Environ. Sci. Pollut. Res. 2014, 21, 7631–7640. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Guo, L.; Chen, Y.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. Effect of magnet powder (Fe3O4) on aerobic granular sludge (AGS) formation and microbial community structure characteristics. ACS Sustain. Chem. Eng. 2018, 6, 9707–9715. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Morin, G.; Bernard, S.; Beyssac, O.; Larquet, E.; Kappler, A.; Guyot, F. Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology 2009, 7, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hage, J.; Schuiling, R.; Vriend, S. Production of magnetite from sodiumjarosite under reducing hydrothermal conditions. The reduction of FeIII to FeII with cellulose. Can. Metall. Q. 1999, 38, 267–276. [Google Scholar] [CrossRef]
- Liang, X.-Y.; Gao, B.-Y.; Ni, S.-Q. Effects of magnetic nanoparticles on aerobic granulation process. Bioresour. Technol. 2017, 227, 44–49. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Jung, H.; Zhao, S.; Pavlostathis, S.G.; Tang, Y. Effect of prestage hydrothermal treatment on the formation of struvite vs vivianite during semicontinuous anaerobic digestion of sewage sludge. ACS Sustain. Chem. Eng. 2021, 9, 9093–9105. [Google Scholar] [CrossRef]
- Prot, T.; Wijdeveld, W.; Eshun, L.E.; Dugulan, A.; Goubitz, K.; Korving, L.; Van Loosdrecht, M. Full-scale increased iron dosage to stimulate the formation of vivianite and its recovery from digested sewage sludge. Water Res. 2020, 182, 115911. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, X.; Deng, S.; Liu, J.; Qiu, B.; Dang, Y.; Holmes, D.E.; Waite, T.D. Inducing in situ crystallization of vivianite in a UCT-MBR system for enhanced removal and possible recovery of phosphorus from sewage. Environ. Sci. Technol. 2019, 53, 9045–9053. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhang, Q.; Aleem, M.; Fang, F.; Xue, Z.; Cao, J. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review. Chemosphere 2019, 226, 246–258. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Bohn, C.M.; Li, G.; Han, D.; Mosier, N.S.; Miller, J.T.; Kenttämaa, H.I.; Abu-Omar, M.M. Speciation and kinetic study of iron promoted sugar conversion to 5-hydroxymethylfurfural (HMF) and levulinic acid (LA). Org. Chem. Front. 2015, 2, 1388–1396. [Google Scholar] [CrossRef]
- An, J.-S.; Back, Y.-J.; Kim, K.-C.; Cha, R.; Jeong, T.-Y.; Chung, H.-K. Optimization for the removal of orthophosphate from aqueous solution by chemical precipitation using ferrous chloride. Environ. Technol. 2014, 35, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Adhikari, D.; Huang, R.; Zhang, H.; Tang, Y.; Roden, E.; Yang, Y. Biochar-facilitated microbial reduction of hematite. Environ. Sci. Technol. 2016, 50, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Y.; Zhao, J.; Jin, S.; Aleem, M.; Zhang, Q.; Fang, F.; Xue, Z.; Luo, J. Phosphorus recovery as vivianite from waste activated sludge via optimizing iron source and pH value during anaerobic fermentation. Bioresour. Technol. 2019, 293, 122088. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Mejia, J.; He, S.; Yang, Y.; Ginder-Vogel, M.; Roden, E.E. Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction. Environ. Sci. Technol. 2018, 52, 5691–5699. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).