Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets, Investigational Design, and Housing
2.2. Ration Chemical Analysis
2.3. Milk Yield and Composition
2.4. Serum Biochemistry
2.5. Statistical Analysis
3. Results
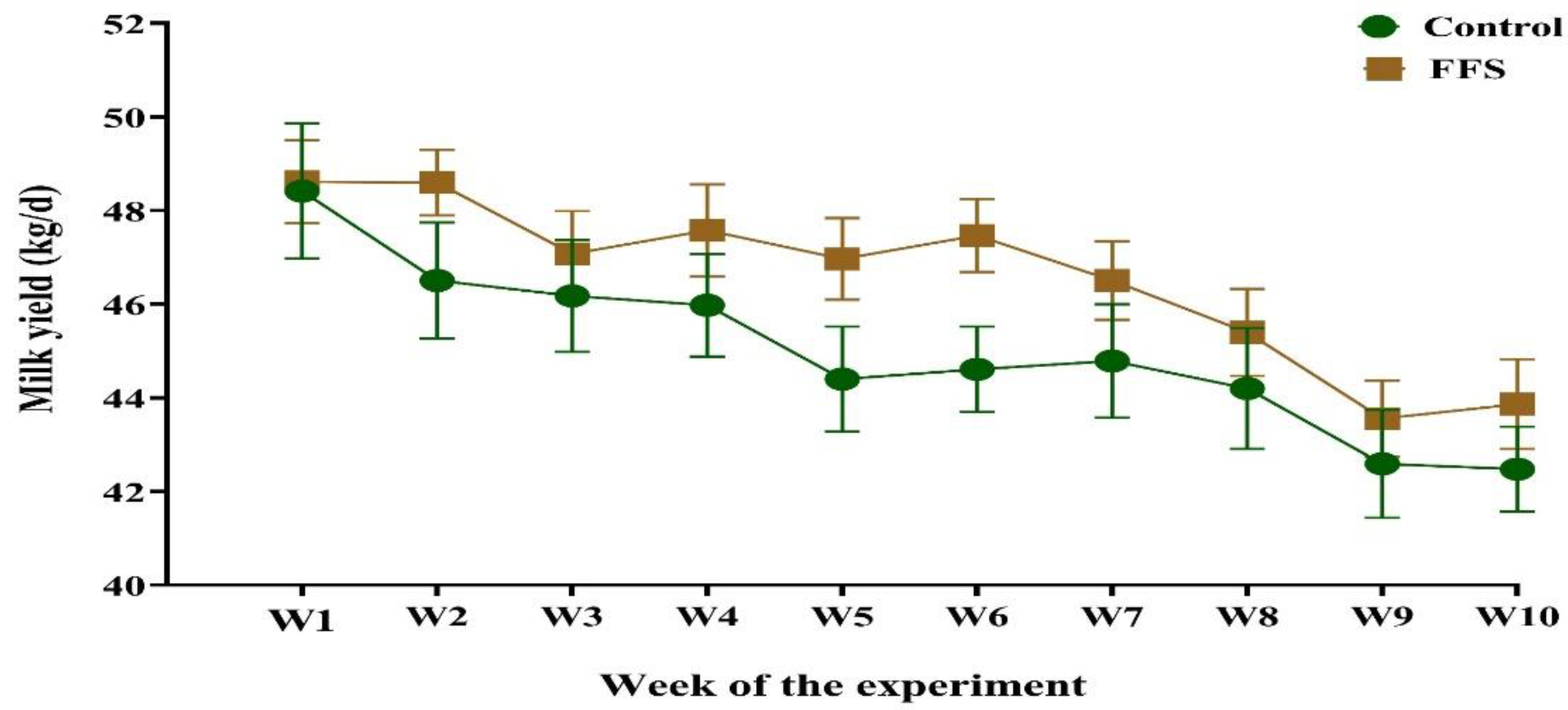
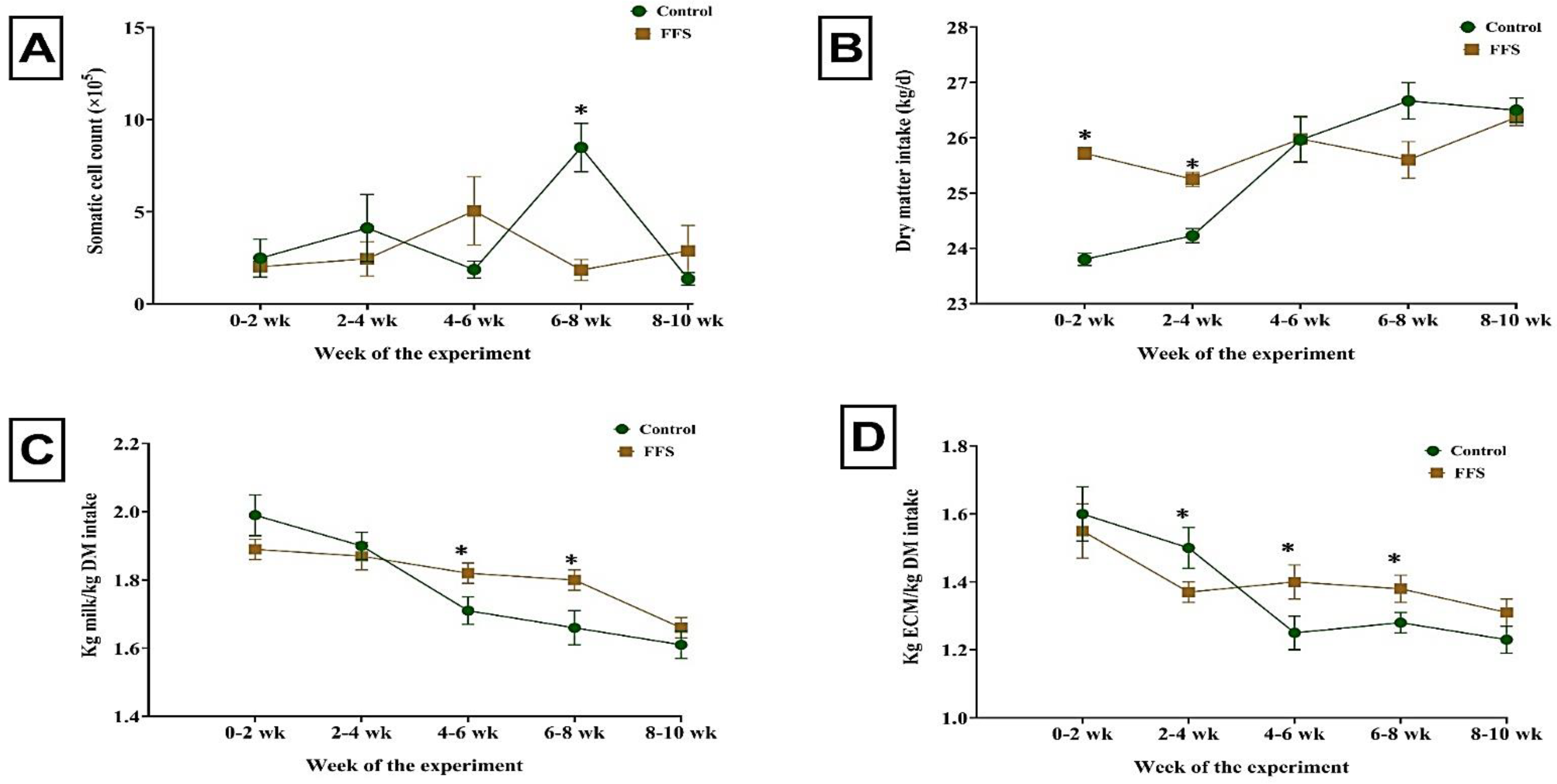
3.1. Lactation Performance, Feed Intake, and Feed Efficiency
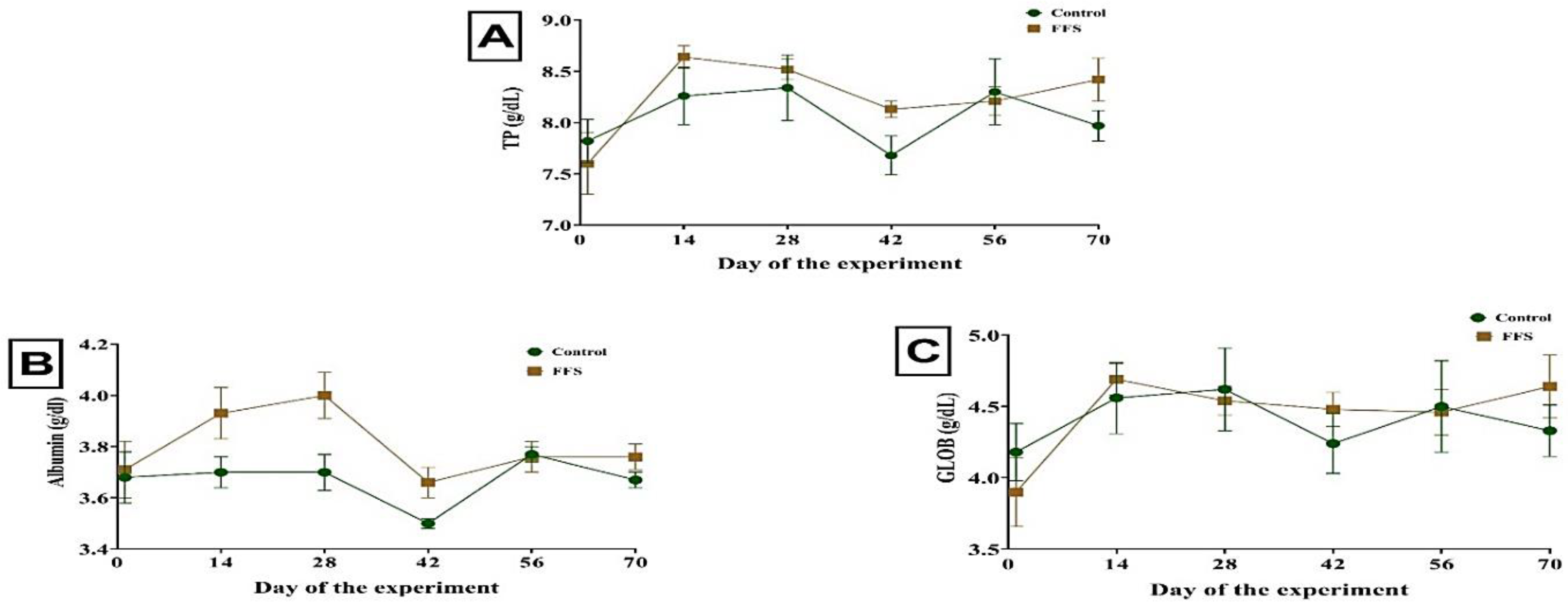
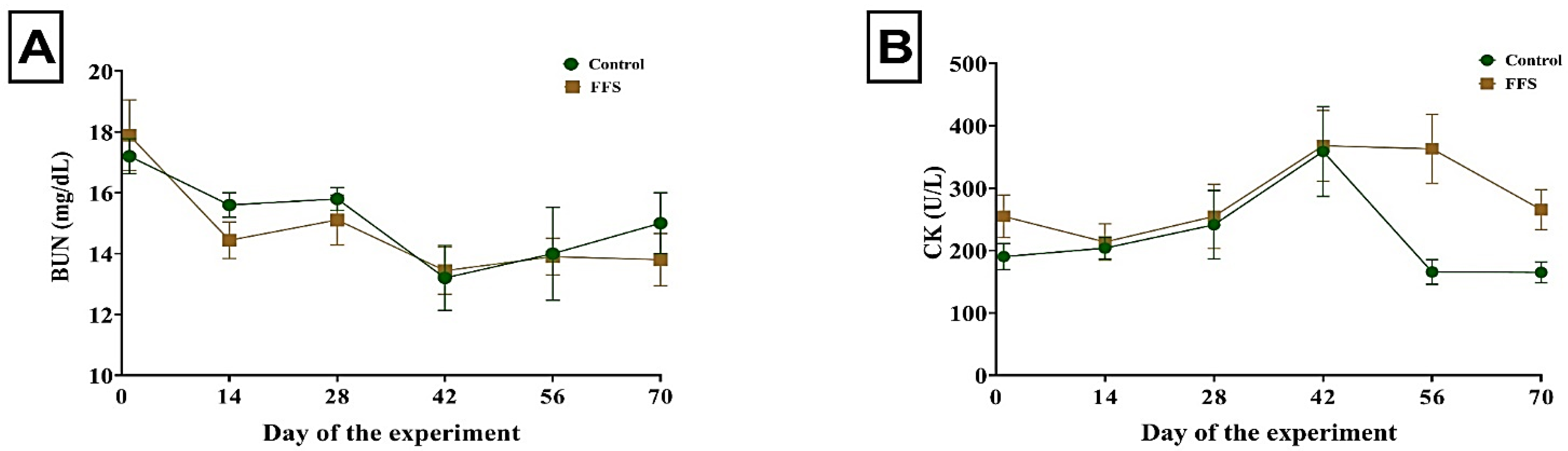
3.2. Blood Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britt, J.; Cushman, R.; Dechow, C.; Dobson, H.; Humblot, P.; Hutjens, M.; Jones, G.; Ruegg, P.; Sheldon, I.; Stevenson, J. Invited review: Learning from the future—A vision for dairy farms and cows in 2067. J. Dairy Sci. 2018, 101, 3722–3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesogan, A.T.; Dahl, G.E. MILK Symposium Introduction: Dairy production in developing countries. J. Dairy Sci. 2020, 103, 9677–9680. [Google Scholar] [CrossRef]
- Mostafa, T.; Al-Sagheer, A.; Arafa, M.; Ayyat, M. Changes in milk production, hematology, metabolites, mineral and hormonal parameters of primiparous and multiparous Maghrebi dairy she-camel during nonbreeding season. Biol. Rhythm. Res. 2022, 53, 216–233. [Google Scholar] [CrossRef]
- Alqaisi, O.; Ndambi, O.A.; Uddin, M.M.; Hemme, T. Current situation and the development of the dairy industry in Jordan, Saudi Arabia, and Syria. Trop. Anim. Health Prod. 2010, 42, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.G.; Takiya, C.S.; Del Valle, T.A.; de Jesus, E.F.; Grigoletto, N.T.; Nakadonari, B.; Cortinhas, C.S.; Acedo, T.S.; Rennó, F.P. Nutrient digestibility, ruminal fermentation, and milk yield in dairy cows fed a blend of essential oils and amylase. J. Dairy Sci. 2018, 101, 9815–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawod, A.; Ahmed, H.; Abou-Elkhair, R.; Elbaz, H.T.; Taha, A.E.; Swelum, A.A.; Alhidary, I.A.; Saadeldin, I.M.; Al-Ghadi, M.Q.; Ba-Awadh, H.A. Effects of extruded linseed and soybean dietary supplementation on lactation performance, first-service conception rate, and mastitis incidence in holstein dairy cows. Animals 2020, 10, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryszak, M.; Szumacher-Strabel, M.; El-Sherbiny, M.; Stochmal, A.; Oleszek, W.; Roj, E.; Patra, A.K.; Cieslak, A. Effects of berry seed residues on ruminal fermentation, methane concentration, milk production, and fatty acid proportions in the rumen and milk of dairy cows. J. Dairy Sci. 2019, 102, 1257–1273. [Google Scholar] [CrossRef] [Green Version]
- Tilahun, M.; Zhao, L.; Guo, Z.; Shen, Y.; Ma, L.; Callaway, T.R.; Xu, J.; Bu, D. Amla (Phyllanthus emblica) fresh fruit as new feed source to enhance ruminal fermentation and milk production in lactating dairy cows. Anim. Feed Sci. Technol. 2022, 283, 115160. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Al-Sagheer, A.; Noreldin, A.E.; Abd El-Hack, M.E.; Khafaga, A.F.; Abdel-Latif, M.A.; Swelum, A.A.; Arif, M.; Salem, A.Z. Beneficial effects of rumen-protected methionine on nitrogen-use efficiency, histological parameters, productivity and reproductive performance of ruminants. Anim. Biotechnol. 2021, 32, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Grazziotin, R.; Halfen, J.; Rosa, F.; Schmitt, E.; Anderson, J.; Ballard, V.; Osorio, J. Altered rumen fermentation patterns in lactating dairy cows supplemented with phytochemicals improve milk production and efficiency. J. Dairy Sci. 2020, 103, 301–312. [Google Scholar] [CrossRef]
- Beauchemin, K.; McGinn, S.; Martinez, T.; McAllister, T. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [Green Version]
- Al-Sagheer, A.A.; Elwakeel, E.A.; Ahmed, M.G.; Sallam, S.M.A. Potential of guava leaves for mitigating methane emissions and modulating ruminal fermentation characteristics and nutrient degradability. Environ. Sci. Pollut. Res. 2018, 25, 31450–31458. [Google Scholar] [CrossRef]
- Elcoso, G.; Zweifel, B.; Bach, A. Effects of a blend of essential oils on milk yield and feed efficiency of lactating dairy cows. Appl. Anim. Sci. 2019, 35, 304–311. [Google Scholar] [CrossRef]
- Silva, S.N.S.e.; Chabrillat, T.; Kerros, S.; Guillaume, S.; Gandra, J.R.; de Carvalho, G.G.P.; Silva, F.F.d.; Mesquita, L.G.; Gordiano, L.A.; Camargo, G.M.F.; et al. Effects of plant extract supplementations or monensin on nutrient intake, digestibility, ruminal fermentation and metabolism in dairy cows. Anim. Feed Sci. Technol. 2021, 275, 114886. [Google Scholar] [CrossRef]
- Xu, J.; Jia, Z.; Chen, A.; Wang, C. Curcumin ameliorates Staphylococcus aureus-induced mastitis injury through attenuating TLR2-mediated NF − κB activation. Microb. Pathog. 2020, 142, 104054. [Google Scholar] [CrossRef]
- Jaguezeski, A.M.; Perin, G.; Bottari, N.B.; Wagner, R.; Fagundes, M.B.; Schetinger, M.R.C.; Morsch, V.M.; Stein, C.S.; Moresco, R.N.; Barreta, D.A.; et al. Addition of curcumin to the diet of dairy sheep improves health, performance and milk quality. Anim. Feed Sci. Technol. 2018, 246, 144–157. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Harper, M.; Giallongo, F.; Bravo, D.; Wall, E.; Hristov, A. Effects of rumen-protected Capsicum oleoresin on immune responses in dairy cows intravenously challenged with lipopolysaccharide. J. Dairy Sci. 2017, 100, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, V.; Roy, D.; Kushwaha, R.; Vaiswani, S. Application of herbal feed additives in animal nutrition-a review. Int. J. Livest. Res. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hosoda, K.; Nishida, T.; Park, W.; Eruden, B. Influence of Mentha× piperita L.(peppermint) supplementation on nutrient digestibility and energy metabolism in lactating dairy cows. Asian-Australas. J. Anim. Sci. 2005, 18, 1721–1726. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists Official Method of Analysis; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.V.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001.
- Sjaunja, L.O.; Baevre, L.; Junkkarinen, L.; Pedersen, J.; Setala, J.A. Nordic proposal for an energy corrected milk (ECM) formula: Performance recording of animals. State Art. EAAP Publ. 1991, 50, 156–157. [Google Scholar]
- Erdman, R. Monitoring feed efficiency in dairy cows using fat-corrected milk per unit of dry matter intake. In Proceedings of the 9th Annual Mid-Atlantic Nutrition Conference, Timonium, MD, USA, 23–24 March 2011; pp. 69–79. [Google Scholar]
- Tyrrell, H.F.; Reid, J.T. Prediction of the energy value of cow’s Milk1, 2. J. Dairy Sci. 1965, 48, 1215–1223. [Google Scholar] [CrossRef]
- SAS. SAS Users Guide Statistical Analyses Systems Institute; SAS: Cary, NC, USA, 2002. [Google Scholar]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, G.; Kadivar, M.; Riasi, A.; Zebeli, Q. Effects of supplementation with a phytobiotics-rich herbal mixture on performance, udder health, and metabolic status of Holstein cows with various levels of milk somatic cell counts. J. Dairy Sci. 2014, 97, 7487–7497. [Google Scholar] [CrossRef] [Green Version]
- Wall, E.H.; Doane, P.H.; Donkin, S.S.; Bravo, D. The effects of supplementation with a blend of cinnamaldehyde and eugenol on feed intake and milk production of dairy cows. J. Dairy Sci. 2014, 97, 5709–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanch, M.; Carro, M.; Ranilla, M.J.; Viso, A.; Vázquez-Añón, M.; Bach, A. Influence of a mixture of cinnamaldehyde and garlic oil on rumen fermentation, feeding behavior and performance of lactating dairy cows. Anim. Feed Sci. Technol. 2016, 219, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [Green Version]
- Alsaht, A.A.; Bassiony, S.M.; Abdel-Rahman, G.A.; Shehata, S.A. Effect of cinnamaldehyde thymol mixture on growth performance and some ruminal and blood constituents in growing lambs fed high concentrate diet. Life Sci. J. 2014, 11, 240–248. [Google Scholar]
- Abulaiti, A.; Ahmed, Z.; Naseer, Z.; El-Qaliouby, H.; Iqbal, M.; Hua, G.; Yang, L. Effect of capsaicin supplementation on lactational and reproductive performance of Holstein cows during summer. Anim. Prod. Sci. 2021, 61, 1321–1328. [Google Scholar] [CrossRef]
- Petit, H. Feed intake, milk production and milk composition of dairy cows fed flaxseed. Can. J. Anim. Sci. 2010, 90, 115–127. [Google Scholar] [CrossRef]
- Spanghero, M.; Robinson, P.H.; Zanfi, C.; Fabbro, E. Effect of increasing doses of a microencapsulated blend of essential oils on performance of lactating primiparous dairy cows. Anim. Feed Sci. Technol. 2009, 153, 153–157. [Google Scholar] [CrossRef]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Wei Tan, A.C.; Leong, W.H.; Yin Chia, A.Y.; Vijayabalan, S.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S.; et al. Synergistic Effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front. Aging Neurosci. 2019, 11, 206. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Hristov, A.; Lee, C.; Cassidy, T.; Heyler, K.; Varga, G.; Pate, J.; Walusimbi, S.; Brzezicka, E.; Toyokawa, K. Immune and production responses of dairy cows to postruminal supplementation with phytonutrients. J. Dairy Sci. 2013, 96, 7830–7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Olives, A.M.; Díaz, J.; Molina, M.; Peris, C. Quantification of milk yield and composition changes as affected by subclinical mastitis during the current lactation in sheep. J. Dairy Sci. 2013, 96, 7698–7708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caboni, P.; Manis, C.; Ibba, I.; Contu, M.; Coroneo, V.; Scano, P. Compositional profile of ovine milk with a high somatic cell count: A metabolomics approach. Int. Dairy J. 2017, 69, 33–39. [Google Scholar] [CrossRef]
- Arif, M.; Al-Sagheer, A.A.; Salem, A.Z.M.; El-Hack, M.E.A.; Swelum, A.A.; Saeed, M.; Jamal, M.; Akhtar, M. Influence of exogenous fibrolytic enzymes on milk production efficiency and nutrient utilization in early lactating buffaloes fed diets with two proportions of oat silage to concentrate ratios. Livest. Sci. 2019, 219, 29–34. [Google Scholar] [CrossRef]
- Sevinc, M.; Basoglu, A.; Birdane, F.; Boydak, M. Liver function in dairy cows with fatty liver. Rev. Med. Vet. (Toulouse) 2001, 152, 297–300. [Google Scholar]
- Liu, P.; He, B.; Yang, X.; Hou, X.; Han, J.; Han, Y.; Nie, P.; Deng, H.; Du, X. Bioactivity evaluation of certain hepatic enzymes in blood plasma and milk of Holstein Cows. Pak. Vet. J. 2012, 32, 601–604. [Google Scholar]
- Noro, M.; Cid, T.P.; Wagemann, F.C.; Arnés, V.V.; Wittwer, M.F. Valoración diagnóstica de enzimas hepáticas en perfiles bioquímicos sanguíneos de vacas lecheras. Rev. MVZ Córdoba 2013, 18, 3474–3479. [Google Scholar] [CrossRef] [Green Version]
- Sattler, T.; Fürll, M. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J. Vet. Medicine. A Physiol. Pathol. Clin. Med. 2004, 51, 132–137. [Google Scholar] [CrossRef]
- Nozad, S.; Ramin, A.-G.; Moghadam, G.; Asri-Rezaei, S.; Babapour, A.; Ramin, S. Relationship between blood urea, protein, creatinine, triglycerides and macro-mineral concentrations with the quality and quantity of milk in dairy Holstein cows. Vet. Res. Forum 2012, 3, 55–59. [Google Scholar]
- Golher, D.; Patel, B.; Bhoite, S.; Syed, M.; Panchbhai, G.; Thirumurugan, P. Factors influencing water intake in dairy cows: A review. Int. J. Biometeorol. 2021, 65, 617–625. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine E-Book: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Rook, J.S. Pregnancy toxemia of ewes, does, and beef cows. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 293–317. [Google Scholar] [CrossRef]
- Plaizier, J.; Krause, D.; Gozho, G.; McBride, B. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Waghorn, G.; Ulyatt, M.; John, A.; Fisher, M. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. Br. J. Nutr. 1987, 57, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Doncel, B.; Capelesso, A.; Giannitti, F.; Cajarville, C.; Macías-Rioseco, M.; Silveira, C.; Costa, R.A.; Riet-Correa, F. Hypomagnesemia in dairy cattle in Uruguay. Pesqui. Vet. Bras. 2019, 39, 564–572. [Google Scholar] [CrossRef]
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef]

| Ingredients | [g/kg Feed] | Nutrient Levels | [g/kg Feed] |
|---|---|---|---|
| Corn grain | 295.6 | Chemical analysis 3 | |
| Wheat bran | 17.7 | Organic matter | 921.6 |
| Soybean meal | 31.9 | Crude protein (N × 6.25) | 158.6 |
| Corn silage | 49.6 | Ether extract | 40.4 |
| Uncorticated cottonseed meal | 85.0 | Neutral detergent fiber | 304.0 |
| Alfalfa hay | 343.4 | Acid detergent fiber | 194.4 |
| Wheat straw | 70.8 | NFC 4 | 418.6 |
| Limestone | 12.3 | Calculated values 5 | |
| Sodium bicarbonate | 7.3 | Digestible crude protein | 669.1 |
| Canola meal oil-free | 70.7 | Metabolizable energy, Mcal/kg | 2.19 |
| Palm oil hydrogenated | 7.8 | Net energy for lactation, Mcal/kg | 1.38 |
| Calcium salt palm oil | 5.9 | NDICP | 18.4 |
| Premix 2 | 1.2 | ADICP | 12.4 |
| Distiller yeast | 0.4 | ||
| Mycotoxin binder | 0.4 |
| Treatment | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | FFS | Treatment | Period | Treatment × Period | ||
| Number of cows | 30 | 30 | - | - | - | - |
| Production (kg/d) | ||||||
| Milk | 45.01 | 46.59 | 0.320 | 0.010 | <0.001 | 0.918 |
| Fat corrected milk 3.5% | 34.77 | 36.43 | 0.743 | 0.100 | <0.001 | 0.206 |
| Energy corrected milk (ECM) | 34.74 | 36.15 | 0.436 | 0.093 | 0.001 | 0.329 |
| Total solids | 4.98 | 5.04 | 0.057 | 0.512 | <0.001 | 0.564 |
| Solids-not-fat | 4.03 | 4.14 | 0.030 | 0.050 | 0.091 | 0.946 |
| Fat | 0.93 | 0.99 | 0.029 | 0.285 | 0.001 | 0.184 |
| Protein | 1.50 | 1.55 | 0.011 | 0.051 | 0.030 | 0.943 |
| Lactose | 2.12 | 2.25 | 0.016 | 0.051 | 0.078 | 0.948 |
| Ash | 0.38 | 0.36 | 0.016 | 0.412 | 0.053 | 0.626 |
| Milk energy output (MJ/d) | 107.86 | 112.21 | 1.367 | 0.098 | 0.001 | 0.333 |
| Milk composition (g/kg) | ||||||
| Total solids | 110.62 | 108.15 | 0.897 | 0.169 | 0.099 | 0.525 |
| Solids-not-fat | 89.48 | 87.46 | 0.311 | 0.349 | <0.001 | 0.860 |
| Fat | 21.14 | 20.69 | 0.584 | 0.692 | 0.032 | 0.225 |
| Protein | 33.42 | 33.22 | 0.123 | 0.348 | <0.001 | 0.869 |
| Lactose | 48.70 | 48.42 | 0.165 | 0.356 | <0.001 | 0.859 |
| Ash | 8.42 | 7.60 | 0.317 | 0.200 | 0.206 | 0.715 |
| Somatic cell count (×105) | 3.68 | 2.85 | 0.55 | 0.452 | 0.395 | 0.049 |
| Milk energy content (MJ/kg) | 2.40 | 2.41 | 0.024 | 0.872 | 0.136 | 0.366 |
| Dry matter intake (kg/d) | 25.40 | 25.76 | 0.733 | 0.536 | 0.279 | 0.064 |
| Feed efficiency | ||||||
| Kg milk/kg DM intake | 1.78 | 1.81 | 0.014 | 0.187 | <0.001 | 0.011 |
| Kg ECM/kg DM intake | 1.37 | 1.40 | 0.018 | 0.361 | <0.001 | 0.046 |
| Treatment | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | FFS | Treatment | Period | Treatment × Period | ||
| Total protein, g/dL | 8.05 | 8.21 | 0.060 | 0.154 | 0.004 | 0.578 |
| Albumin, g/dL | 3.65 | 3.80 | 0.024 | 0.007 | 0.039 | 0.599 |
| Globulin, g/dL | 4.41 | 4.42 | 0.053 | 0.813 | 0.081 | 0.803 |
| Urea nitrogen, mg/dL | 15.35 | 14.93 | 0.255 | 0.424 | 0.001 | 0.927 |
| Creatine kinase, U/L | 221.23 | 273.23 | 26.123 | 0.326 | 0.659 | 0.988 |
| Alkaline phosphatase, U/L | 40.06 | 38.66 | 1.158 | 0.628 | 0.568 | 0.956 |
| Alanine aminotransferase, U/L | 70.55 | 74.66 | 1.515 | 0.114 | <0.001 | 0.566 |
| Gamma glutamyl transferase, U/L | 27.68 | 29.26 | 0.919 | 0.221 | 0.774 | 0.999 |
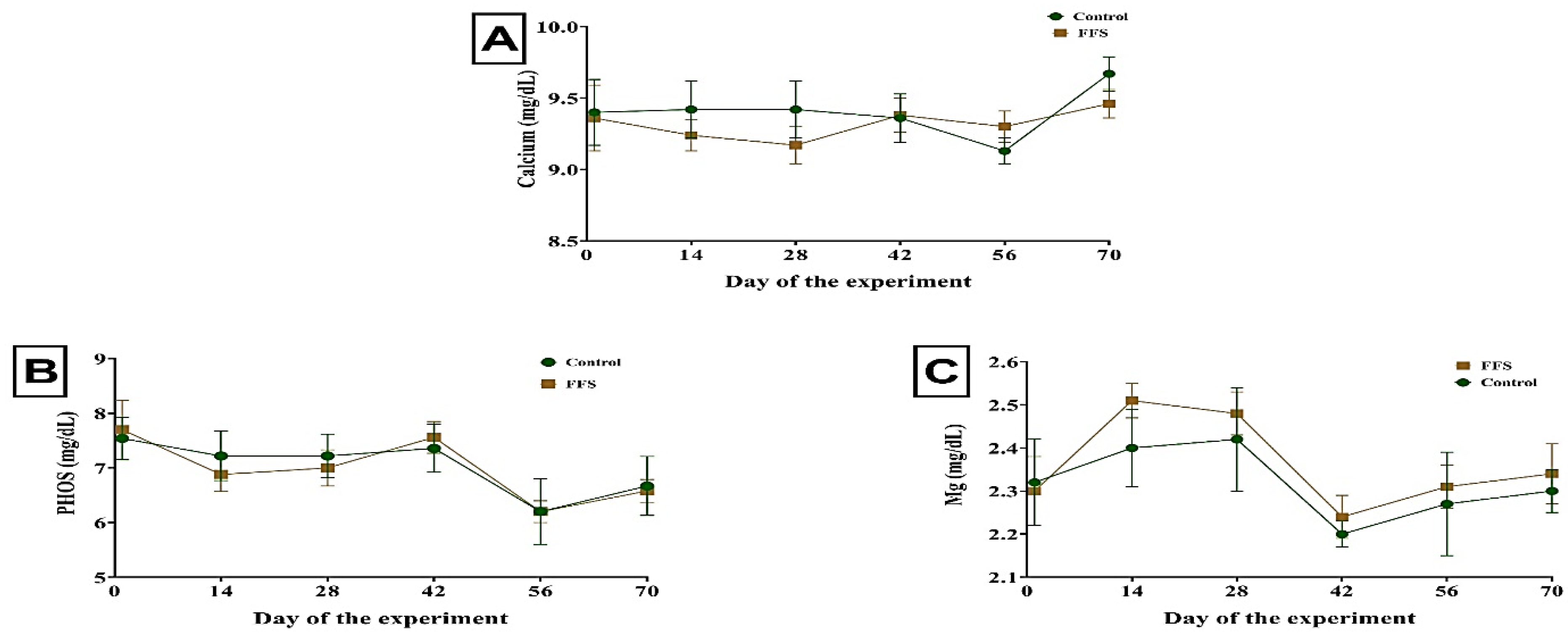
| Calcium, mg/dL | 9.42 | 9.34 | 0.042 | 0.417 | 0.530 | 0.928 |
| Phosphorus, mg/dL | 7.06 | 7.13 | 0.109 | 0.642 | 0.030 | 0.656 |
| Magnesium, mg/dL | 2.31 | 2.37 | 0.019 | 0.148 | 0.009 | 0.959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSuwaiegh, S.B.; Almotham, A.M.; Alyousef, Y.M.; Mansour, A.T.; Al-Sagheer, A.A. Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation. Sustainability 2022, 14, 8444. https://doi.org/10.3390/su14148444
AlSuwaiegh SB, Almotham AM, Alyousef YM, Mansour AT, Al-Sagheer AA. Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation. Sustainability. 2022; 14(14):8444. https://doi.org/10.3390/su14148444
Chicago/Turabian StyleAlSuwaiegh, Shaker B., Abdalrahman M. Almotham, Yousef Mohammad Alyousef, Abdallah Tageldein Mansour, and Adham A. Al-Sagheer. 2022. "Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation" Sustainability 14, no. 14: 8444. https://doi.org/10.3390/su14148444
APA StyleAlSuwaiegh, S. B., Almotham, A. M., Alyousef, Y. M., Mansour, A. T., & Al-Sagheer, A. A. (2022). Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation. Sustainability, 14(14), 8444. https://doi.org/10.3390/su14148444








