Comparative Study on the Isothermal Reduction Kinetics of Iron Oxide Pellet Fines with Carbon-Bearing Materials
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.1.1. Iron Oxide Pellet Fines
2.1.2. Carbon-Bearing Materials
Reactivity Behavior
2.2. Compact Preparation
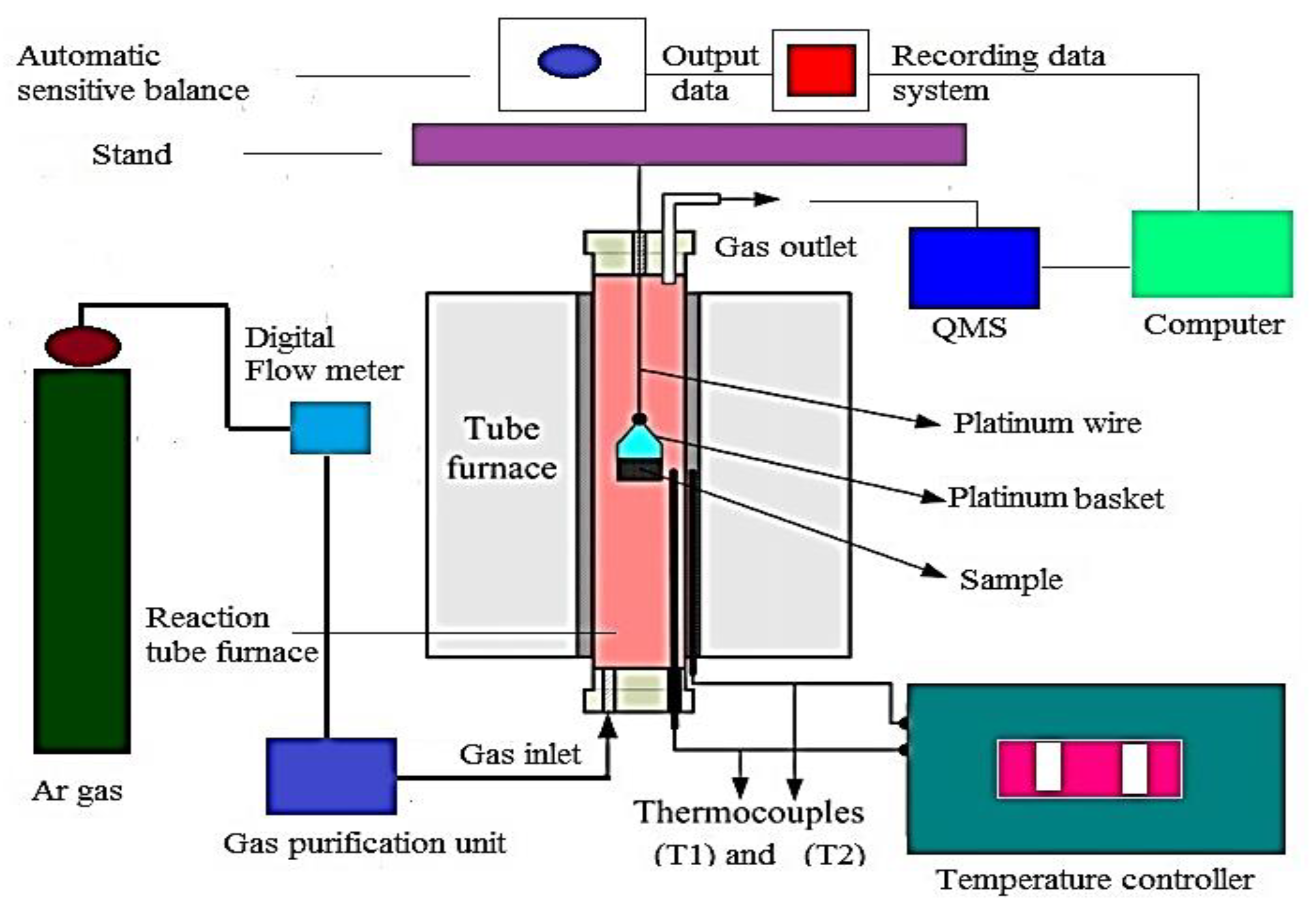
2.3. Reduction System and Procedure
3. Results and Discussion
3.1. Effect of Temperature on the Reduction of Composites
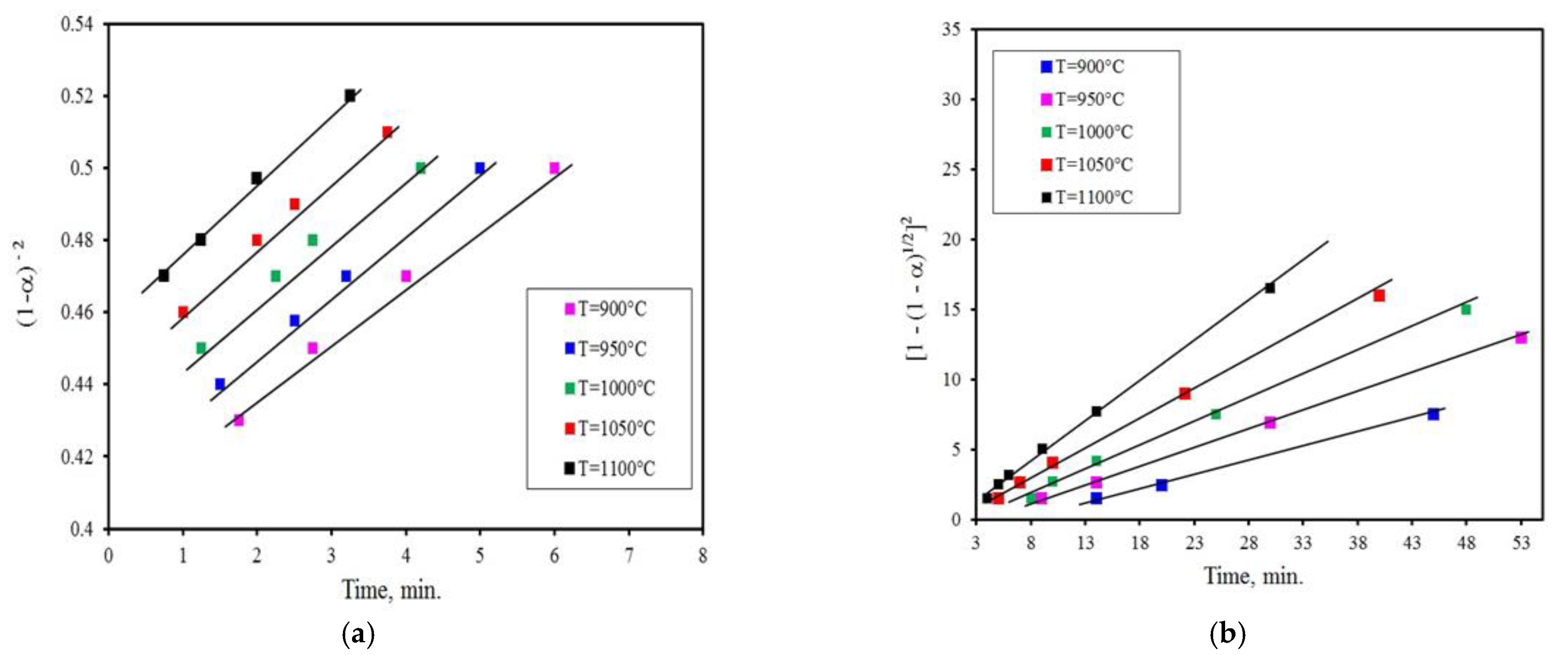
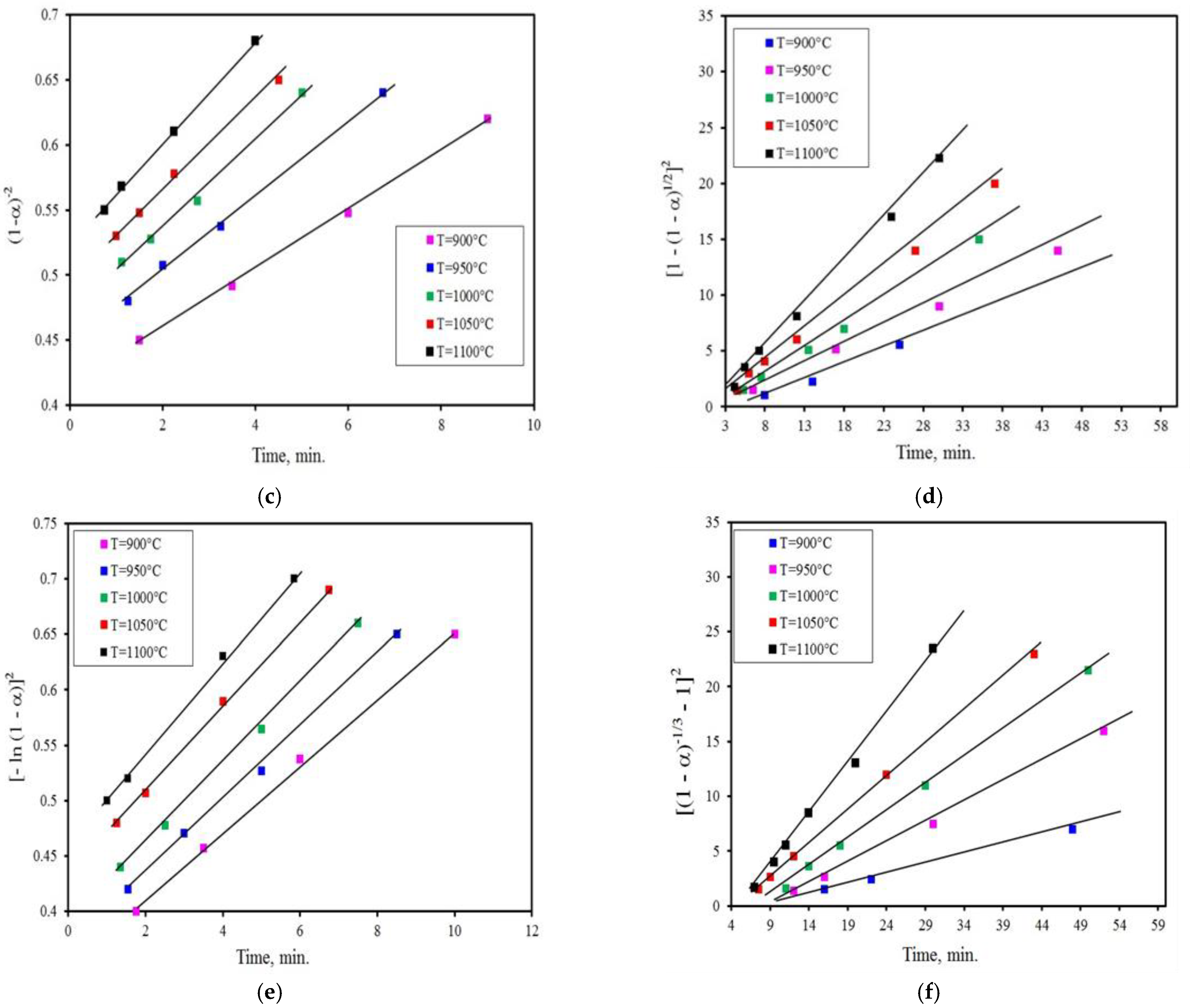
3.2. Kinetics Analysis
3.3. Determination of Activation Energy Values Ea
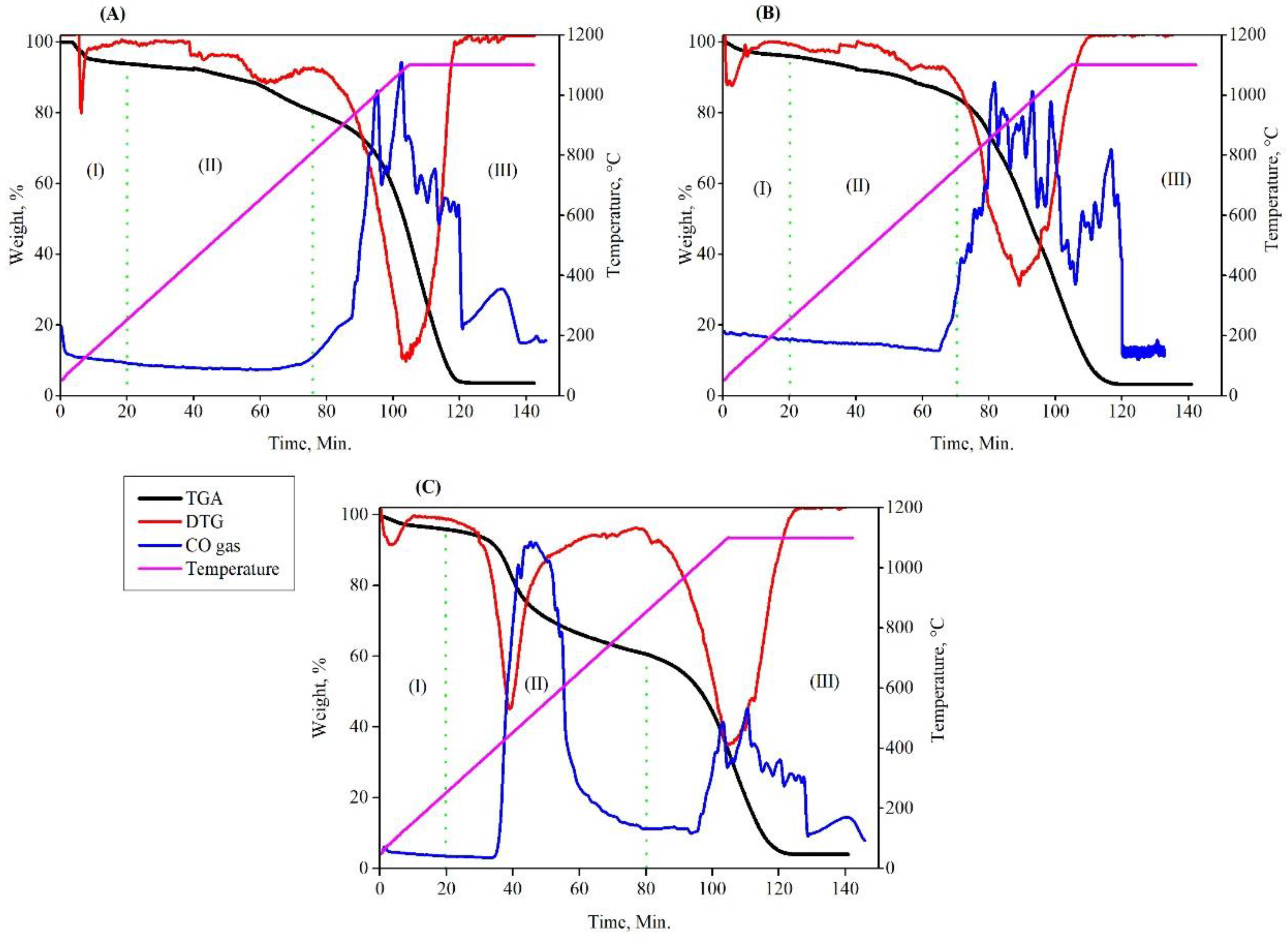
3.4. Measurements Based on (QMS) Analysis
- i.
- The reduction of Fe2O3 was accomplished in the first step by a direct interaction between Fe2O3 and carbon (3Fe2O3 + C = 2Fe3O4 + CO). As a result, CO gas was produced at the expense of nearby carbon particles. The CO gas generated in situ can also help in the reduction of hematite to magnetite (3Fe2O3 + CO = 2Fe3O4 + CO2). As a result, the amount of CO2 in the output gas increased.
- ii.
- In the second step, a portion of produced CO2 gas reacted with solid carbon to produce new and active CO gas (CO2 + C = 2CO). As a result, the CO concentration in the output gas increased. Depending on the gas concentration and the applied temperature, the produced CO gas could participate in the reduction of magnetite to wustite and part of FeO to metallic iron.
- iii.
- The unreduced FeO reacted with CO gas in the third step to form metallic iron (FeO + CO = Fe +CO2). The conversion of FeO to metallic iron took place at a rather modest pace. As a result, CO and CO2 concentrations in the output gas are comparatively low.
4. Conclusions
- The impact of a reducing agent on the reduction process is remarkable at relatively low temperatures.
- The rate of reduction increased upon raising the temperature at the initial stage, and then progressively decreased during the final stages.
- At the same temperature, the reduction rate and degree of reduction with activated charcoal and charcoal are higher than those with coal due to higher fixed carbon, surface area, and porosity.
- The reduction reaction takes place step by step from hematite to metallic iron (Fe2O3 → Fe3O4 → FeO → Fe).
- The reduction reaction, whether using activated charcoal or charcoal, has two different characteristics. The first one is obtained up to a conversion degree of α ≤ 0.4 and corresponds to the chemical reaction model (1−α)−2 indicating that the chemical reaction is the regulating mechanism at the first stage. At the latter stage, it occurs up to the conversion degree of 0.4 < α ≤ 0.9 for the gas diffusion model, namely [1 − (1 − α)1/2]2 is the rate-controlling mechanism. In the case of reduction with coal, the reduction reaction is regulated by the Avrame–Erofeev model [−ln(1 − α)2] in the early stage, whereas in the last stage, the rate-controlling mechanism is the 3-D diffusion model (Z-L-T), namely [(1 − α)−1/3 − 1]2.
- The obtained activation energy values Ea for the reduction of composites I, II, and III at the initial stage are 62.39, 77.45, and 88.66 kJ/mole, respectively, while at the final stage, the computed Ea values are 172.29, 196.66, and 219.19 kJ/mole, respectively.
- In QMS measurements, the examination of CO and CO2 concentrations reveals that the reduction process is significantly influenced by carbon gasification, which accelerates the rate of reduction.
- The utilization of biomass carbon-bearing materials rather than fossil-origin resources is a vital option for the reduction of iron oxide pellet fines.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Luo, F.; Liu, S.; Zhang, M.; Zhou, D. Comparative study on the kinetics of the isothermal reduction of iron ore composite pellets using coke, charcoal, and biomass as reducing agents. Metals 2021, 11, 340. [Google Scholar] [CrossRef]
- Gielen, D. Energy Technology Perspectives 2008: Scenarios and Strategies for CO2 Emissions Reduction. Int. J. Gas Turbine Propuls. Power Syst. 2010, 3, 1–9. [Google Scholar] [CrossRef]
- Ahmed, H. New trends in the application of carbon-bearing materials in blast furnace iron-making. Minerals 2018, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Wang, X.; Lin, B. How to reduce CO2 emissions in China’s iron and steel industry. Renew. Sustain. Energy Rev. 2016, 57, 1496–1505. [Google Scholar] [CrossRef]
- Coleti, J.L.; Manfredi, G.V.P.; Vinhal, J.T.; Junca, E.; Espinosa, D.C.R.; Tenório, J.A.S. Kinetic investigation of self-reduction basic oxygen furnace dust briquettes using charcoals from different biomass. J. Mater. Res. Technol. 2020, 9, 13282–13293. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Lu, P. Experimental investigation on flow properties of different biomass and torrefied biomass powders. Biomass-Bioenergy 2019, 122, 63–75. [Google Scholar] [CrossRef]
- Safarian, S.; Unnþórsson, R.; Richter, C. A review of biomass gasification modelling. Renew. Sustain. Energy Rev. 2019, 110, 378–391. [Google Scholar] [CrossRef]
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Lovel, R.; Vining, K.; Dell’Amico, M. Iron ore sintering with charcoal. Miner. Process. Extr. Metall. Rev. 2007, 116, 85–92. [Google Scholar] [CrossRef]
- Helle, H.; Helle, M.; Pettersson, F.; Saxén, H. Optimisation study of ironmaking using biomass. Ironmak. Steelmak. 2010, 37, 590–598. [Google Scholar] [CrossRef]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Inoue, R.; Ariyama, T. Improvement of reactivity of carbon iron ore composite with biomass char for blast furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Zhou, Y.; Yi, C. Two-step direct reduction of iron ore pellets by utilization of biomass: Effects of preheating temperature, pellet size and composition. J. Renew. Sustain. Energy 2013, 560, 441–446. [Google Scholar] [CrossRef]
- Luo, S.; Yi, C.; Zhou, Y. Direct reduction of mixed biomass—Fe2O3 briquettes using biomass-generated syngas. Renew. Energy 2011, 36, 3332–3336. [Google Scholar] [CrossRef]
- Barella, S.; Bondi, E.; Di Cecca, C.; Ciuffini, A.F.; Gruttadauria, A.; Mapelli, C.; Mombelli, D. New perspectivein steelmaking activity to increase competitiveness and reduce environmental impact. La Met. Ital. 2014, 16, 31–40. [Google Scholar]
- Sarkar, S.; Mazumder, D. Solid waste management in steel industry-challenges and opportunities. Eng. Technol. Int. J. Soc. Behav. Educ. Econ. Bus. Ind. Eng. 2015, 9, 978–981. [Google Scholar]
- Rieger, J.; Schenk, J.; Rieger, J.; Schenk, J. Residual processing in the European steel industry: A technological overview. J. Sustain. Met. 2019, 5, 295–309. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and recycling of by-products in the steel sector: Recent achievements paving the way to circular economy and industrial symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.M.; Viswanathan, N.N.; Björkman, B. Isothermal reduction kinetics of self-reducing mixtures. Ironmak. Steelmak. 2016, 44, 66–75. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Halim, K.S.A.; Bahgat, M.; Mousa, E.A.; Shereafy, E.E.E.; El-Tawi, A.A. Carbothermic reduction of Fe2O3/C compacts: Comparative approach to kinetics and mechanism. Ironmak. Steelmak. 2013, 40, 534–544. [Google Scholar] [CrossRef]
- Chowdhury, G.M.; Roy, G.G.; Roy, S.K. Reduction kinetics of iron ore-graphite composite pellets in a packed-bed reactor under inert and reactive atmospheres. Metall. Mater. Trans. B 2008, 39, 160–178. [Google Scholar] [CrossRef]
- Ueda, S.; Yanagiya, K.; Watanabe, K.; Murakami, T.; Inoue, R.; Ariyama, T. Reaction model and reduction behavior of carbon iron ore composite in blast furnace. ISIJ Int. 2009, 49, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Nishimura, T.; Kasai, E. Lowering reduction temperature of iron ore and carbon composite by using ores with high combined water content. ISIJ Int. 2009, 49, 1686–1693. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y.; Sawayama, M.; Kasai, A.; Yamagata, Y.; Noma, F. Reduction behavior of carbon composite iron ore hot briquette in shaft furnace and scope on blast furnace performance reinforcement. ISIJ Int. 2003, 43, 1904–1912. [Google Scholar] [CrossRef]
- Dutta, S.K.; Ghosh, A. A new method for measurement of degree of reduction in composite pellets of iron ore with carbonaceous matter. ISIJ Int. 1993, 33, 1104–1106. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Nogami, H.; Yagi, J. Numerical analysis on charging carbon composite agglomerates into blast furnace. ISIJ Int. 2004, 44, 510–517. [Google Scholar] [CrossRef]
- Iguchi, Y.; Takada, Y. Rate of direct reactions measured in vacuum of iron ore-carbon composite pellets heated at high temperatures: Influence of carbonaceous materials, oxidation degree of iron oxides and temperature. ISIJ Int. 2004, 44, 673–681. [Google Scholar] [CrossRef]
- Narçin, N.; Aydln, S.; Şeşen, K.; Dikeç, F. Reduction of iron ore pellets with domestic lignite coal in a rotary tube furnace. Int. J. Miner. Process. 1995, 43, 49–59. [Google Scholar] [CrossRef]
- Jung, S. Effects of the Content and Particle Size of Char in the Composite on the Carbothermic Reduction of Titanomagnetite at 1100 °C. ISIJ Int. 2014, 54, 2933–2935. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Roy, G.G. Effect of Amount of Carbon on the Reduction Efficiency of Iron Ore-Coal Composite Pellets in Multi-layer Bed Rotary Hearth Furnace (RHF). Met. Mater. Trans. A 2016, 47, 2347–2356. [Google Scholar] [CrossRef] [Green Version]
- Sharma, T. Reduction of iron ore fines with coal fines. Ironmak. Steelmak. 1993, 20, 362–365. [Google Scholar]
- Kasai, E.; Kitajima, T.; Kawaguchi, T. Carbothermic reduction in the combustion bed packed with composite pellets of iron oxide and coal. ISIJ Int. 2000, 40, 842–849. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Mori, T.; Kuwabara, M. Mechanism of Carbothermic Reduction of Hematite in Hematite–Carbon Composite Pellets. ISIJ Int. 2007, 47, 1394–1400. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Strzov, V.; Lucas, J.A.; Wibberley, L.J. Thermal investigations of direct iron ore reduction with coal. Thermochem. Acta 2004, 410, 133. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Li, G.F. Investigation of kinetics of coal based reduction of oolitic iron ore. Ironmak. Steelmak. 2014, 41, 763–768. [Google Scholar] [CrossRef]
- Hammam, A.; Cao, Y.; El-Geassy, A.-H.; El-Sadek, M.; Li, Y.; Wei, H.; Omran, M.; Yu, Y. Non-Isothermal Reduction Kinetics of Iron Ore Fines with Carbon-Bearing Materials. Metals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Gao, P.; Wei, X.; Li, G. Thermogravimetric study of coal-based reduction of oolitic iron ore: Kinetics and mechanisms. Int. J. Miner. Processing 2015, 143, 87–97. [Google Scholar] [CrossRef]
- Tanaka, H. Thermal analysis and kinetics of solid state reactions. Thermochim. Acta 1995, 267, 29–44. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Park, H.; Sahajwalla, V. Effect of Alumina and Silica on the Reaction Kinetics of Carbon Composite Pellets at 1473 K. ISIJ Int. 2014, 54, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.K. Principles of Blast Furnace Ironmaking; Cootha Publishing House: Brisbane, Australia, 1981. [Google Scholar]
- Fruehan, R.J. The rate of reduction of iron oxides by carbon. Met. Mater. Trans. A 1977, 8, 279–286. [Google Scholar] [CrossRef]







| Carbon Source | Moisture | Volatiles | Ash | Fixed Carbon |
|---|---|---|---|---|
| Act. charcoal | 4.5 | 12 | 2.5 | 81 |
| Charcoal | 9 | 14 | 3 | 74 |
| Coal | 4.5 | 33.5 | 4 | 58 |
| Computed Ea (kJ/mol) | At Initial Stage | At Final Stage |
|---|---|---|
| Composite I | 62.39 | 172.29 |
| Composite II | 77.45 | 196.48 |
| Composite III | 88.66 | 219.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammam, A.; Nasr, M.I.; El-Sadek, M.H.; Omran, M.; Ahmed, A.; Li, Y.; Xiong, Y.; Yu, Y. Comparative Study on the Isothermal Reduction Kinetics of Iron Oxide Pellet Fines with Carbon-Bearing Materials. Sustainability 2022, 14, 8647. https://doi.org/10.3390/su14148647
Hammam A, Nasr MI, El-Sadek MH, Omran M, Ahmed A, Li Y, Xiong Y, Yu Y. Comparative Study on the Isothermal Reduction Kinetics of Iron Oxide Pellet Fines with Carbon-Bearing Materials. Sustainability. 2022; 14(14):8647. https://doi.org/10.3390/su14148647
Chicago/Turabian StyleHammam, Abourehab, Mahmoud I. Nasr, Mohamed H. El-Sadek, Mamdouh Omran, Abdallah Ahmed, Ying Li, Yuandong Xiong, and Yaowei Yu. 2022. "Comparative Study on the Isothermal Reduction Kinetics of Iron Oxide Pellet Fines with Carbon-Bearing Materials" Sustainability 14, no. 14: 8647. https://doi.org/10.3390/su14148647
APA StyleHammam, A., Nasr, M. I., El-Sadek, M. H., Omran, M., Ahmed, A., Li, Y., Xiong, Y., & Yu, Y. (2022). Comparative Study on the Isothermal Reduction Kinetics of Iron Oxide Pellet Fines with Carbon-Bearing Materials. Sustainability, 14(14), 8647. https://doi.org/10.3390/su14148647








