Application of the Response Surface Methodology (RSM) in the Optimization of Acenaphthene (ACN) Removal from Wastewater by Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stock Solution Preparation
2.3. Analytical Method (Chromatographic Analysis Using HPLC)
2.4. Synthesis of Activated Carbon
2.5. Lab Experiments
2.6. Optimization Using Central Composite Design (CCD) with the Response Surface Methodology (RSM)
3. Results and Discussion
3.1. HPLC Results
3.2. Effect of Solution pH
3.3. Effect of Activated Carbon Dosage
3.4. Effect of Contact Time
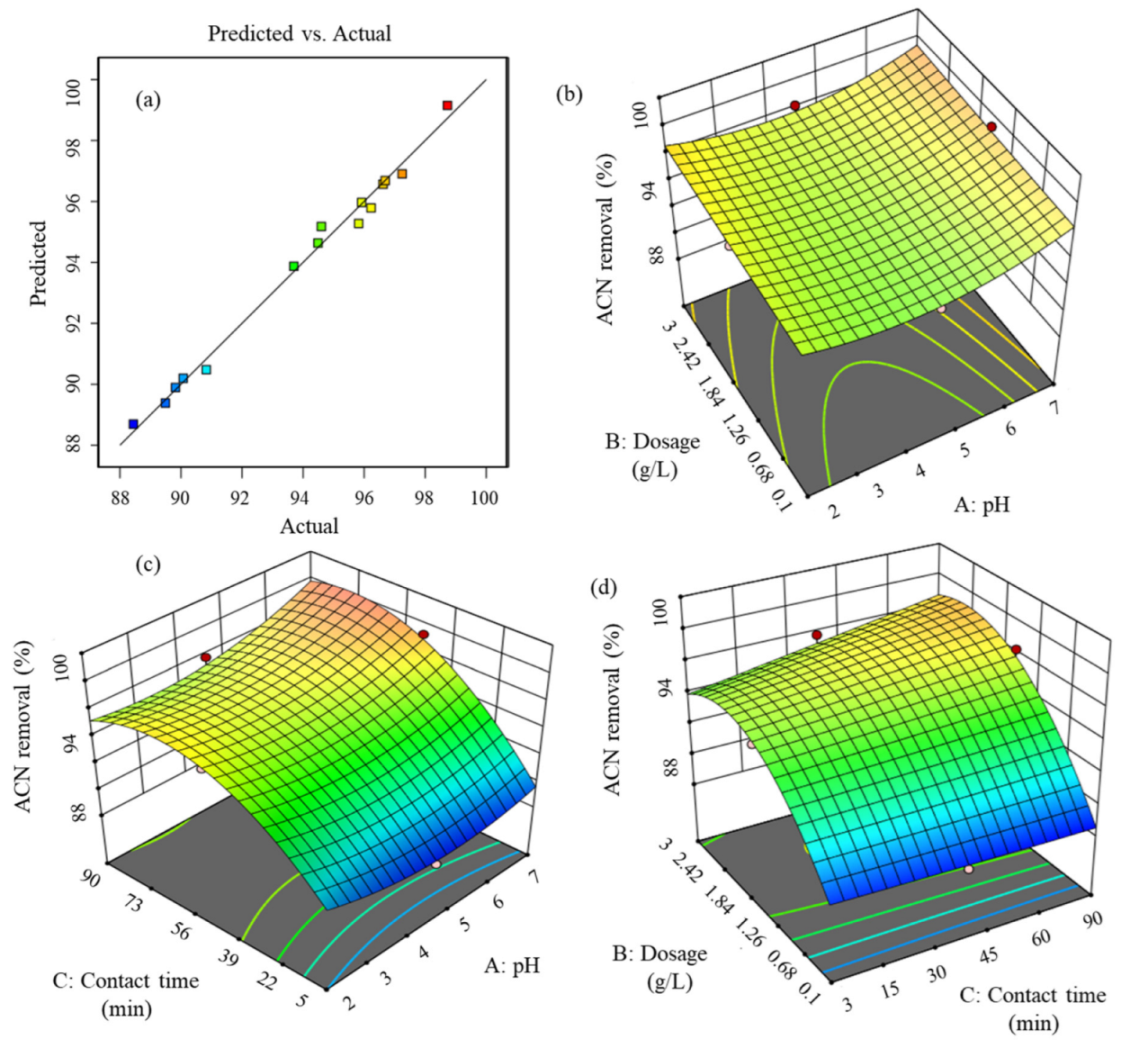
3.5. CCD-Based RSM and Analysis of Variance (ANOVA)
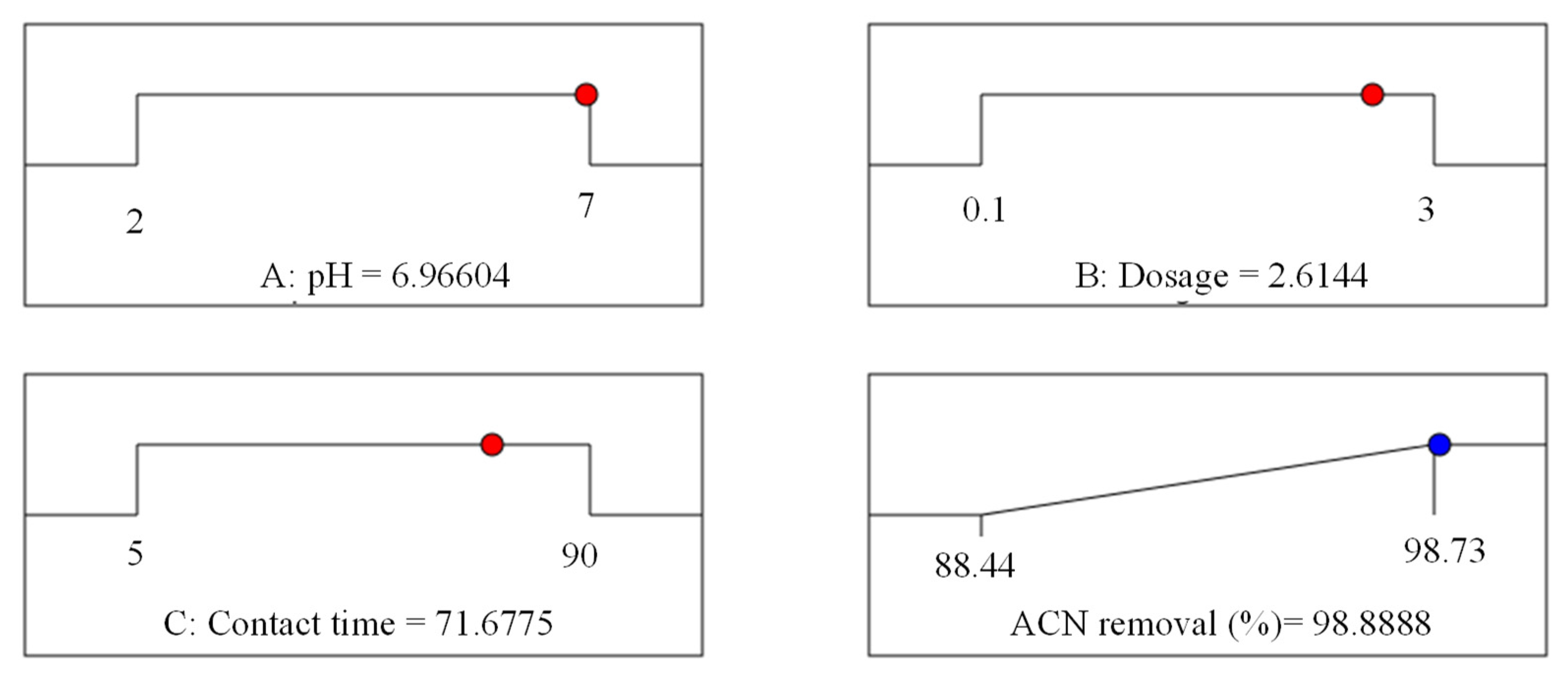
3.6. Three-Dimensional Surface Plots and Optimization Using the RSM
3.7. ACN Adsorption Efficiency of Different Types of Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valderrama, C.; Gamisans, X.; de las Heras, X.; Farrán, A.; Cortina, J.L. Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: Intraparticle diffusion coefficients. J. Hazard. Mater. 2008, 157, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Soclo, H.H.; Garrigues, P.; Ewald, M. Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: Case studies in Cotonou (Benin) and Aquitaine (France) Areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Huang, Y.; Fulton, A.N.; Keller, A.A. Simultaneous removal of PAHs and metal contaminants from water using magnetic nanoparticle adsorbents. Sci. Total Environ. 2016, 571, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haneef, T.; Ul Mustafa, M.R.; Yusof, K.W.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of polycyclic aromatic hydrocarbons (PAHs) from produced water by Ferrate (VI) oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Malakahmad, A.; Law, M.X.; Ng, K.W.; Manan, T.S.A. The Fate and Toxicity Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Water Streams of Malaysia. Procedia Eng. 2016, 148, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Tang, Y.M.; Junaid, M.; Niu, A.; Deng, S.; Pei, D.S. Diverse toxicological risks of PAHs in surface water with an impounding level of 175 m in the Three Gorges Reservoir Area, China. Sci. Total Environ. 2017, 580, 1085–1096. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Zhou, Q.; Zhao, X.; Sun, Z. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater. 2018, 343, 276–284. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Acedo, B. Chemical and photochemical degradation of acenaphthylene. Intermediate identification. J. Hazard. Mater. 2000, 75, 89–98. [Google Scholar] [CrossRef]
- Bayati, F.; Shayegan, J.; Noorjahan, A. Treatment of oilfield produced water by dissolved air precipitation/solvent sublation. J. Pet. Sci. Eng. 2011, 80, 26–31. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of polycyclic aromatic hydrocarbons in a heterogeneous fenton like oxidation system using nanoscale zero-valent iron as a catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Zeledón-Toruño, Z.C.; Lao-Luque, C.; de las Heras, F.X.C.; Sole-Sardans, M. Removal of PAHs from water using an immature coal (leonardite). Chemosphere 2007, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, R.; Yavari, S.; Kamyab, H.; Ashokkumar, V.; Chelliapan, S.; Yuzir, A. Study of oil sorption behaviour of esterified oil palm empty fruit bunch (OPEFB) fibre and its kinetics and isotherm studies. Environ. Technol. Innov. 2021, 22, 101397. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Ngo, H.H.; Liang, S.; Zhang, J. Adsorption of phenanthrene from aqueous solutions by biochar derived from an ammoniation-hydrothermal method. Sci. Total Environ. 2020, 733, 139267. [Google Scholar] [CrossRef]
- Deb, A.; Kanmani, M.; Debnath, A.; Bhowmik, K.L.; Saha, B. Ultrasonic assisted enhanced adsorption of methyl orange dye onto polyaniline impregnated zinc oxide nanoparticles: Kinetic, isotherm and optimization of process parameters. Ultrason. Sonochem. 2019, 54, 290–301. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V. Bioremediation of polycyclic aromatic hydrocarbon (PAH) compounds: (acenaphthene and fluorene) in water using indigenous bacterial species isolated from the Diep and Plankenburg rivers, Western Cape, South Africa. Brazilian J. Microbiol. 2017, 48, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Schocken, M.J.; Gibson, D.T. Bacterial oxidation of the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Appl. Environ. Microbiol. 1984, 48, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Mallick, S. Biodegradation of acenaphthene by Sphingobacterium sp. strain RTSB involving trans-3-carboxy-2-hydroxybenzylidenepyruvic acid as a metabolite. Chemosphere 2019, 219, 748–755. [Google Scholar] [CrossRef]
- Mortazavi, M.; Baghdadi, M.; Seyed Javadi, N.H.; Torabian, A. The black beads produced by simultaneous thermal reducing and chemical bonding of graphene oxide on the surface of amino-functionalized sand particles: Application for PAHs removal from contaminated waters. J. Water Process Eng. 2019, 31, 100798. [Google Scholar] [CrossRef]
- Khurshid, H.; Mustafa, M.R.U.; Isa, M.H. A Comprehensive Insight on Adsorption of Polyaromatic Hydrocarbons, Chemical Oxygen Demand, Pharmaceuticals, and Chemical Dyes in Wastewaters Using Biowaste Carbonaceous Adsorbents. Adsorpt. Sci. Technol. 2022, 2022, 9410266. [Google Scholar] [CrossRef]
- Isabirye, M.; Raju, D.V.; Kitutu, M.; Yemeline, V.; Deckers, J.; Poesen, J. Additional We are IntechOpen, the world’ s leading publisher of Open Access books Built by scientists, for scientists TOP 1%. Intech 2012, 13. [Google Scholar]
- Şahan, T. Application of RSM for Pb(II) and Cu(II) adsorption by bentonite enriched with [sbnd]SH groups and a binary system study. J. Water Process Eng. 2019, 31, 100867. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Saha, B. Ultrasound-aided rapid and enhanced adsorption of anionic dyes from binary dye matrix onto novel hematite/polyaniline nanocomposite: Response surface methodology optimization. Appl. Organomet. Chem. 2020, 34, e5353. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Bhowmik, K.L.; Rudra Paul, S.; Saha, B. Application of polyaniline impregnated mixed phase Fe2O3, MnFe2O4 and ZrO2 nanocomposite for rapid abatement of binary dyes from aqua matrix: Response surface optimisation. Int. J. Environ. Anal. Chem. 2021, 101, 1–19. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Thanh Ho, V.T.; Bach, L.G. Application of response surface methodology to optimize the fabrication of ZnCl2-activated carbon from sugarcane bagasse for the removal of Cu2+. Water Sci. Technol. 2017, 75, 2047–2055. [Google Scholar] [CrossRef]
- Mohammad Hosein, H.; Mohammad Reza, H. Determination of some polycyclic aromatic hydrocarbons in the Caspian seawater by HPLC following preconcentration with solid-phase extraction. Iran. J. Chem. Chem. Eng. 2008, 27, 91–96. [Google Scholar]
- Khurshid, H.; Mustafa, M.R.U.; Isa, M.H. Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water. Sustainbility 2022, 14, 1986. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kumar, P.S.; Krithiga, T.; Prabu, D.; Amarnath, D.J.; Sathish, S.; Venkatesan, D.; Hosseini-Bandegharaei, A.; Prashant, P. Acenaphthene adsorption onto ultrasonic assisted fatty acid mediated porous activated carbon-characterization, isotherm and kinetic studies. Chemosphere 2021, 284, 131249. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Feasibility study of acenaphthene degradation in a novel slurry UV photocatalytic membrane reactor: Effect of operating parameters and optimization using response surface modeling. Chem. Eng. Process.-Process Intensif. 2020, 155, 108051. [Google Scholar] [CrossRef]
- Lu, L.; Lin, Y.; Chai, Q.; He, S.; Yang, C. Removal of acenaphthene by biochar and raw biomass with coexisting heavy metal and phenanthrene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 103–109. [Google Scholar] [CrossRef]
- Kumar, J.A.; Amarnath, D.J.; Sathish, S.; Jabasingh, S.A.; Saravanan, A.; Hemavathy, R.V.; Anand, K.V.; Yaashikaa, P.R. Enhanced PAHs removal using pyrolysis-assisted potassium hydroxide induced palm shell activated carbon: Batch and column investigation. J. Mol. Liq. 2019, 279, 77–87. [Google Scholar] [CrossRef]
- Tangsiri, R.; Nezamzadeh-Ejhieh, A. Cadmium sulfide nanoparticles: Synthesis, brief characterization and experimental design by response surface methodology (RSM) in the photodegradation of ranitidine hydrochloride. Chem. Phys. Lett. 2020, 758, 137919. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Saha, B. Sono-assisted enhanced adsorption of eriochrome Black-T dye onto a novel polymeric nanocomposite: Kinetic, isotherm, and response surface methodology optimization. J. Dispers. Sci. Technol. 2021, 42, 1579–1592. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Z. Impacts of charcoal characteristics on sorption of polycyclic aromatic hydrocarbons. Chemosphere 2008, 71, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Ofman, P.; Struk-Sokołowska, J.; Skoczko, I.; Wiater, J. Alternated biodegradation of naphthalene (NAP), acenaphthylene (ACY) and acenaphthene (ACE) in an aerobic granular sludge reactor (GSBR). J. Hazard. Mater. 2020, 383, 121184. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.R.; Das, P.; Mukhopadhayay, A. Biochar from waste Sterculia foetida and its application as adsorbent for the treatment of PAH compounds: Batch and optimization. Fuel 2021, 306, 121623. [Google Scholar] [CrossRef]

| Parameters | Unit | Lowest Value | Highest Value | |
|---|---|---|---|---|
| Numeric 1 | pH | 2 | 7 | |
| Numeric 2 | Dosage | g/L | 0.1 | 3 |
| Numeric 3 | Contact time | min | 5 | 90 |
| Run | pH | Dosage (g/L) | Contact Time (min) |
|---|---|---|---|
| 1 | 2 | 3 | 5 |
| 2 | 2 | 1.55 | 47.5 |
| 3 | 7 | 3 | 5 |
| 4 | 4.5 | 1.55 | 47.5 |
| 5 | 7 | 3 | 90 |
| 6 | 4.5 | 1.55 | 90 |
| 7 | 7 | 0.1 | 5 |
| 8 | 4.5 | 0.1 | 47.5 |
| 9 | 4.5 | 1.55 | 47.5 |
| 10 | 7 | 0.1 | 90 |
| 11 | 4.5 | 1.55 | 5 |
| 12 | 4.5 | 1.55 | 47.5 |
| 13 | 2 | 3 | 90 |
| 14 | 4.5 | 1.55 | 47.5 |
| 15 | 4.5 | 1.55 | 47.5 |
| 16 | 7 | 1.55 | 47.5 |
| 17 | 2 | 0.1 | 90 |
| 18 | 4.5 | 1.55 | 47.5 |
| 19 | 4.5 | 3 | 47.5 |
| 20 | 2 | 0.1 | 5 |
| pH | Dosage (g/L) | Contact Time (min) | Initial ACN Concentration (mg/L) | Final ACN Concentration (mg/L) | Removal of ACN (%) |
|---|---|---|---|---|---|
| 2 | 0.1 | 5 | 9.58 ± 0.5 | 0.88 ± 0.2 | 90.83 ± 1 |
| 2 | 0.1 | 90 | 9.58 ± 0.5 | 0.60 ± 0.2 | 93.69 ± 1 |
| 2 | 1.55 | 47.5 | 9.58 ± 0.5 | 0.39 ± 0.2 | 95.92 ± 1 |
| 2 | 3 | 5 | 9.58 ± 0.5 | 0.95 ± 0.2 | 90.07 ± 1 |
| 2 | 3 | 90 | 9.58 ± 0.5 | 0.317 ± 0.2 | 96.69 ± 1 |
| 4.5 | 0.1 | 47.5 | 9.58 ± 0.5 | 0.53 ± 0.2 | 94.49 ± 1 |
| 4.5 | 1.55 | 5 | 9.58 ± 0.5 | 1.11 ± 0.2 | 88.44 ± 1 |
| 4.5 | 1.55 | 47.5 | 9.58 ± 0.5 | 0.52 ± 0.2 | 94.60 ± 1 |
| 4.5 | 1.55 | 90 | 9.58 ± 0.5 | 0.40 ± 0.2 | 95.82 ± 1 |
| 4.5 | 3 | 47.5 | 9.58 ± 0.5 | 0.36 ± 0.2 | 96.23 ± 1 |
| 7 | 0.1 | 5 | 9.58 ± 0.5 | 0.98 ± 0.2 | 89.82 ± 1 |
| 7 | 0.1 | 90 | 9.58 ± 0.5 | 0.32 ± 0.2 | 96.62 ± 1 |
| 7 | 1.55 | 47.5 | 9.58 ± 0.5 | 0.26 ± 0.2 | 97.25 ± 1 |
| 7 | 3 | 5 | 9.58 ± 0.5 | 1.01 ± 0.2 | 89.49 ± 1 |
| 7 | 3 | 90 | 9.58 ± 0.5 | 0.12 ± 0.2 | 98.73 ± 1 |
| Source | Sum of Squares | df * | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 134.26 | 9 | 14.92 | 60.69 | 0.0425 |
| A-pH | 37.40 | 1 | 37.40 | 7.91 | 0.0374 |
| B-Dosage | 1.91 | 1 | 1.91 | 11.87 | 0.0343 |
| C-Contact time | 0.0012 | 1 | 0.0012 | 388.20 | 0.0023 |
| AB | 1.30 | 1 | 1.30 | 0.09 | 0.0145 |
| AC | 0.0021 | 1 | 0.0021 | 19.27 | 0.0230 |
| BC | 3.71 | 1 | 3.71 | 17.16 | 0.0430 |
| A2 | 4.25 | 1 | 4.25 | 14.62 | 0.0112 |
| B2 | 6.42 | 1 | 6.42 | 0.011 | 0.0122 |
| C2 | 12.80 | 1 | 12.80 | 93.98 | 0.0032 |
| Residual | 0.0001 | 10 | 5.75 × 10−6 | ||
| Lack of fit | 0.0001 | 5 | 0.000 | ||
| Core total | 134.26 | 19 | R2 | 0.99 |
| Adsorbent | Adsorption Capacity (%) | Reference |
|---|---|---|
| Activated carbon from oil palm leaves | 98.88 | This study |
| Ultrasonic-assisted fatty-acid-mediated porous activated carbon | 99 | [28] |
| Rice bran biochar | 98 | [30] |
| Banana biochar | 74.6 | [30] |
| Biochar from waste Sterculia foetida | 92 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moria, K.M.; Khurshid, H.; Mustafa, M.R.U.; Alhothali, A.; Bamasag, O.O. Application of the Response Surface Methodology (RSM) in the Optimization of Acenaphthene (ACN) Removal from Wastewater by Activated Carbon. Sustainability 2022, 14, 8581. https://doi.org/10.3390/su14148581
Moria KM, Khurshid H, Mustafa MRU, Alhothali A, Bamasag OO. Application of the Response Surface Methodology (RSM) in the Optimization of Acenaphthene (ACN) Removal from Wastewater by Activated Carbon. Sustainability. 2022; 14(14):8581. https://doi.org/10.3390/su14148581
Chicago/Turabian StyleMoria, Kawthar Mostafa, Hifsa Khurshid, Muhammad Raza Ul Mustafa, Areej Alhothali, and Omaimah Omar Bamasag. 2022. "Application of the Response Surface Methodology (RSM) in the Optimization of Acenaphthene (ACN) Removal from Wastewater by Activated Carbon" Sustainability 14, no. 14: 8581. https://doi.org/10.3390/su14148581
APA StyleMoria, K. M., Khurshid, H., Mustafa, M. R. U., Alhothali, A., & Bamasag, O. O. (2022). Application of the Response Surface Methodology (RSM) in the Optimization of Acenaphthene (ACN) Removal from Wastewater by Activated Carbon. Sustainability, 14(14), 8581. https://doi.org/10.3390/su14148581








