Hydrogen Fuel for Future Mobility: Challenges and Future Aspects
Abstract
:1. Introduction
2. Issues and Concerns of Present Vehicle Fuel
3. Motivation for Hydrogen Energy
3.1. FCEV vs. BEV
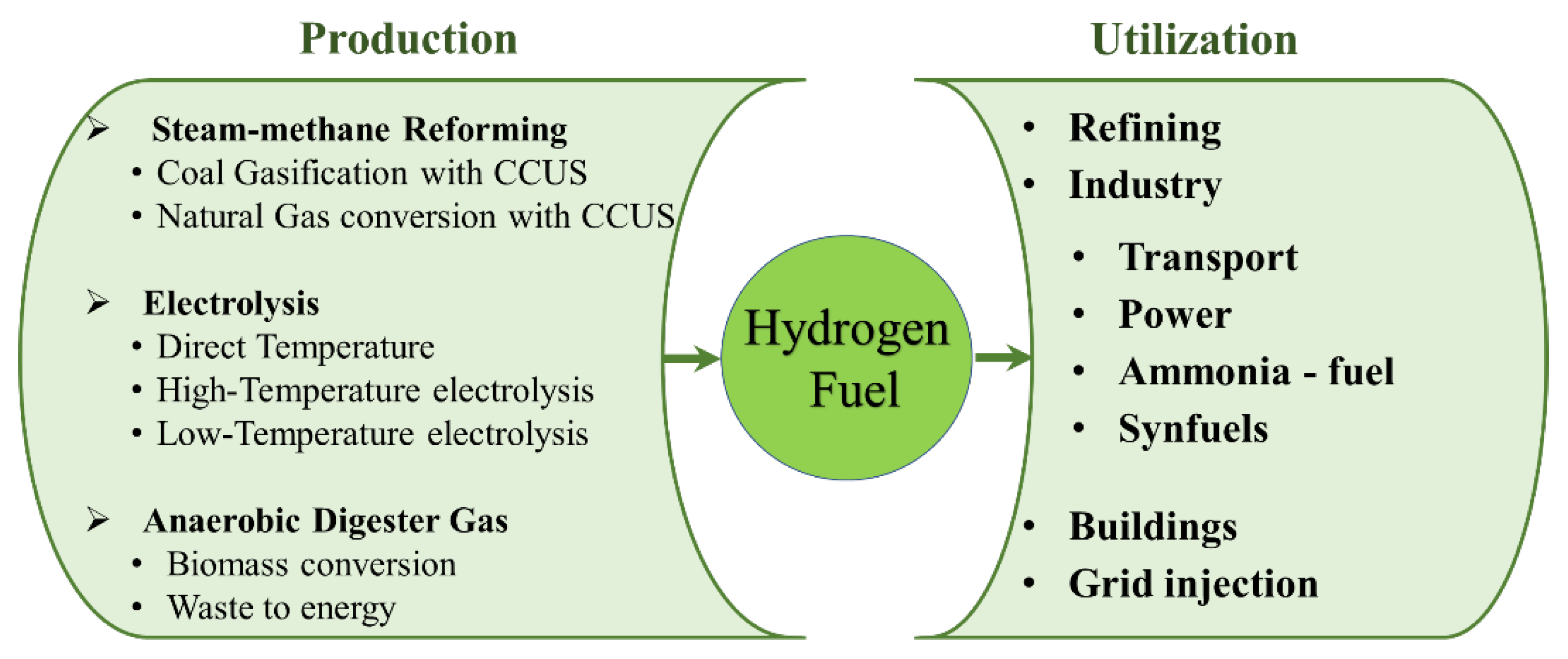
3.2. Present Status of Hydrogen Fuel Utilization
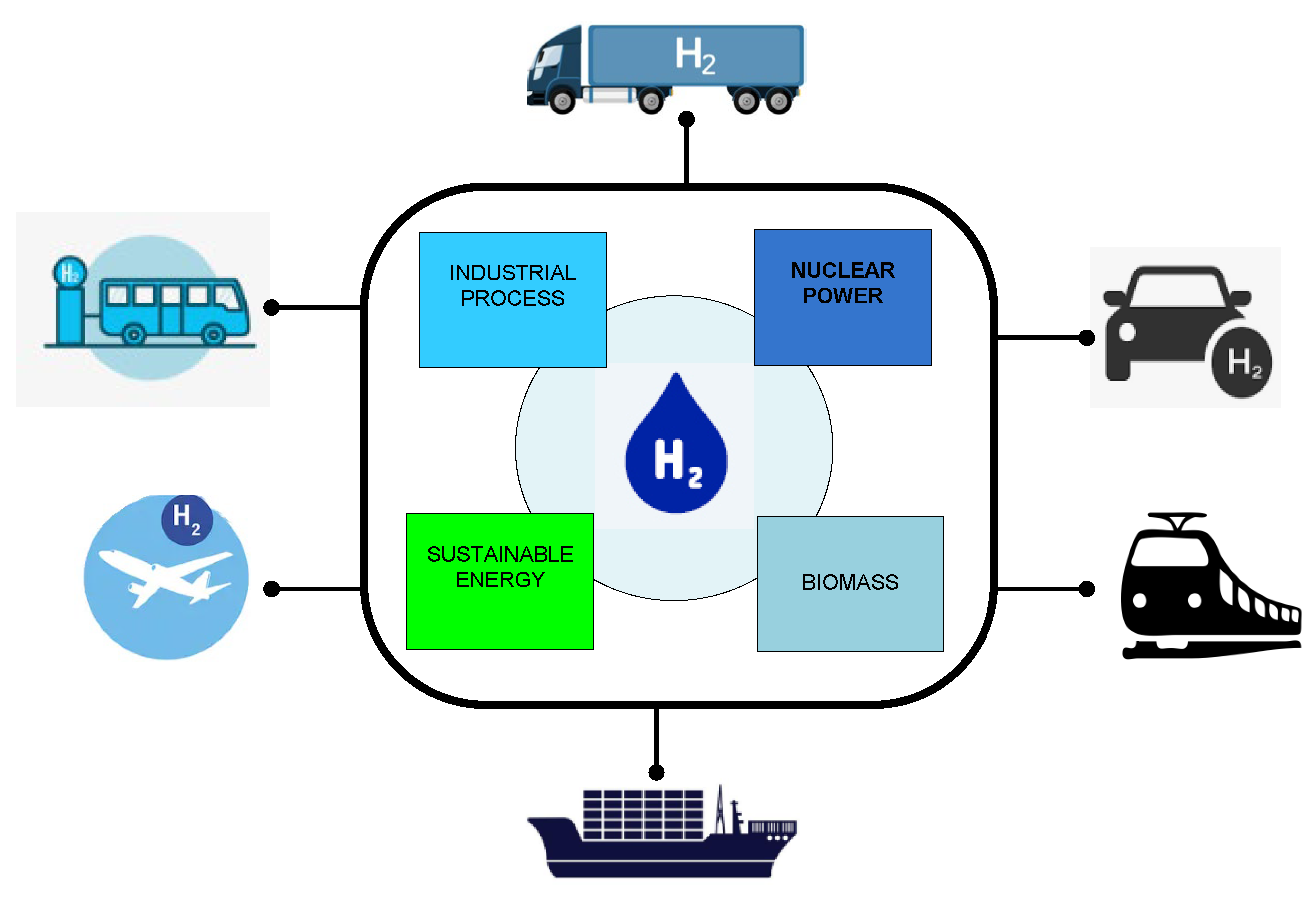
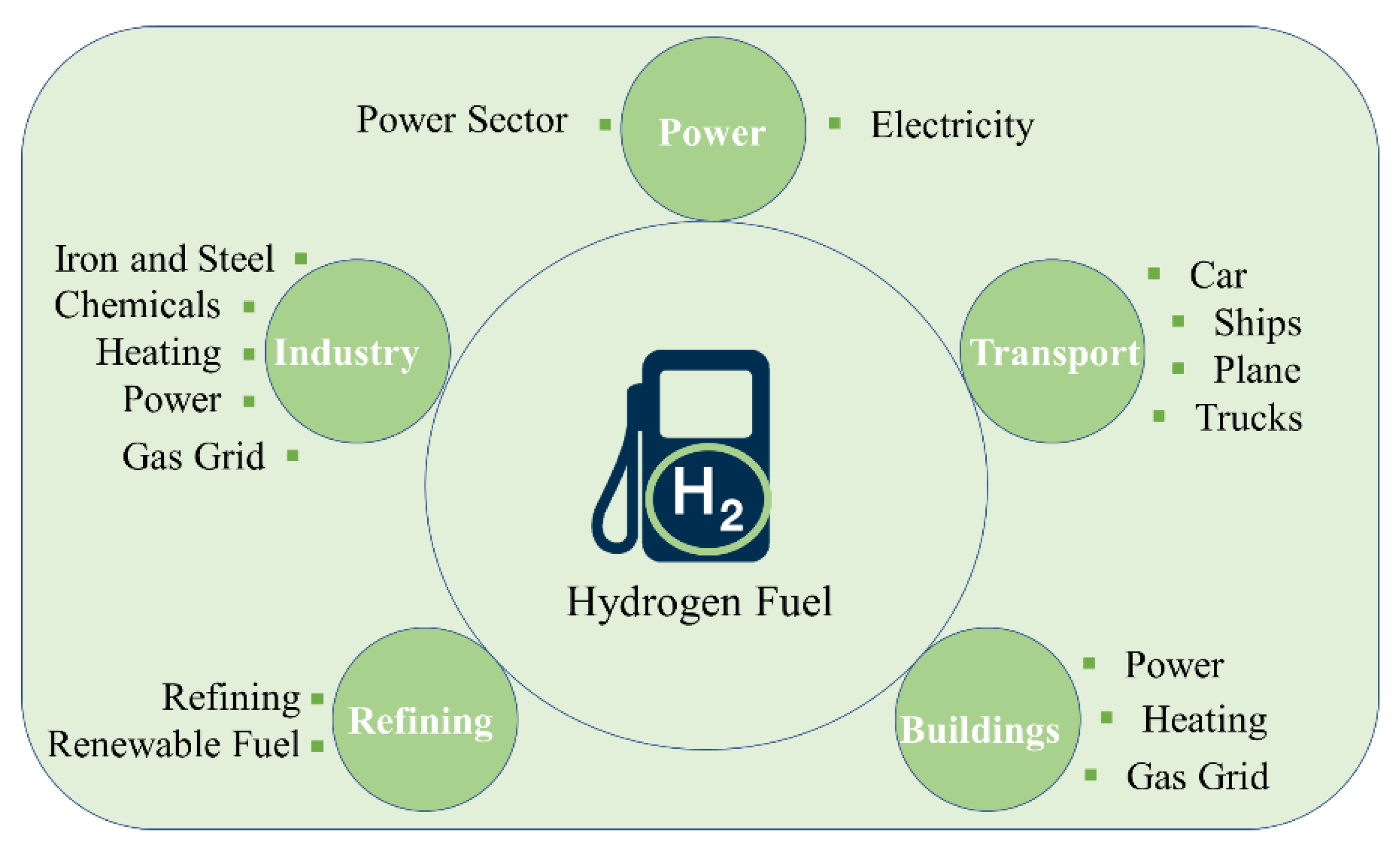
3.3. Utilization Hydrogen Fuel in Different Countries and Sectors
3.4. Consumer Perspective
- Due to the non-availability, storage, and emission concerns with hydrogen fuel in many nations, most light-duty vehicles (cars and vans) and heavy-duty vehicles (trucks and buses) now use non-renewable fuel. This opens up several opportunities to minimize the refueling time, storage requirements, and emissions in hydrogen fuel production and utilization. In addition, hydrogen fuel cells use less material than lithium batteries. However, 11,200 automobiles, predominantly in California, Europe, and Japan, use hydrogen fuel [55]. Long-distance travel and poor filling station usage are still major issues in hydrogen-based transportation [70].
- The use of hydrogen fuel in maritime transportation has been studied and proven in small ships. By 2030, the use of hydrogen fuel in ships will reduce air pollution while simultaneously increasing freight activity by roughly 45 percent. Because hydrogen fuel has a lower density than conventional liquid fuels, it has significant hurdles in terms of storage costs and cargo capacity.
- In rail transportation, hydrogen fuel-based trains are utilized in Germany. As a result, there are several opportunities to use hydrogen fuel in rail freight. Both hydrogen and battery electric trains with partial line electrification are viable replacements for non-electrified operations, which are prevalent in many areas [61].
- In aviation, hydrogen fuel utilization is demonstrated using small projects. Therefore, this brings a major opportunity to use hydrogen fuel in a fast-growing passenger transport mode. The redesign of the aviation model is needed to accommodate large storage volume. Additionally, the use of hydrogen fuel can be applied in airports for on-board energy supply.
4. Adaptation of On-Board Hydrogen Production Technology for Vehicles
4.1. System Designs for On-Board Hydrogen Production
4.2. Power Electronics for On-Board Hydrogen Production
5. Adaptation of Internal Combustion Production Technology for Vehicles
- Hydrogen’s lack of lubrication damages the injector nozzle’s sealing surface.
- Injectors must be very tiny to fit into the engine head where the four valves on each cylinder are placed.
- A strong dynamic reaction is required for multi-injection.
- Due to frictional heat, the internal pump of the liquid hydrogen tank would fail while delivering liquid hydrogen (LH2) to the required high-pressure levels.
5.1. Properties of Hydrogen as Fuel for Internal Combustion Engine
5.2. Hydrogen Use in Internal Combustion Engines
5.2.1. Spark Ignition Engines
5.2.2. Compression Ignition Engines
5.2.3. Natural Gas–Hydrogen Mixtures Engines
6. Comparative Analysis (On-Board Hydrogen Vehicle vs. Hydrogen-Fueled ICEs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Igliński, B.; Pietrzak, M.B.; Kiełkowska, U.; Skrzatek, M.; Gajdos, A.; Zyadin, A.; Natarajan, K. How to Meet the Green Deal Objectives—Is It Possible to Obtain 100% RES at the Regional Level in the EU? Energies 2022, 15, 2296. [Google Scholar] [CrossRef]
- Saarloos, B.A.; Quinn, J.C. Achieving Optimal Value of Solar: A Municipal Utility Rate Analysis. Solar 2022, 2, 7. [Google Scholar] [CrossRef]
- Saidin, M.I.S.; O’Neill, J. Climate Change and the Diversification of Green Social Capital in the International Political Economy of the Middle East and North Africa: A Review Article. Sustainability 2022, 14, 3756. [Google Scholar] [CrossRef]
- Ruiz Duarte, J.L.; Fan, N. Operation of a Power Grid with Embedded Networked Microgrids and Onsite Renewable Technologies. Energies 2022, 15, 2350. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Wang, J.; Baldick, R. Research on resilience of power systems under natural disasters—A review. IEEE Trans. Smart Grid 2016, 31, 1604–1613. [Google Scholar] [CrossRef]
- Yue, C.-D.; Wang, I.-C.; Huang, J.-S. Feasibility of Replacing Nuclear and Fossil Fuel Energy with Offshore Wind Energy: A Case for Taiwan. Energies 2022, 15, 2385. [Google Scholar] [CrossRef]
- Chen, F.; Lu, S.-M.; Tseng, K.-T.; Lee, S.-C.; Wang, E. Assessment of renewable energy reserves in Taiwan. Renew. Sustain. Energy Rev. 2010, 14, 2511–2528. [Google Scholar] [CrossRef]
- Mikkili, S.; Kanjune, A.; Bonthagorla, P.K.; Senjyu, T. Non-Symmetrical (NS) Reconfiguration Techniques to Enhance Power Generation Capability of Solar PV System. Energies 2022, 15, 2124. [Google Scholar] [CrossRef]
- Jordehi, A.R. Maximum power point tracking in photovoltaic (PV) systems: A review of different approaches. Renew. Sustain. Energy Rev. 2016, 65, 1127–1138. [Google Scholar] [CrossRef]
- Dash, S.K.; Ray, P.K. A New PV-Open-UPQC Configuration for Voltage Sensitive Loads Utilizing Novel Adaptive Controllers". IEEE Trans. Ind. Inform. 2021, 17, 421–429. [Google Scholar] [CrossRef]
- Malathy, S.; Ramaprabha, R. Reconfiguration strategies to extract maximum power from photovoltaic array under partially shaded conditions. Renew. Sustain. Energy Rev. 2018, 81, 2922–2934. [Google Scholar] [CrossRef]
- Priyanka, G.; Dash, S.K. A Detailed Review on Intelligent Maximum Power Point Tracking Algorithms. In Proceedings of the 2020 2nd International Conference on Innovative Mechanisms for Industry Applications (ICIMIA), Bangalore, India, 5–7 March 2020; pp. 47–53. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- European Economic Area. Renewable Energy in Europe 2016; European Environment Agency, EU Publication: Copenhagen, Denmark, 2016. [Google Scholar]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Piela, P.; Mitzel, J. Polymer electrolyte membrane fuel cell efficiency at the stack level. J. Power Sources 2015, 292, 95–103. [Google Scholar] [CrossRef]
- Pavelka, M.; Marsik, F. Detailed thermodynamic analysis of polymer electrolyte membrane fuel cell efficiency. Int. J. Hydrogen Energy 2013, 38, 7102–7113. [Google Scholar] [CrossRef]
- Hwang, J.-J. Effect of hydrogen delivery schemes on fuel cell efficiency. J. Power Sources 2013, 239, 54–63. [Google Scholar] [CrossRef]
- Fuel Cells. Available online: https://www.energy.gov/eere/fuelcells/fuel-cells (accessed on 5 May 2022).
- Larminie, J.; Lowry, J. Front matter. In Electric Vehicle Technology Explained, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Bowers, B.J.; Zhao, J.L.; Ruffo, M.; Khan, R.; Dattatraya, D.; Dushman, N.; Beziat, J.C.; Boudjemaa, F. Onboard fuel processor for PEM fuel cell vehicles. Int. J. Hydrogen Energy 2007, 32, 1437–1442. [Google Scholar] [CrossRef]
- Global Hydrogen Review. 2021. Available online: https://www.iea.org/reports/global-hydrogen-review-2021 (accessed on 5 May 2022).
- Li, M.; Zhang, X.; Li, G. A comparative assessment of battery and fuel cell electric vehicles using a well-to-wheel analysis. Energy 2016, 94, 693–704. [Google Scholar] [CrossRef]
- Brunet, J.; Kotelnikova, A.; Ponssard, J.P. The deployment of BEV and FCEV in 2015. Available online: https://www.researchgate.net/profile/Jean-Ponssard/publication/282865470_The_deployment_of_BEV_and_FCEV_in_2015/links/5bbf165692851c4efd5651dd/The-deployment-of-BEV-and-FCEV-in-2015.pdf (accessed on 5 May 2022).
- Hydrogen or Battery? A Clear Case, Until Further Notice (2019). VolksWagen Website. Available online: https://www.volkswagenag.com/en/news/stories/2019/08/hydrogen-or-battery--that-is-the-question.html (accessed on 5 May 2022).
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Van Mierlo, J.; Maggetto, G. Fuel cell or battery: Electric cars are the future. Fuel Cells 2007, 7, 165–173. [Google Scholar] [CrossRef]
- Chen, Y.; Melaina, M. Model-based techno-economic evaluation of fuel cell vehicles considering technology uncertainties. Transp. Res. Part D Transp. Environ. 2019, 74, 234–244. [Google Scholar] [CrossRef]
- Hasan, M.K.; Mahmud, M.; Habib, A.A.; Motakabber, S.M.A.; Islam, S. Review of electric vehicle energy storage and management system: Standards, issues, and challenges. J. Energy Storage 2021, 41, 102940. [Google Scholar] [CrossRef]
- Mongird, K.; Viswanathan, V.; Alam, J.; Vartanian, C.; Sprenkle, V.; Baxter, R. 2020 Grid Energy Storage Technology Cost and Performance Assessment. Energy. 2020. Available online: https://esmap.org/sites/default/files/resources-document/final-esgc-cost-performance-report-12-11-2020_0.pdf (accessed on 5 May 2022).
- Battery Electric, vs. Hydrogen—Which Is the Future for Electric Vehicles? Available online: https://www.lexology.com/library/detail.aspx?g=1bf1cbf0-ac2f-4b39-a3de-2df77a9a515e (accessed on 5 May 2022).
- Gnann, T.; Plötz, P.; Kühn, A.; Wietschel, M. How to decarbonise heavy road transport. In Proceedings of the ECEEE 2017 Summer Study, Hyères, France, 13 August 2017. [Google Scholar]
- Chakraborty, S.; Kumar, N.M.; Jayakumar, A.; Dash, S.K.; Elangovan, D. Selected Aspects of Sustainable Mobility Reveals Implementable Approaches and Conceivable Actions. Sustainability 2021, 13, 12918. [Google Scholar] [CrossRef]
- Dash, S.K.; Ray, P.K. Photovoltaic tied unified power quality conditioner topology based on a novel notch filter utilized control algorithm for power quality improvement. Trans. Inst. Meas. Control 2019, 41, 1912–1922. [Google Scholar] [CrossRef]
- Ala, G.; Castiglia, V.; Di Filippo, G.; Miceli, R.; Romano, P.; Viola, F. From electric mobility to hydrogen mobility: Current state and possible future expansions. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference (MELECON), Palermo, Italiy, 16–18 June 2020; IEEE: Piscataway, NJ, USA; pp. 1–6. [Google Scholar]
- Fuel Cell Technologies Office. Fuel Cells. 2015. Available online: https://www.energy.gov/sites/prod/files/2015/11/f27/fcto_fuel_cells_fact_sheet.pdf (accessed on 22 February 2022).
- European Commission, Press Release Database. 2018. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_18_3708 (accessed on 5 May 2022).
- Moro, A.; Lonza, L. Electricity carbon intensity in European Member States: Impacts on GHG emissions of electric vehicles. Transp. Res. Part D Transp. Environ. 2018, 64, 5–14. [Google Scholar] [CrossRef]
- Harrison, G.; Vilchez, J.J.G.; Thiel, C. Industry strategies for the promotion of E-mobility under alternative policy and economic scenarios. Eur. Transp. Res. Rev. 2018, 10, 19. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Singh, B.; Majeau-Bettez, G.; Strømman, A.H. Comparative environmental life cycle assessment of conventional and electric vehicles. J. Ind. Ecol. 2013, 17, 53–64. [Google Scholar] [CrossRef]
- Egnér, F.; Trosvik, L. Electric vehicle adoption in Sweden and the impact of local policy instruments. Energy Policy 2018, 121, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Held, T.; Gerrits, L. On the road to electrification–A qualitative comparative analysis of urban e-mobility policies in 15 European cities. Transp. Policy 2019, 81, 12–23. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.; Pan, H. A global comparison and assessment of incentive policy on electric vehicle promotion. Sustain. Cities Soc. 2019, 44, 597–603. [Google Scholar] [CrossRef]
- Fanti, M.P.; Mangini, A.M.; Roccotelli, M.; Nolich, M.; Ukovich, W. Modeling Virtual Sensors for Electric Vehicles Charge Services. In Proceedings of the 2018 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 3853–3858. [Google Scholar]
- Fanti, M.P.; Mangini, A.M.; Roccotelli, M.; Silvestri, B. Innovative Approaches for Electric Vehicles Relocation in Sharing Systems. IEEE Trans. Autom. Sci. Eng. 2021, 19, 21–36. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Cantuarias-Villessuzanne, C.; Weinberger, B.; Roses, L.; Vignes, A.; Brignon, J.M. Social cost-benefit analysis of hydrogen mobility in Europe. Int. J. Hydrogen Energy 2016, 41, 19304–19311. [Google Scholar] [CrossRef] [Green Version]
- Stiller, C.; Bünger, U.; Møller-Holst, S.; Svensson, A.M.; Espegren, K.A.; Nowak, M. Pathways to a hydrogen fuel infrastructure in Norway. Int. J. Hydrogen Energy 2010, 35, 2597–2601. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef] [Green Version]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Hydrogen Refuelling Infrastructure. ITM Power. 2017. Available online: https://www.level-network.com/wp-content/uploads/2017/02/ITM-Power.pdf (accessed on 5 May 2022).
- Gardiner, M. Energy Requirements for Hydrogen Gas Compression and Liquefaction as Related to Vehicle Storage Needs. DOE Hydrogen and Fuel Cells Program Record. 2009. Available online: https://www.hydrogen.energy.gov/pdfs/9013_energy_requirements_for_hydrogen_gas_compression.pdf (accessed on 5 May 2022).
- Office of Energy Efficiency and Renewable Energy. All-Electric Vehicles. Available online: https://www.fueleconomy.gov/feg/evtech.shtml (accessed on 22 February 2022).
- Curry, C. Lithium-Ion Costs and Market. 2017. Available online: https://data.bloomberglp.com/bnef/sites/14/2017/07/BNEF-Lithium-ion-battery-costs-and-market.pdf (accessed on 22 February 2022).
- Tajitsu, N.; Shiraki, M. Toyota Plans to Expand Production, Shrink Cost of Hydrogen Fuel Cell Vehicles; Business News; Reuters: London, UK, 2018. [Google Scholar]
- Brown, E.G. Joint Agency Staff Report on Assembly Bill 8: Assessment of Time and Cost Needed to Attain 100 Hydrogen Refueling Stations In California; California Energy Commission: Sacramento, CA, USA, 2015. [Google Scholar]
- Thomas, C.E. Fuel cell and battery electric vehicles compared. Int. J. Hydrogen Energy 2009, 34, 6005–6020. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Number of Hydrogen Fueling Stations for Road Vehicles Worldwide as of 2021. Available online: https://www.statista.com/statistics/1026719/number-of-hydrogen-fuel-stations-by-country/ (accessed on 5 May 2022).
- Tlili, O.; Mansilla, C.; Frimat, D.; Perez, Y. Hydrogen market penetration feasibility assessment: Mobility and natural gas markets in the US, Europe, China and Japan. Int. J. Hydrogen Energy 2019, 44, 16048–16068. [Google Scholar] [CrossRef]
- Fueling the Future of Mobility Hydrogen and Fuel Cell Solutions for Transportation. Volume 1, Deloitte, China. Available online: https://www2.deloitte.com/content/dam/Deloitte/cn/Documents/finance/deloitte-cn-fueling-the-future-of-mobility-en-200101.pdf (accessed on 22 February 2022).
- Medisetty, V.M.; Kumar, R.; Ahmadi, M.H.; Vo, D.-V.N.; Ochoa, A.A.V.; Solanki, R. Overview on the Current Status of Hydrogen Energy Research and Development in India. Chem. Eng. Technol. 2020, 43, 613–624. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen fuel and transport system: A sustainable and environmental future. Int. J. Hydrogen Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Așchilean, I.; Cobîrzan, N.; Bolboacă, A.M.; Raboaca, M.S. Optimal Synergy between Photovoltaic Panels and Hydrogen Fuel Cells for Green Power Supply of a Green Building—A Case Study. Sustainability 2021, 13, 6304. [Google Scholar] [CrossRef]
- IEA. “The Future of Hydrogen”, Paris. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 24 March 2022).
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Zahed, A.; Bashir, M.; Alp, T.; Najjar, Y. A perspective of solar hydrogen and its utilization in Saudi Arabia. Int. J. Hydrogen Energy 1991, 16, 277–281. [Google Scholar] [CrossRef]
- Collera, A.A.; Agaton, C.B. Opportunities for production and utilization of green hydrogen in the Philippines. Int. J. Energy Econ. Policy 2021, 11, 37–41. [Google Scholar] [CrossRef]
- Imasiku, K.; Farirai, F.; Olwoch, J.; Agbo, S.N. A Policy Review of Green Hydrogen Economy in Southern Africa. Sustainability 2021, 13, 13240. [Google Scholar] [CrossRef]
- Hikima, K.; Tsujimoto, M.; Takeuchi, M.; Kajikawa, Y. Transition Analysis of Budgetary Allocation for Projects on Hydrogen-Related Technologies in Japan. Sustainability 2020, 12, 8546. [Google Scholar] [CrossRef]
- Breuer, J.L.; Scholten, J.; Koj, J.C.; Schorn, F.; Fiebrandt, M.; Samsun, R.C.; Albus, R.; Görner, K.; Stolten, D.; Peters, R. An Overview of Promising Alternative Fuels for Road, Rail, Air, and Inland Waterway Transport in Germany. Energies 2022, 15, 1443. [Google Scholar] [CrossRef]
- Aprea, J.L.; Bolcich, J.C. The energy transition towards hydrogen utilization for green life and sustainable human development in Patagonia. Int. J. Hydrogen Energy 2020, 45, 25627–25645. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Jenn, A.; Fulton, L. Low Carbon Scenario Analysis of a Hydrogen-Based Energy Transition for On-Road Transportation in California. Energies 2021, 14, 7163. [Google Scholar] [CrossRef]
- Yang, M.; Hu, S.; Yang, F.; Xu, L.; Bu, Y.; Yuan, D. On-Board Liquid Hydrogen Cold Energy Utilization System for a Heavy-Duty Fuel Cell Hybrid Truck. World Electr. Veh. J. 2021, 12, 136. [Google Scholar] [CrossRef]
- IEA. “Hydrogen,” Paris. 2021. Available online: https://www.iea.org/reports/hydrogen (accessed on 27 March 2022).
- IEA. “Global Hydrogen Demand by Sector in The Net Zero Scenario” 2020–2023. Available online: https://www.iea.org/data-and-statistics/charts/global-hydrogen-demand-by-sector-in-the-net-zero-scenario-2020–2030 (accessed on 27 March 2022).
- Ingersoll, J.G. The Renewable Hydrogen–Methane (RHYME) Transportation Fuel: A Practical First Step in the Realization of the Hydrogen Economy. Hydrogen 2022, 3, 8. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Ahn, K.; Rakha, H.A. Developing a Hydrogen Fuel Cell Vehicle (HFCV) Energy Consumption Model for Transportation Applications. Energies 2022, 15, 529. [Google Scholar] [CrossRef]
- Miah, S.; Lipu, M.S.H.; Meraj, S.T.; Hasan, K.; Ansari, S.; Jamal, T.; Masrur, H.; Elavarasan, R.M.; Hussain, A. Optimized Energy Management Schemes for Electric Vehicle Applications: A Bibliometric Analysis towards Future Trends. Sustainability 2021, 13, 12800. [Google Scholar] [CrossRef]
- Cerniauskas, S.; Grube, T.; Praktiknjo, A.; Stolten, D.; Robinius, M. Future Hydrogen Markets for Transportation and Industry: The Impact of CO2 Taxes. Energies 2019, 12, 4707. [Google Scholar] [CrossRef] [Green Version]
- Achour, H.; Carton, J.G.; Olabi, A.G. Estimating vehicle emissions from road transport, case study: Dublin City. Appl. Energy 2011, 88, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Qi, A.; Peppley, B.; Karan, K. Integrated fuel processors for fuel cell application: A review. Fuel Process. Technol. 2007, 88, 3–22. [Google Scholar] [CrossRef]
- Boettner, D.D.; Moran, M.J. Proton exchange membrane (PEM) fuel cell-powered vehicle performance using direct-hydrogen fueling and on-board methanol reforming. J. Energy 2004, 29, 2317–2330. [Google Scholar] [CrossRef]
- Wu, W.; Chuang, B.N.; Hwang, J.J.; Lin, G.K.; Yang, S.B. Techno-economic evaluation of a hybrid fuel cell vehicle with on-board MeOH-to-H2 processor. Appl. Energy 2019, 238, 401–412. [Google Scholar] [CrossRef]
- Purnima, P.; Jayanti, S. A high-efficiency, auto-thermal system for onboard hydrogen production for low temperature PEM fuel cells using dual reforming of ethanol. Int. J. Hydrogen Energy 2016, 41, 13800–13810. [Google Scholar] [CrossRef]
- Zhang, T.; Ou, K.; Jung, S.; Choi, B.; Kim, Y.B. Dynamic analysis of a PEM fuel cell hybrid system with an on-board dimethyl ether (DME) steam reformer (SR). Int. J. Hydrogen Energy 2018, 43, 13521–13531. [Google Scholar] [CrossRef]
- Karakaya, M.; Avci, A.K. Comparison of compact reformer configurations for on-board fuel processing. Int. J. Hydrogen Energy 2010, 35, 2305–2316. [Google Scholar] [CrossRef]
- Darwish, N.A.; Hilal, N.; Versteeg, G.; Heesink, B. Feasibility of the direct generation of hydrogen for fuel-cell-powered vehicles by on-board steam reforming of naphtha. Fuel 2004, 83, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Parvasi, P.; Mohammad Jokar, S.; Basile, A.; Iulianelli, A. An On-Board Pure H2 Supply System Based on A Membrane Reactor for A Fuel Cell Vehicle: A Theoretical Study. Membranes 2020, 10, 159. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol production and transformation: A critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Lu, N.; Xie, D. Novel membrane reactor concepts for hydrogen production from hydrocarbons: A review. Int. J. Chem. React. Eng. 2016, 14, 1–31. [Google Scholar] [CrossRef]
- Chan, C.C.; Bouscayrol, A.; Chen, K. Electric, hybrid, and fuel-cell vehicles: Architectures and modeling. IEEE Trans. Veh. Technol. 2010, 59, 589–598. [Google Scholar] [CrossRef]
- Mazzone, S.; Campbell, A.; Zhang, G.; García-García, F. Ammonia cracking hollow fibre converter for on-board hydrogen production. Int. J. Hydrogen Energy 2021, 46, 37697–37704. [Google Scholar] [CrossRef]
- Han, X.; Li, F.; Zhang, T.; Zhang, T.; Song, K. Economic energy management strategy design and simulation for a dual-stack fuel cell electric vehicle. Int. J. Hydrogen Energy 2017, 42, 11584–11595. [Google Scholar] [CrossRef]
- De Bernardinis, A.; Frappe, E.; Bethoux, O.; Marchand, C.; Coquery, G. Electrical Architecture for High Power Segmented PEM Fuel Cell in Vehicle Application. In Proceedings of the 2012 First International Conference on Renewable Energies and Vehicular Technology, Hammamet, Tunisia, 26–28 March 2012; pp. 15–22. [Google Scholar] [CrossRef]
- Marx, N.; Boulon, L.; Gustin, F.; Hissel, D.; Agbossou, K. A review of multi-stack and modular fuel cell systems: Interests, application areas and on-going research activities. Int. J. Hydrogen Energy 2014, 39, 12101–12111. [Google Scholar] [CrossRef]
- Jian, B.; Wang, H. Hardware-in-the-loop real-time validation of fuel cell electric vehicle power system based on multi-stack fuel cell construction. J. Clean. Prod. 2022, 331, 129807. [Google Scholar] [CrossRef]
- Al-Baghdadi, M. An Overview of Hydrogen as an Alternative Fuel. Encyclopedia. 2020. Available online: https://encyclopedia.pub/revision/9798/v1 (accessed on 12 June 2021).
- Verhelst, S. Recent progress in the use of hydrogen as a fuel for internal combustion engines. Int. J. Hydrogen Energy 2014, 39, 1071–1085. [Google Scholar] [CrossRef] [Green Version]
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A review of synthetic fuels for passenger vehicles. Energy Rep. 2019, 5, 555–569. [Google Scholar] [CrossRef]
- Helmolt, R.V.; Eberle, U. Fuel cell vehicles: Status. J. Power Sources 2007, 165, 833–843. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef] [Green Version]
- Wallner, T.; Matthias, N.S.; Scarcelli, R.; Kwon, J.C. Evaluation of the efficiency and the drive cycle emissions for a hydrogen direct-injection engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2012, 227, 99–109. [Google Scholar] [CrossRef]
- Sopena, C.; Dieguez, P.; Sainz, D.; Urroz, J.; Guelbenzu, E.; Gandía, L.M. Conversion of a commercial spark ignition engine to run on hydrogen: Performance comparison using hydrogen and gasoline. Int. J. Hydrogen Energy 2010, 35, 1420–1429. [Google Scholar] [CrossRef]
- Yamane, K. Hydrogen-Fueled ICE, Successfully Overcoming Challenges through High-Pressure Direct Injection Technologies: 40 Years of Japanese Hydrogen ICE Research and Development; No. 2018-01-1145; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Dimitriou, P.; Tsujimura, T. A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrogen Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/1628 of the European Parliament and of the Council. Available online: https://www.legislation.gov.uk/eur/2016/1628/chapter/XIV (accessed on 17 February 2022).
- Korn, T.; Nobile, R.-F.; Grassinger, D. Zero-emission, maximum performance—The latest generation of hydrogen combustion engines. In Proceedings of the 42nd International Vienna Motor Symposium, Vienna, Austria, 29–30 April 2021. [Google Scholar]
- Pauer, T.; Weller, H.; Schünemann, E.; Eichlseder, H.; Grabner, P.; Schaffer, K. H2 ICE für zukünftige PKWs und leichte Nutzfahrzeuge. In Proceedings of the 41st Internationales Wiener Motorensymposium, Vienna, Austria, 22–24 April 2020. [Google Scholar]
- Boretti, A. Transient positive ignition internal combustion engines have now surpassed the 50% fuel conversion efficiency barrier. Int. J. Hydrogen Energy 2019, 44, 7051–7052. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A Fully Renewable and Efficient Backup Power System with a Hydrogen-Biodiesel-Fueled IC Engine. Energy Procedia 2019, 157, 1305–1319. [Google Scholar] [CrossRef]
- Gandhi, R.D. Use of hydrogen in internal combustion engine. Int. J. Eng. Manag. Sci. 2015, 3, 207–216. [Google Scholar]
- Huang, W.; Zheng, D.; Chen, X.; Shi, L.; Dai, X.; Chen, Y.; Jing, X. Standard Thermodynamic Properties for the Energy Grade Evaluation of Fossil Fuels and Renewable Fuels. Renew. Energy 2020, 147, 2160–2170. [Google Scholar] [CrossRef]
- Faizal, M.; Chuah, L.S.; Lee, C.; Hameed, A.; Lee, J.; Shankar, M. Review of hydrogen fuel for internal combustion engines. J. Mech. Eng. Res. Dev. 2019, 42, 35–46. [Google Scholar]
- Sanli, A.; Yılmaz, I.T.; Gümüş, M. Assessment of Combustion and Exhaust Emissions in a Common-Rail Diesel Engine Fueled with Methane and Hydrogen/Methane Mixtures under Different Compression Ratio. Int. J. Hydrogen Energy 2020, 45, 3263–3283. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G. Performance and emission study in manifold hydrogen injection with diesel as an ignition source for different start of injection. Renew. Energy 2009, 34, 328–334. [Google Scholar] [CrossRef]
- Shi, C.; Ji, C.; Ge, Y.; Wang, S.; Bao, J.; Yang, J. Numerical Study on Ignition Amelioration of a Hydrogen-Enriched Wankel Engine under Lean-Burn Condition. Appl. Energy 2019, 255, 113800. [Google Scholar] [CrossRef]
- Benajes, J.; Novella, R.; Gomez-Soriano, J.; Barbery, I.; Libert, C. Advantages of Hydrogen Addition in a Passive Pre-chamber Ignited SI Engine for Passenger Car Applications. Int. J. Energy Res. 2021, 45, 13219–13237. [Google Scholar] [CrossRef]
- Probir, B.K.; Dines, M. An experimental investigation on engine performance and emissions of a single cylinder diesel engine using hydrogen as inducted fuel and diesel as injected fuel with exhaust gas recirculation. Int. J. Hydrogen Energy 2009, 34, 4847–4854. [Google Scholar]
- Desantes, J.M.; Molina, S.; Novella, R.; Lopez-Juarez, M. Comparative Global Warming Impact and NOX Emissions of Conventional and Hydrogen Automotive Propulsion Systems. Energy Convers. Manag. 2020, 221, 113137. [Google Scholar] [CrossRef]
- Lee, J.; Park, C.; Bae, J.; Kim, Y.; Choi, Y.; Lim, B. Effect of Different Excess Air Ratio Values and Spark Advance Timing on Combustion and Emission Characteristics of Hydrogen-Fueled Spark Ignition Engine. Int. J. Hydrogen Energy 2019, 44, 25021–25030. [Google Scholar] [CrossRef]
- Dhyani, V.; Subramanian, K.A. Control of Backfire and NOx Emission Reduction in a Hydrogen Fueled Multi-Cylinder Spark Ignition Engine Using Cooled EGR and Water Injection Strategies. Int. J. Hydrogen Energy 2019, 44, 6287–6298. [Google Scholar] [CrossRef]
- Xu, P.; Ji, C.; Wang, S.; Bai, X.; Cong, X.; Su, T.; Shi, L. Realizing Low NOx Emissions on a Hydrogen-Fuel Spark Ignition Engine at the Cold Start Period through Excess Air Ratios Control. Int. J. Hydrogen Energy 2018, 43, 21617–21626. [Google Scholar] [CrossRef]
- Pani, A. VSSUT Burla Formation, Kinetics and Control Strategies of Nox Emission in Hydrogen Fueled IC Engine. Int. J. Eng. Res. Technol. 2020, 9, 132–141. [Google Scholar] [CrossRef]
- Nguyen, D.; Choi, Y.; Park, C.; Kim, Y.; Lee, J. Effect of Supercharger System on Power Enhancement of Hydrogen-Fueled Spark-Ignition Engine under Low-Load Condition. Int. J. Hydrogen Energy 2021, 46, 6928–6936. [Google Scholar] [CrossRef]
- Ayad, S.M.M.E.; Belchior, C.R.P.; da Silva, G.L.R.; Lucena, R.S.; Carreira, E.S.; de Miranda, P.E.V. Analysis of Performance Parameters of an Ethanol Fueled Spark Ignition Engine Operating with Hydrogen Enrichment. Int. J. Hydrogen Energy 2020, 45, 5588–5606. [Google Scholar] [CrossRef]
- Chaichan, M.T. Performance and Emission Characteristics of CIE Using Hydrogen, Biodiesel, and Massive EGR. Int. J. Hydrogen Energy 2018, 43, 5415–5435. [Google Scholar] [CrossRef]
- Boretti, A. Hydrogen Internal Combustion Engines to 2030. Int. J. Hydrogen Energy 2020, 45, 23692–23703. [Google Scholar] [CrossRef]
- Winkler, S.L.; Anderson, J.E.; Garza, L.; Ruona, W.C.; Vogt, R.; Wallington, T.J. Vehicle criteria pollutant (PM, NOx, CO, HCs) emissions: How low should we go? NPJ Clim. Atmos. Sci. 2018, 1, 26. [Google Scholar] [CrossRef]
- Shivaprasad, K.V.; Rajesh, R.; Anteneh Wogasso, W.; Nigatu, B.; Addisu, F. Usage of Hydrogen as a Fuel in Spark Ignition Engine. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012037. [Google Scholar] [CrossRef]
- Jhang, S.-R.; Lin, Y.-C.; Chen, K.-S.; Lin, S.-L.; Batterman, S. Evaluation of Fuel Consumption, Pollutant Emissions and Well-to-Wheel GHGs Assessment from a Vehicle Operation Fueled with Bioethanol, Gasoline and Hydrogen. Energy 2020, 209, 118436. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Pan, J.; Wei, H.; Zhou, L.; Liu, C. Effects of Direct-Injected Hydrogen Addition on Methane Combustion Performance in an Optical SI Engine with High Compression-Ratio. Int. J. Hydrogen Energy 2020, 45, 3284–3293. [Google Scholar] [CrossRef]
- Jabbr, A.I.; Koylu, U.O. Influence of Operating Parameters on Performance and Emissions for a Compression-Ignition Engine Fueled by Hydrogen/Diesel Mixtures. Int. J. Hydrogen Energy 2019, 44, 13964–13973. [Google Scholar] [CrossRef]
- Aydin, K.; Kenanoğlu, R. Effects of Hydrogenation of Fossil Fuels with Hydrogen and Hydroxy Gas on Performance and Emissions of Internal Combustion Engines. Int. J. Hydrogen Energy 2018, 43, 14047–14058. [Google Scholar] [CrossRef]
- Castro, N.; Toledo, M.; Amador, G. An Experimental Investigation of the Performance and Emissions of a Hydrogen-Diesel Dual Fuel Compression Ignition Internal Combustion Engine. Appl. Therm. Eng. 2019, 156, 660–667. [Google Scholar] [CrossRef]
- Imran, S.; Korakianitis, T.; Shaukat, R.; Farooq, M.; Condoor, S.; Jayaram, S. Experimentally Tested Performance and Emissions Advantages of Using Natural-Gas and Hydrogen Fuel Mixture with Diesel and Rapeseed Methyl Ester as Pilot Fuels. Appl. Energy 2018, 229, 1260–1268. [Google Scholar] [CrossRef]
- Murugesan, P.; Hoang, A.T.; Venkatesan, E.P.; Kumar, D.S.; Balasubramanian, D.; Le, A.T.; Pham, V.V. Role of hydrogen in improving performance and emission characteristics of homogeneous charge compression ignition engine fueled with graphite oxide nanoparticle-added microalgae biodiesel/diesel blends. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Dimitrov, E.; Pantchev, S.; Michaylov, P.; Peychev, M. Economic Aspects of Using Hydrogen in Compression Ignition Engine Operating on Gas-Diesel Cycle. IOP Conf. Ser. Mater. Sci. Eng. 2019, 664, 012022. [Google Scholar] [CrossRef]
- Sharma, P.; Dhar, A. Advances in Hydrogen-Fuelled Compression Ignition Engine. In Prospects of Alternative Transportation Fuels; Springer Singapore: Singapore, 2018; pp. 55–78. ISBN 9789811075179. [Google Scholar]
- Cruccolini, V.; Discepoli, G.; Cimarello, A.; Battistoni, M.; Mariani, F.; Grimaldi, C.N.; Dal Re, M. Lean Combustion Analysis Using a Corona Discharge Igniter in an Optical Engine Fueled with Methane and a Hydrogen-Methane Blend. Fuel 2020, 259, 116290. [Google Scholar] [CrossRef]
- Dimitriou, P.; Kumar, M.; Tsujimura, T.; Suzuki, Y. Combustion and Emission Characteristics of a Hydrogen-Diesel Dual-Fuel Engine. Int. J. Hydrogen Energy 2018, 43, 13605–13617. [Google Scholar] [CrossRef]
- Nag, S.; Dhar, A.; Gupta, A. Hydrogen-Diesel Co-Combustion Characteristics, Vibro-Acoustics and Unregulated Emissions in EGR Assisted Dual Fuel Engine. Fuel 2022, 307, 121925. [Google Scholar] [CrossRef]
- Nag, S.; Sharma, P.; Gupta, A.; Dhar, A. Experimental Study of Engine Performance and Emissions for Hydrogen Diesel Dual Fuel Engine with Exhaust Gas Recirculation. Int. J. Hydrogen Energy 2019, 44, 12163–12175. [Google Scholar] [CrossRef]
- Di Battista, D.; Di Bartolomeo, M.; Cipollone, R. Flow and Thermal Management of Engine Intake Air for Fuel and Emissions Saving. Energy Convers. Manag. 2018, 173, 46–55. [Google Scholar] [CrossRef]
- Ouchikh, S.; Lounici, M.S.; Tarabet, L.; Loubar, K.; Tazerout, M. Effect of Natural Gas Enrichment with Hydrogen on Combustion Characteristics of a Dual Fuel Diesel Engine. Int. J. Hydrogen Energy 2019, 44, 13974–13987. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Liu, B.; Zeng, K.; Yu, J.; Jiang, D. Combustion Characteristics of a Direct-Injection Engine Fueled with Natural Gas−Hydrogen Mixtures. Energy Fuels 2006, 20, 540–546. [Google Scholar] [CrossRef]
- Fan, B.; Pan, J.; Liu, Y.; Chen, W.; Lu, Y.; Otchere, P. Numerical Investigation of Mixture Formation and Combustion in a Hydrogen Direct Injection plus Natural Gas Port Injection (HDI + NGPI) Rotary Engine. Int. J. Hydrogen Energy 2018, 43, 4632–4644. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.; Seo, D.; Lee, S.; Hong, S.; Ahn, Y.-H.; Park, Y. Critical Hydrogen Concentration of Hydrogen-Natural Gas Blends in Clathrate Hydrates for Blue Hydrogen Storage. Renew. Sustain. Energy Rev. 2021, 141, 110789. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. A Comprehensive Study on Using New Hydrogen-Natural Gas and Ammonia-Natural Gas Blends for Better Performance. J. Nat. Gas Sci. Eng. 2020, 81, 103362. [Google Scholar] [CrossRef]
- Hydrogen Internal Combustion Engines and Hydrogen Fuel Cells. Available online: https://www.cummins.com/news/2022/01/27/hydrogen-internal-combustion-engines-and-hydrogen-fuel-cells (accessed on 20 April 2022).
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Rafa, N.; Musharrat, A.; Lam, S.S.; Boretti, A. Sustainable Hydrogen Production: Technological Advancements and Economic Analysis. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
| Overall Efficiency | Current Costs | Refueling Time | Range Autonomy | Energy Density | Sustainability | |
|---|---|---|---|---|---|---|
| Fuel cell | Around 30% | ~53 USD/kW (technology cost) 0.24 USD/kWh (refueling cost) | <10 min | Up to 600 km | 550 Wh/kg | Common and safe materials (except for platinum) |
| Battery | Around 75% | ~130 USD/kW (technology cost) 0.14–0.30 USD/kWh (refueling cost) | 1–14 h (depend on charging power) | 200–400 km (most common cases) | 150 Wh/kg | Use of pollutant metals such as cobalt |
| Reference | Fuel for Hydrogen Production | Economic Benefit | Commercial Potential | Efficiency |
|---|---|---|---|---|
| [75] | Methanol fuel processors | ✓ | ✓ | High |
| [76] | Methanol | ✓ | ✓ | High |
| [77] | Ethanol | ✕ | ✕ | Low |
| [78] | Dimethyl ether (DME) a | ✓ | ✓ | Average |
| [79] | N-heptane fuel processor | ✕ | ✕ | Low |
| [80] | Naphtha | ✕ | ✕ | Low |
| Parameter | Hydrogen | Diesel | Gasoline | Methane |
|---|---|---|---|---|
| % Mass of carbon | 0 | 86 | 84 | 75 |
| Molecular weight | 2.016 | ~170 | ~110 | 16.043 |
| Octane number | 130+ | - | 86–94 | 120+ |
| Cetane number | - | 40–55 | 13–17 | - |
| Density at STP (kg/m3) | 0.089 | 830.0 | 730–780 | 0.720 |
| Volumetric energy at STP (MJ/m3) | 1.07 × 10 | 3.5 × 104 | 3.3 × 104 | 3.3 × 10 |
| Net lower heating value (MJ/kg) | 119.9 | 42.5 | 43.9 | 45.8 |
| Boiling point (K) | 20.0 | 453–633 | 298–488 | 111.0 |
| Auto-ignition temperature (K) | 853 | ~523 | ~623 | 813 |
| Minimum ignition energy in air at 1 bar and stoichiometry (mJ) | 0.020 | 0.240 | 0.240 | 0.290 |
| Stoichiometry air/fuel mass ratio | 34.4 | 14.5 | 14.7 | 17.2 |
| Quenching distance at NTP (mm) | 0.64 | - | ~2 | 2.1 |
| Laminar flame speed in air at NTP (m/s) | 1.85 | 0.37–0.43 | 0.37–0.43 | 0.38 |
| Diffusion coefficient in air at STP (m2/s) | 8.5 × 10−6 | - | - | 1.9 × 10−6 |
| Flammability limits in air (% vol) | 4–76 | 0.6–5.5 | 1–7.6 | 5.3–15 |
| Adiabatic flame temperature at NTP (K) | 2480 | ~2300 | 2580 | 2214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, S.K.; Chakraborty, S.; Roccotelli, M.; Sahu, U.K. Hydrogen Fuel for Future Mobility: Challenges and Future Aspects. Sustainability 2022, 14, 8285. https://doi.org/10.3390/su14148285
Dash SK, Chakraborty S, Roccotelli M, Sahu UK. Hydrogen Fuel for Future Mobility: Challenges and Future Aspects. Sustainability. 2022; 14(14):8285. https://doi.org/10.3390/su14148285
Chicago/Turabian StyleDash, Santanu Kumar, Suprava Chakraborty, Michele Roccotelli, and Umesh Kumar Sahu. 2022. "Hydrogen Fuel for Future Mobility: Challenges and Future Aspects" Sustainability 14, no. 14: 8285. https://doi.org/10.3390/su14148285







