Phytoremediation Using Willow in Industrial Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
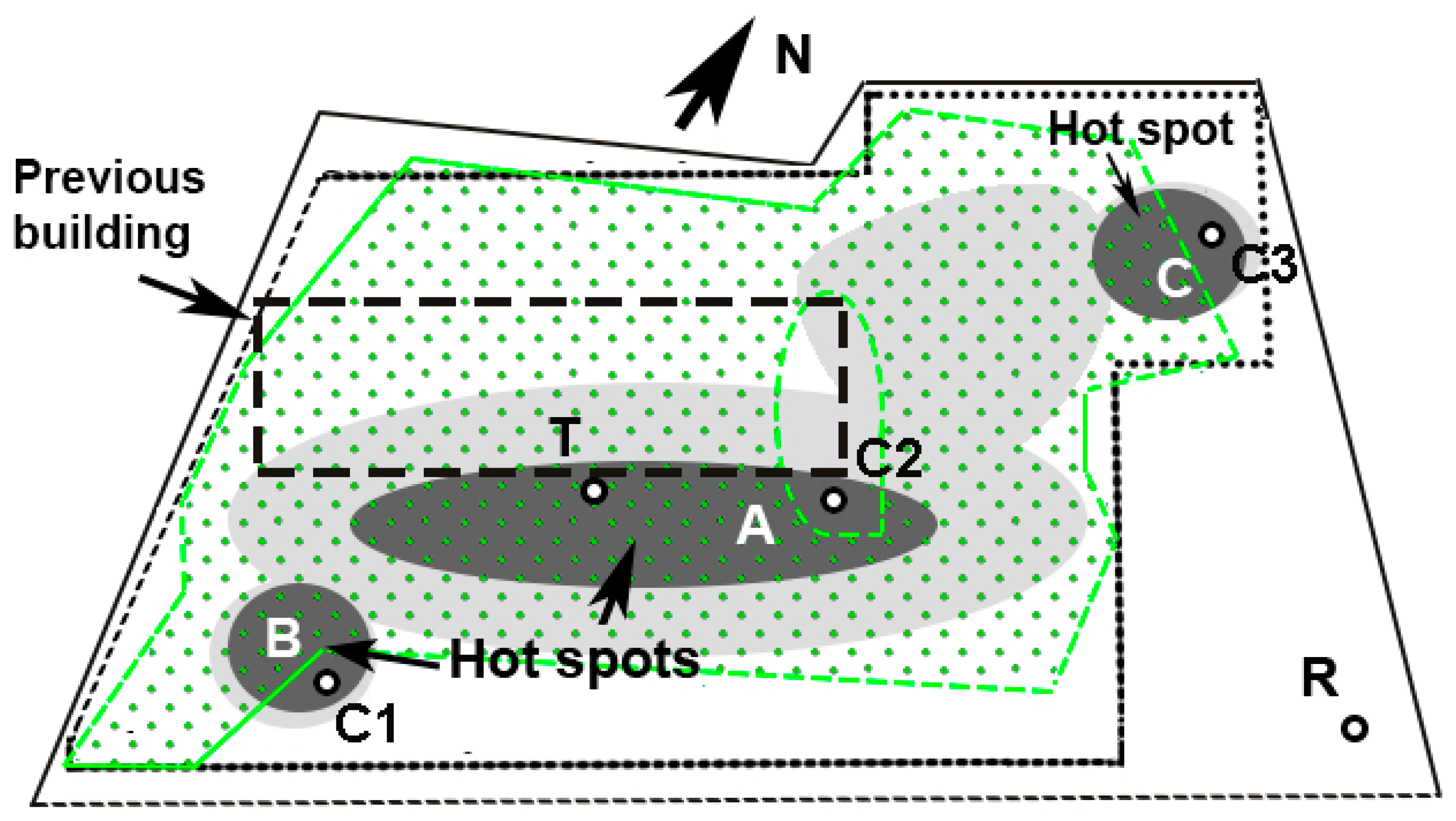
2.1. The Site
2.2. Plant Materials
2.3. Plantation and Cultivation
2.4. Sample Collection
2.5. Analysed Contaminants
- Metals and metalloids: Arsenic, cadmium, chromium, copper, lead, nickel and zinc.
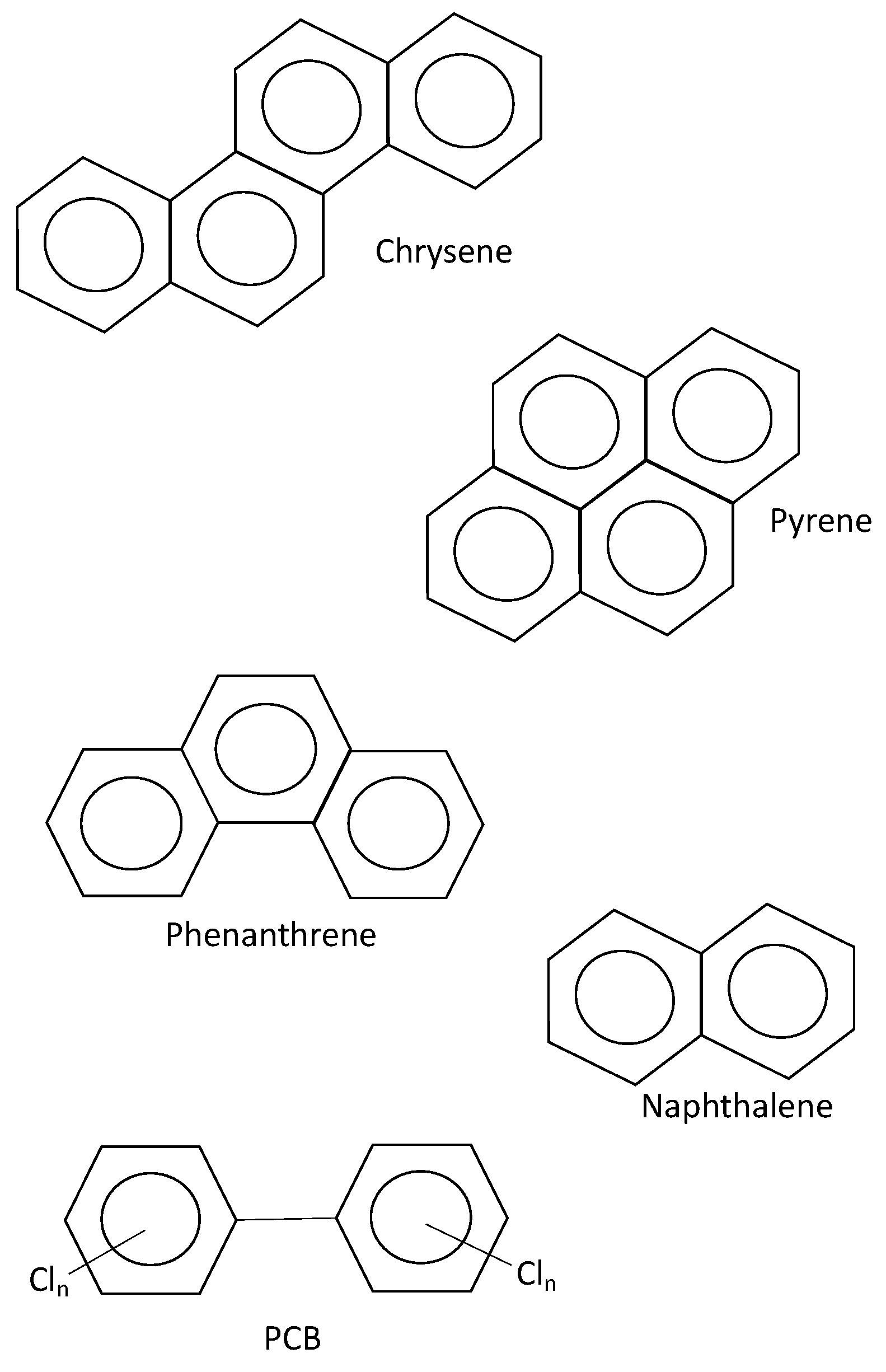
- PAHs: Naphthalene, acenaphthene, acenaphthylene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(k)fluoranthene, benzo(g,h,i)perylene, benzo(a)pyrene, indenol(1,2,3-c,d)pyrene, dibenzo(a,h)anthracene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene (the last six of these are carcinogenic).
- PCBs: PCB-28, PCB-52, PCB-101, PCB-118, PCB-138, PCB-153 and PCB-180.
2.6. Calculations and Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennen, K.; Kirkwood, N. Phyto Principles and Resources for Site Remediation and Landscape Design; Routledge: New York, NY, USA, 2015; ISBN 978-0-415-81415-7. [Google Scholar]
- Riaza, U.; Athar, T.; Mustafa, T.; Iqbsl, R. Economic feasibility of phytoremediation. In Biotechnological Strategies for Promoting Invigorating Environs; Bhat, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 481–502. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.R. Remediation of contaminated soils with green plants: An overview. In Vitro Cell. Develop. Biol.-Plant 1993, 29, 207–212. [Google Scholar] [CrossRef]
- Thijs, S.; Witters, N.; Janssen, J.; Ruttens, A.; Weyens, N.; Herzig, R.; Mench, M.; Van Slycken, S.; Meers, E.; Meiresonne, L.; et al. Tobacco, sunflower and high biomass SRC clones show potential for trace metal phytoextraction on a moderately contaminated field site in Belgium. Front. Plant Sci. 2018, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Arya, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Alkorta, I.; Garbis, C. Phytoremediation of organic contaminants in soils. Biores. Technol. 2001, 79, 273–276. [Google Scholar] [CrossRef]
- Raskin, I.; Kumar, K.; Dushenkov, S.; Salt, D.E. Bioconcentration of heavy metals by plants. Cur. Opin. Biotechnol. 1994, 5, 285–290. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar]
- Landberg, T.; Greger, M. Can heavy metal tolerant clones of Salix be used as vegetation filters on heavy contaminated land? In Willow Vegetation Filters for Municipal Wastewaters and Sludges. A Biological Purification System, Proceedings of a Study Tour, Conference and Workshop, Ultuna, Uppsala, Sweden, 5–10 June 1994; Aronsson, P., Perttu, K., Eds.; Rapport 50, Avd. f. Skoglig Intensivodling, SLU; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 133–144. ISBN 91-576-4916-2. [Google Scholar]
- Landberg, T.; Greger, M. Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. Appl. Geochem. 1996, 11, 175–180. [Google Scholar] [CrossRef]
- Felix, H. Vor-Ort-Reinigung schwermetallbelasteter Böden mit Hilfe von metallakkumulierenden Pflanzen (Hyperakkumulatoren). Terra Tech 1997, 2, 47–49. [Google Scholar]
- Greger, M.; Landberg, T. Use of willow in phytoextraction. Int. J. Phytorem. 1999, 1, 115–123. [Google Scholar] [CrossRef]
- Hammar, D.; Kayser, A.; Keller, C. Phytoextraction of Cd and Zn with Salix viminalis in field trials. Soil Use Manag. 2003, 19, 187–192. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Mleczk, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazynska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T. Novel field data on phytoextraction: Precultivation with Salix reduces cadmium in wheat grains. Int. J. Phytorem. 2015, 17, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.A.; Sofi, Z.M.; Malik, J.A.; Wani, J.A. Phytoremediation of heavy metals using Salix (willows). In Bioremediation and Biotechnology; Bhat, R., Hakeem, K., Dervash, M., Eds.; Springer: Cham, Swizerland, 2020; Volume 2, pp. 257–268. [Google Scholar]
- Brieger, G.; Wells, J.R.; Hunter, R.D. Content in fly ash ecosystem. Water Air. Soil Pollut. 1992, 63, 87–103. [Google Scholar] [CrossRef]
- Ledin, S. Willow wood properties, production and economy. Biomass Bioenergy 1996, 11, 75–83. [Google Scholar] [CrossRef]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy metal uptake by willow clones from sewage sludge-treated soil: The potential for phytoremediation. Int. J. Phytoremed. 2002, 4, 59–72. [Google Scholar] [CrossRef]
- Vyslouilová, M.; Tlusto, P.; Száková, J.; Pavlíková, D. As, Cd, Pb and Zn uptake by Salix spp. clones grown in soils enriched by high loads of these elements. Plant Soil Environ. 2003, 49, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Tanac, Q.; Zhang, F.; Maa, C.; Xiao, J.; Chen, G. Phytoremediation potential evaluation of multiple Salix clones for heavy metals (Cd, Zn and Pb) in flooded soils. Sci. Total Environ. 2022, 813, 152482. [Google Scholar] [CrossRef]
- Wieshammer, G.; Unterbrunner, R.; Garcia, T.B.; Zivkovic, M.F.; Puschenreiter, M.; Wenzel, W.W. Phytoextraction of Cd and Zn from agricultural soils by Salix ssp. and intercropping of Salix caprea and Arabidopsis halleri. Plant Soil 2007, 298, 255–264. [Google Scholar] [CrossRef]
- Wang, Y.D.; Greger, M.; Haglund, P.; Lundstedt, S.; Bergknut, M.; Kitty, A. Studies on phytodegradation of PAHs in creosote contaminated soil. In Proceedings of the COST Action 837 Workshop, Grainau, Germany, 23–25 May 2002. [Google Scholar]
- Vervaeke, P.; Luyssaert, S.; Mertens, J.; Meers, E.; Tack, F.M.G.; Lust, N. Phytoremediation prospects of willow stands on contaminated sediment: A field trial. Environ. Pollut. 2003, 126, 275–282. [Google Scholar] [CrossRef]
- Faubert, M.F.; Desjardins, D.; Labrecque, M. Willows used for phytoremediation increased organic contaminant concentrations in soil surface. Appl. Sci. 2021, 11, 2979. [Google Scholar] [CrossRef]
- Telenius, B.F. Implications of vertical distribution and within-stand variation in moisture content for biomass estimations of some willow and hybrid poplar clones. Scand. J. Forest Res. 1997, 12, 336–339. [Google Scholar] [CrossRef]
- Tao, S.; Liu, W.X.; Chen, Y.J.; Xu, F.L.; Dawson, R.W.; Li, B.G.; Cao, J.; Wang, X.; Hu, J.; Fang, J. Evaluation of factors influence root-induced changes of copper fractionation in rhizosphere of a calcareous soil. Environ. Pollut. 2004, 129, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press of Elsevier: London, UK; Waltham, MA, USA, 2012; Chapter 14; p. 353. ISBN 978-0-12-384905-2. [Google Scholar]
- Greger, M. Influence of willow (Salix viminalis L.) roots on soil metal chemistry: Effects of clones with varying metal uptake potential. In Biogeochemistry of Trace Elements in the Rhizosphere; Huang, P.M., Gobran, G.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 301–312. [Google Scholar]
- Chrostowski, P.; Durda, J.L.; Edelmann, K.G. The use of natural processes for the control of chromium migration. Remediat. J. 1991, 2, 341–351. [Google Scholar] [CrossRef]
- Smith, L.A.; Means, J.L.; Chen, A. Remedial Options for Metals-Contaminated Sites; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Park, K.S.; Sims, R.C.; Dupont, R. Transformation of PAHs in soil systems. J. Environ, Eng. (ASCE) 1990, 116, 623–640. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, M.S.; Mudliar, S.; Chakrabarti, T. Biodegradation of chlorinated compounds—A review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 165–198. [Google Scholar] [CrossRef]
| Site | Pb | Cd | Cu | As | PAH | PCB |
|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |
| Total conc. range | 2.3–2600 | <0.2–4.5 | 3.7–290 | <1.9–8.9 | <0.3–150 | <0.01–0.05 |
| KM | 50 | 0.8 | 80 | 10 | 1.0 | 0.008 |
| MKM | 400 | 12 | 200 | 25 | 10 | 0.2 |
| Contaminants | Reference Location | Location with S. viminalis | Control Location, C1 | Control Location, C2 | Control Location, C3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2015 | Removal | 2005 | 2015 | Removal | 2005 | 2022 | Removal | 2005 | 2022 | Removal | 2005 | 2022 | Removal | |
| (mg kg−1) | (%) | (mg kg−1) | (%) | (mg kg−1) | (%) | (mg kg−1) | (%) | (mg kg−1) | (%) | ||||||
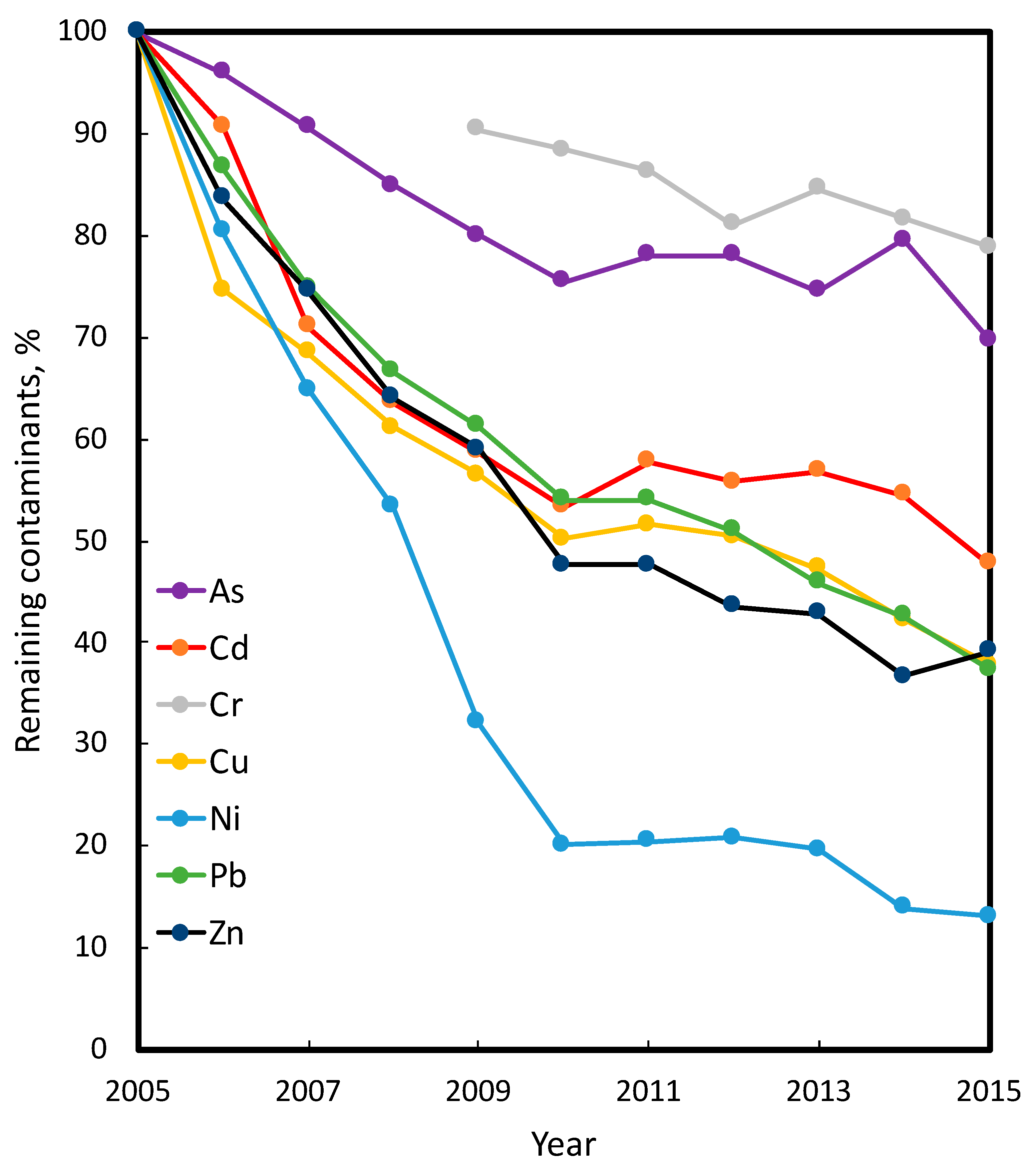
| Metals and metalloids | |||||||||||||||
| Cr | 0.25 | 0.26 | 0 | 9.0 | 7.1 | 21.1 ** | 7.96 | 8.23 | 0 | 7.72 | 7.54 | 2.3 | 5.68 | 6.11 | 0 |
| As | 0.15 | 0.15 | 0 | 5.3 | 3.7 | 30.2 ** | 1.28 | 1.21 | 5.4 | 3.77 | 3.63 | 3.7 | 1.84 | 1.97 | 0 |
| Cd | 0.13 | 0.13 | 0 | 4.4 | 2.1 | 54.5 *** | 0.76 | 0.74 | 2.6 | 0.38 | 0.39 | 0 | 0.34 | 0.35 | 0 |
| Zn | 52 | 52 | 0 | 64 | 25 | 60.9 *** | 62 | 60 | 3.2 | 60 | 58 | 3.3 | 50 | 49 | 2 |
| Cu | 32 | 33 | 0 | 294 | 111 | 62.2 *** | 4.3 | 4.4 | 0 | — | 2.1 | 0 | 3.7 | 3.5 | 5.4 |
| Pb | 25 | 24 | 0 | 2350 | 879 | 62.6 *** | 130 | 132 | 0 | 42 | 44.5 | 0 | 56 | 55.2 | 1.4 |
| Ni | 5.5 | 5.5 | 0 | 15.3 | 2.0 | 86.9 *** | 11.9 | 12.3 | 0 | 11.3 | 10.7 | 5.3 | 9.61 | 9.73 | 0 |
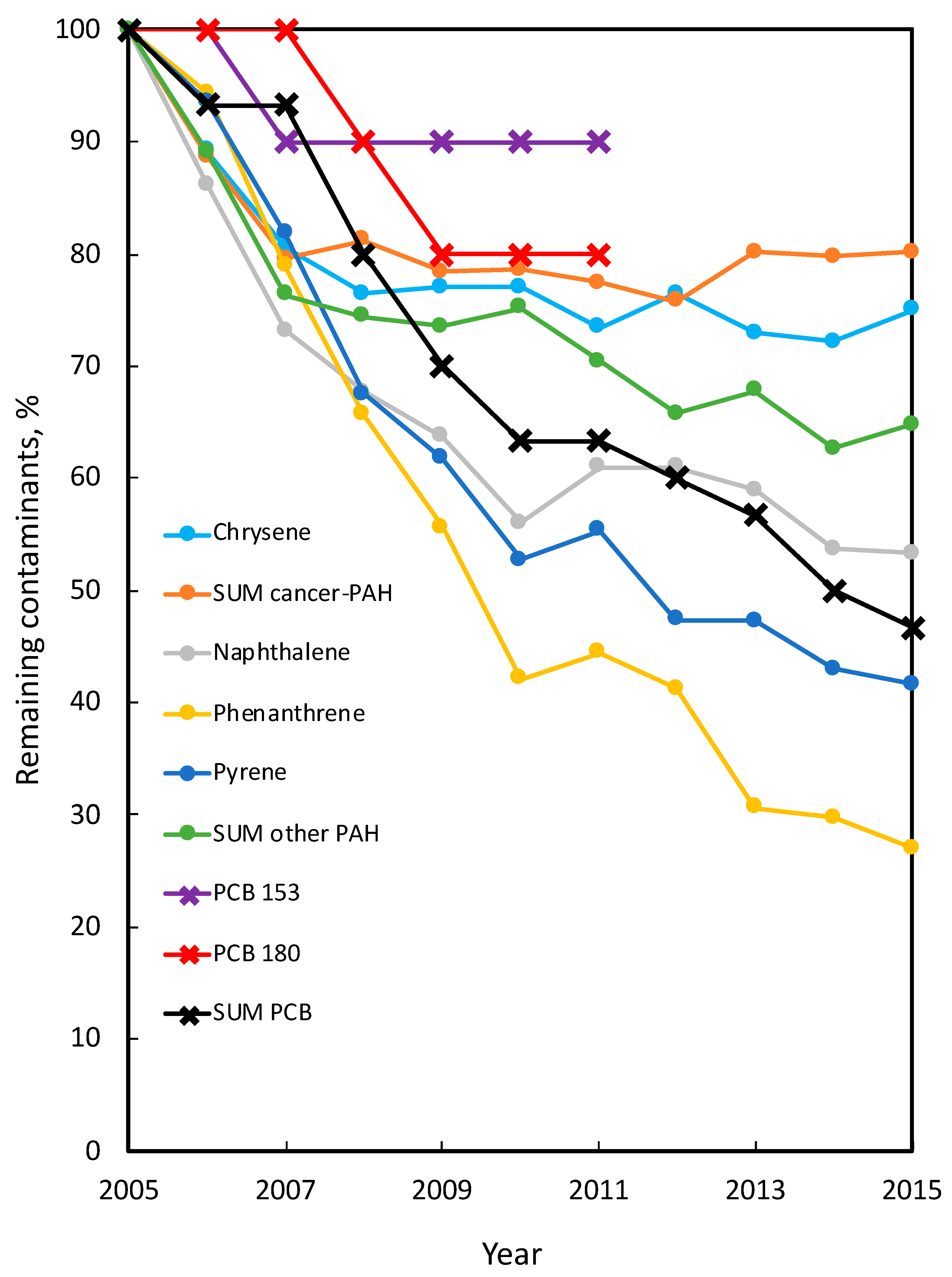
| PAHs | |||||||||||||||
| Chrysene | 0.09 | 0.09 | 0 | 0.36 | 0.27 | 25.0 * | 0.32 | 0.31 | 3.1 | 0.24 | 0.22 | 8.3 | 0.41 | 0.41 | 0 |
| Sum of carcinogenic PAHs | 0.31 | 0.31 | 0 | 0.96 | 0.77 | 19.8 | 0.83 | 0.79 | 4.8 | 0.79 | 0.77 | 2.5 | 1.25 | 1.29 | 0 |
| Naphthalene | 0.25 | 0.24 | 0 | 0.92 | 0.49 | 46.7 *** | 0.30 | 0.31 | 0 | 0.45 | 0.47 | 0 | 0.91 | 0.89 | 2.2 |
| Phenanthrene | 0.08 | 0.08 | 0 | 0.37 | 0.10 | 73.0 *** | — | — | — | — | — | — | — | — | — |
| Pyrene | 0.18 | 0.18 | 0 | 0.77 | 0.32 | 54.3 *** | 0.31 | 0.29 | 6.5 | 0.55 | 0.54 | 1.8 | 0.75 | 0.76 | 0 |
| Sum of other PAHs | 0.86 | 0.82 | 0 | 2.67 | 1.73 | 35.2 *** | 1.20 | 1.22 | 0 | 1.79 | 1.83 | 0 | 3.28 | 3.32 | 0 |
| PCBs | |||||||||||||||
| PCB 153 | — | — | 0.01 | 0.009 Y | 10.0 Y,*** | — | — | — | — | — | — | — | — | — | |
| PCB 180 | — | — | 0.01 | 0.008 Y | 20.0 Y,* | — | — | — | — | — | — | — | — | — | |
| Sum of PCBs | — | — | 0.03 | 0.014 | 53.3 *** | 0.03 | 0.03 | 0 | 0.03 | 0.03 | 0 | 0.03 | 0.03 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landberg, T.; Greger, M. Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability 2022, 14, 8449. https://doi.org/10.3390/su14148449
Landberg T, Greger M. Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability. 2022; 14(14):8449. https://doi.org/10.3390/su14148449
Chicago/Turabian StyleLandberg, Tommy, and Maria Greger. 2022. "Phytoremediation Using Willow in Industrial Contaminated Soil" Sustainability 14, no. 14: 8449. https://doi.org/10.3390/su14148449
APA StyleLandberg, T., & Greger, M. (2022). Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability, 14(14), 8449. https://doi.org/10.3390/su14148449






