Psychophysiological Responses of Adults According to Cognitive Demand Levels for Horticultural Activities
Abstract
:1. Introduction
1.1. Effects of Human Contact with Nature
1.2. Cognitive Effects of Horticultural Activities
1.3. Electroencephalogram-Based Cognitive Load Assessment
2. Materials and Methods
2.1. Research Participants
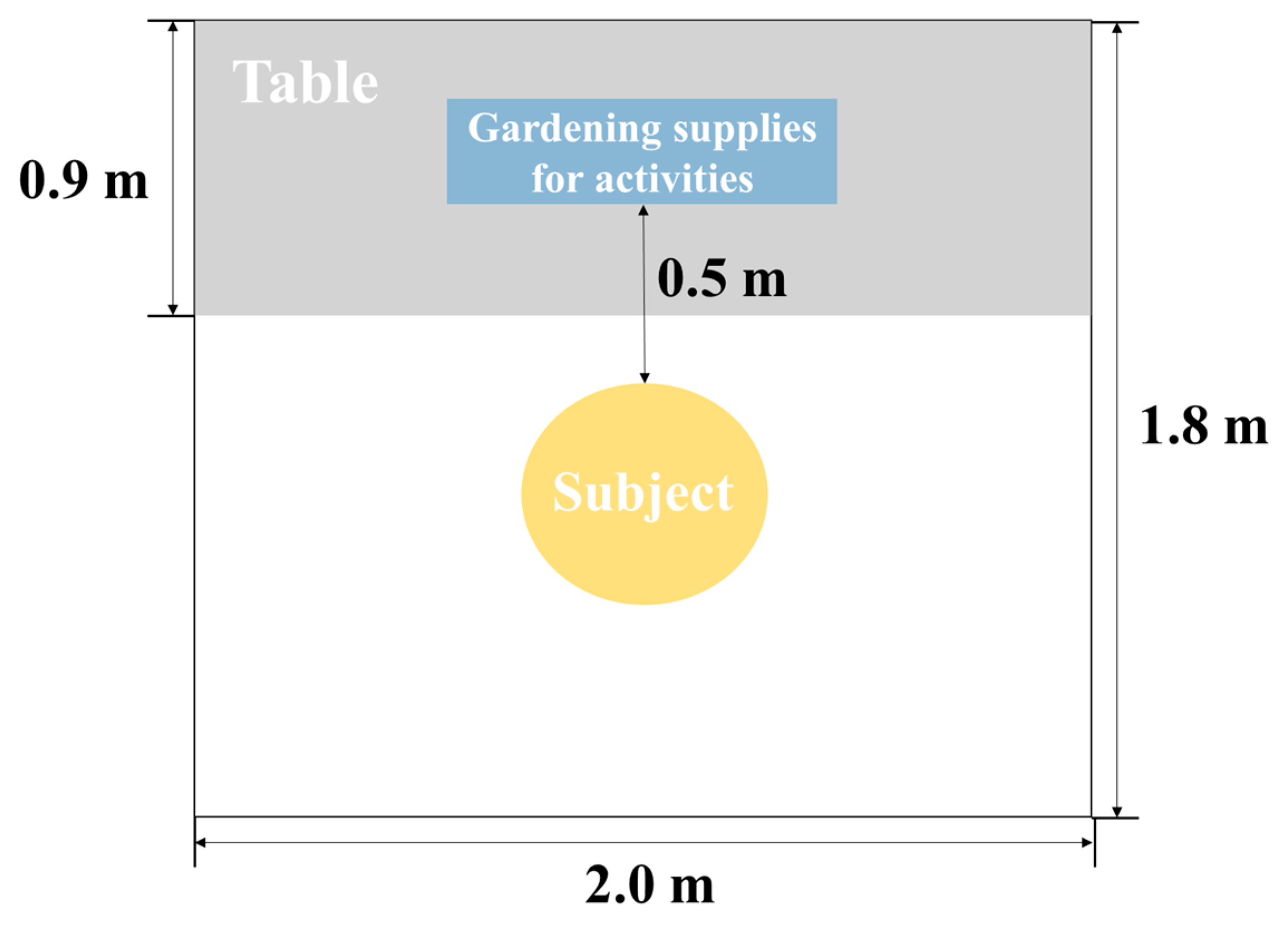
2.2. Experimental Condition
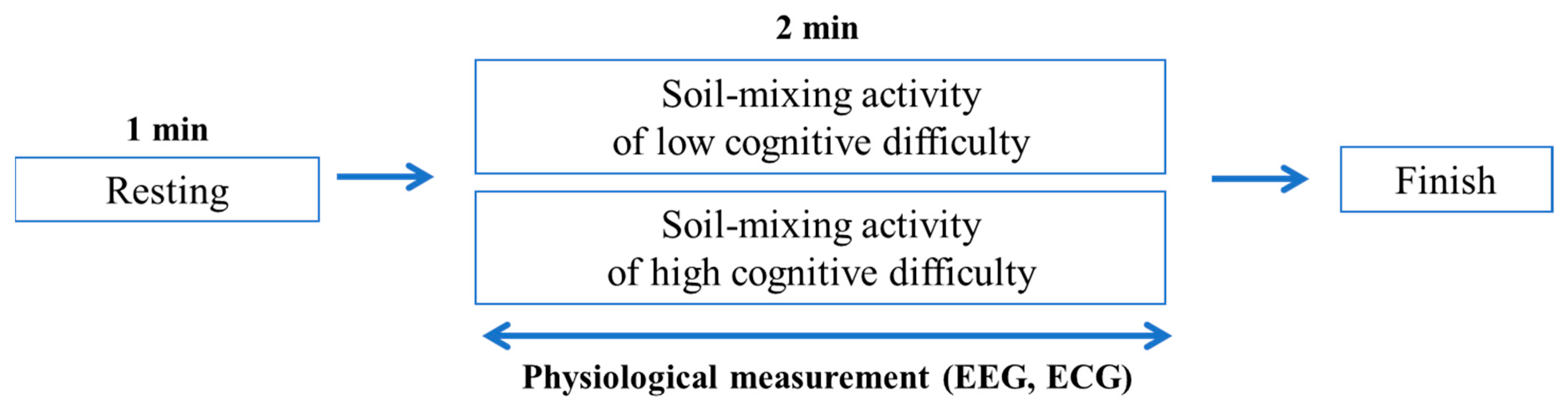
2.3. Experimental Procedure
2.4. Measurement Items
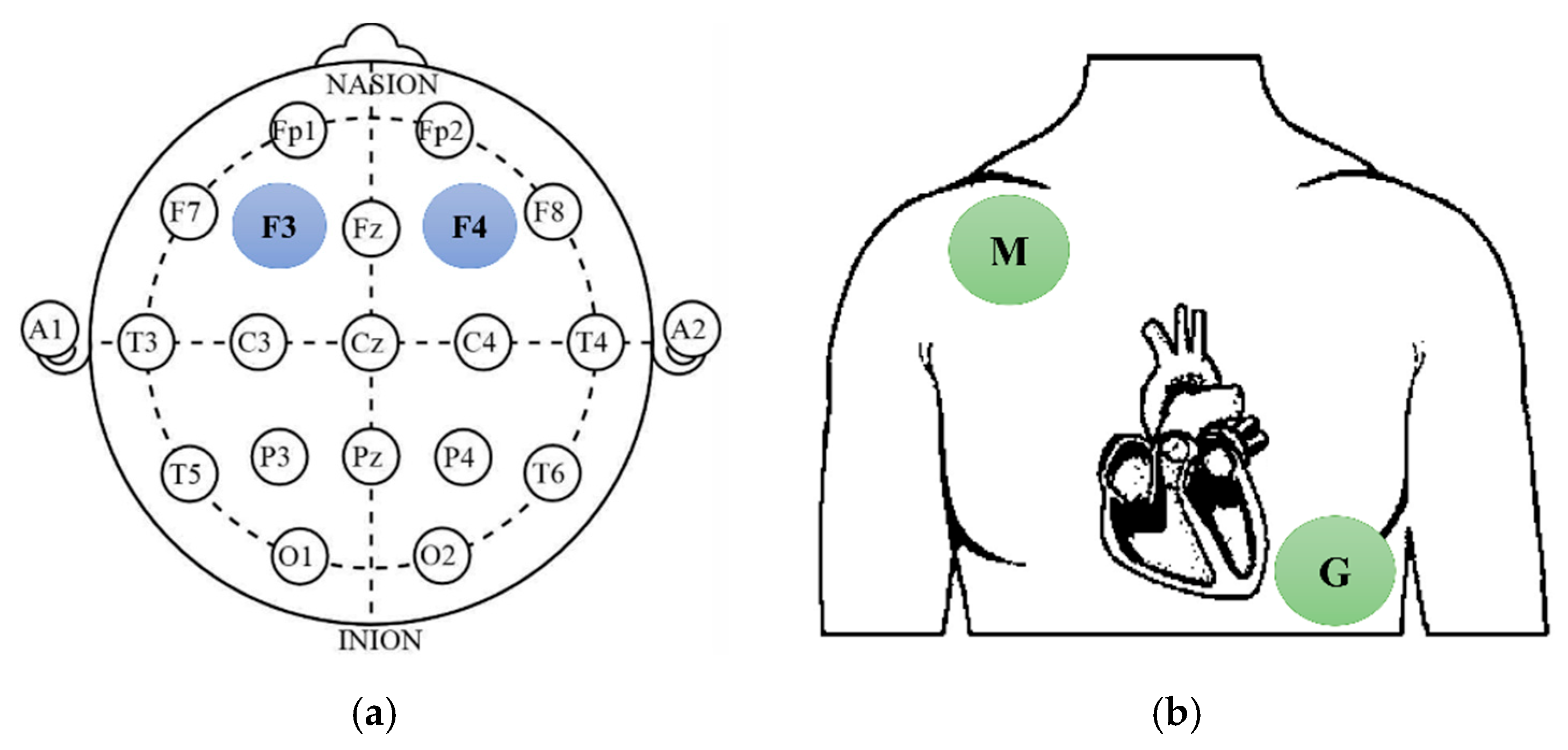
2.4.1. Physiological Measurement
2.4.2. Psychological Measurement
2.5. Data Processing and Analysis
3. Results
3.1. Demographic Information
3.2. Electroencephalography (EEG)
3.3. Electrocardiography (ECG)
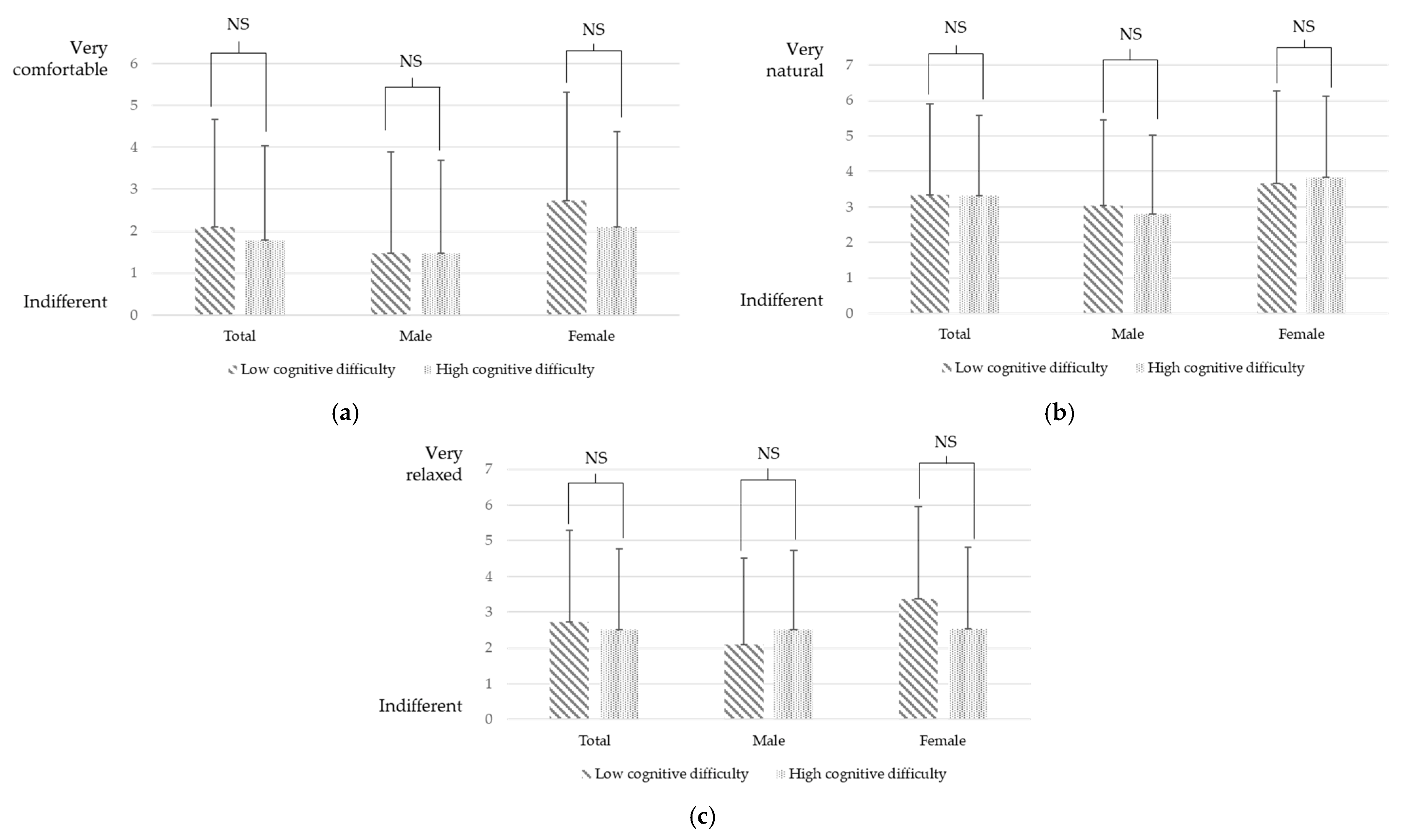
3.4. Semantic Differential Method (SDM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, S.J.; Kim, S.J.; Kim, D.S. Priority analysis of activation policies for agro-healing services. J. Korea Soc. Rural. Plan. 2019, 25, 89–102. [Google Scholar] [CrossRef]
- Stilgoe, J.R. Gone barefoot lately? Am. J. Prev. Med. 2001, 20, 243–244. [Google Scholar] [CrossRef]
- Chiesura, A. The role of urban parks for the sustainable city. Landscape Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- Tyrväinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The influence of urban green environments on stress relief measures: A field experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Ulrich, R.S. Health benefits of gardens in hospitals. In Proceedings of the Plants for People International Exhibition Floriade, Haarlemmermeer, The Netherlands, 6 April–20 October 2002. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Orians, G.H. An ecological and evolutionary approach to landscape aesthetics. In Landscape Meanings and Values; Routledge: London, UK, 1986; pp. 3–25. [Google Scholar]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, UK, 1984. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Lee, M.J.; Kim, J.S.; Oh, W.; Jang, J.S. Effects of indoor horticultural activities on improvement of attention and concentration in elementary school students. Hortic. Sci. Technol. 2013, 31, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Park, S.A.; Lee, A.Y.; Park, H.G.; Lee, W.L. Benefits of gardening activities for cognitive function according to measurement of brain nerve growth factor levels. Int. J. Environ. Res. Public Health 2019, 16, 760. [Google Scholar] [CrossRef] [Green Version]
- Jarrott, E.S.; Gigliotti, C. From the garden to the table: Evaluation of a dementia-specific HT program. Acta Hortic. 2004, 639, 139–144. [Google Scholar] [CrossRef]
- Zarjam, P.; Epps, J.; Chen, F. Spectral EEG featuresfor evaluating cognitive load. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3841–3844. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: The interface between memory and cognition. J. Cogn. Neurosci. 1992, 4, 281–288. [Google Scholar] [CrossRef]
- Paas, F.; Tuovinen, J.E.; Tabbers, H.; van Gerven, P.W.M. Cognitive load measurement as a means to advance cognitive load theory. Educ. Psychol. 2003, 38, 63–71. [Google Scholar] [CrossRef]
- Faller, J.; Cummings, J.; Saproo, S.; Sajda, P. Regulation of arousal via online neurofeedback improves human performance in a demanding sensory-motor task. Proc. Natl. Acad. Sci. USA 2019, 116, 6482–6490. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ruiz, N.; Taib, R.; Choi, E.; Chen, F. Galvanic skin response (gsr) as an index of cognitive load. In Proceedings of the CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 28 April–3 May 2007; pp. 2651–2656. [Google Scholar] [CrossRef]
- Backs, R.W.; Walrath, L.C. Eye movement and pupillary response indices of mental workload during visual search of symbolic displays. Appl. Ergon. 1992, 23, 243–254. [Google Scholar] [CrossRef]
- Fuentes-Garcia, J.P.; Pereira, T.; Castro, M.A.; Santos, A.C.; Villafaina, S. Psychophysiological stress response of adolescent chess players during problem-solving tasks. Physiol. Behav. 2019, 209, 112609. [Google Scholar] [CrossRef]
- Antonenko, P.; Paas, F.; Grabner, R.; van Gog, T. Using electroencephalography to measure cognitive load. Educ. Psychol. Rev. 2010, 22, 425–438. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Berka, C.; Levendowski, D.J.; Cvetinovic, M.M.; Petrovic, M.M.; Davis, G.; Lumicao, M.N.; Zivkovic, V.T.; Popovic, M.V.; Olmstead, R. Real-time analysis of EEG indexes of alertness, cognition, and memory acquired with a wireless EEG headset. Int. J. Hum.-Comput. Interact. 2004, 17, 151–170. [Google Scholar] [CrossRef]
- Mills, C.; Fridman, I.; Soussou, W.; Waghray, D.; Olney, A.M.; D’Mello, S.K. Put your thinking cap on: Detecting cognitive load using EEG during learning. In Proceedings of the Seventh International Learning Analytics & Knowledge Conference, Vancouver, BC, Canada, 13–17 March 2017; pp. 80–89. [Google Scholar] [CrossRef] [Green Version]
- Shagass, C. Electrical activity of the brain. In Handbook of Psychophysiology; Hort Rinehart & Winston: New York, NY, USA, 1972. [Google Scholar]
- Gale, A.; Edwards, J. The EEG and human behavior. Physiol. Corr. Hum. Behav. 1983, 2, 99–127. [Google Scholar]
- Duraisingam, A.; Palaniappan, R.; Andrews, S. Cognitive task difficulty analysis using EEG and data mining. In Proceedings of the 2017 Conference on Emerging Devices and Smart Systems (ICEDSS), Mallasamudram, India, 3–4 March 2017; pp. 52–57. [Google Scholar] [CrossRef]
- Rebsamen, B.; Kwok, K.; Penney, T.B. Evaluation of cognitive workload from EEG during a mental arithmetic task. Proc. Hum. Factors Ergonomics Soc. Ann. M. 2011, 55, 1342–1345. [Google Scholar] [CrossRef]
- Friedman, N.; Fekete, T.; Gal, Y.K.; Shriki, O. EEG-based prediction of cognitive load in intelligence tests. Front. Hum. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Kim, S.O.; Oh, Y.A.; Park, S.A. Foliage plants improve concentration and emotional condition of elementary school students performing an intensive assignment. HortScience 2020, 55, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1,3,7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- American Society of Heating, Refrigerating and Air-Conditioning Engineers. ASHRAE Handbook-Fundamentals; ASHRAE: Atlanta, GA, USA, 2009. [Google Scholar]
- Park, S.A.; Lee, A.Y.; Lee, K.S.; Son, K.C. Gardening tasks performed by adults are moderate- to high-intensity physical activities. HortTechnology 2014, 24, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, 498–516. [Google Scholar] [CrossRef] [Green Version]
- Onton, J.A.; Kang, D.Y.; Coleman, T.P. Visualization of whole-night sleep EEG from 2-channel mobile recording device reveals distinct deep sleep stages with differential electrodermal activity. Front. Hum. Neurosci. 2016, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.; Galloway, T.; Halpin, D.; Willemsen-Dunlap, A. Healthcare teams neurodynamically reorganize when resolving uncertainty. Entropy 2016, 18, 427. [Google Scholar] [CrossRef] [Green Version]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- Park, B.W. Introduction to Neurofeedback; Korea Mental Health Institute: Seoul, Korea, 2005. [Google Scholar]
- Yu, J.; Kang, H.; Jung, J. Effects of neurofeedback on brain waves and cognitive functions of children with cerebral palsy: A randomized control trial. J. Phys. Ther. Sci. 2012, 24, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.; Kim, S.O.; Park, S.A. Attention and emotional states during horticultural activities of adults in 20s using electroencephalography: A pilot study. Sustainability 2021, 13, 12968. [Google Scholar] [CrossRef]
- Malik, M. Heart rate variability. Curr. Opin. Cardiol. 1996, 1, 151–181. [Google Scholar] [CrossRef]
- Chang, K.S.; Lee, K.; Lim, H.S. The status of diabetes mellitus and effects of related factors on heart rate variability in a community. Kor. Diabetes J. 2009, 33, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Park, S.A.; Jung, S.J.; Lee, J.Y.; Son, K.C.; An, Y.J.; Lee, S.W. Physiological and psychological responses of humans to the index of greenness of an interior space. Complement. Ther. Med. 2016, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- García-Monge, A.; Rodríguez-Navarro, H.; González-Calvo, G.; Bores-García, D. Brain activity during different throwing games: EEG exploratory study. Int. J. Environ. Res. Public Health 2020, 17, 6796. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, S.M.; Brouwer, A.M.; Keizer, A.W.; Van, N.W. Effect of beta and gamma neurofeedback on memory and intelligence in the elderly. Biol. Psychol. 2014, 95, 74–85. [Google Scholar] [CrossRef]
- Chayer, C.; Freedman, M. Frontal lobe functions. Curr. Neurol. Neurosci. Rep. 2001, 1, 547–552. [Google Scholar] [CrossRef]
- Choi, N.Y.; Wu, Y.T.; Park, S.A. Effects of olfactory stimulation with aroma oils on psychophysiological responses of female adults. Int. J. Environ. Res. Public Health 2022, 19, 5196. [Google Scholar] [CrossRef]
- Schwarz, G.; Voit-Augustin, H.; Litscher, G.; Baumgartner, A. Specific problems in interpretation of absolute values of spectral edge frequency (SEF) in comparison to bispectral index (BIS) for assessing depth of anesthesia. Internet J. Neuromonitoring 2004, 99, 781–787. [Google Scholar] [CrossRef]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016, 7, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.S.; Amin, H.U. Designing EEG Experiments for Studying the Brain: Design Code and Example Datasets; Elsevier: London, UK, 2017. [Google Scholar]
- Egner, T.; Gruzelier, J. Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. Neuroreport 2001, 12, 4155–4159. [Google Scholar] [CrossRef] [Green Version]
- Vernon, D.J. Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Appl. Psychophys. Biof. 2005, 30, 347–364. [Google Scholar] [CrossRef]
- Kim, S.O.; Pyun, S.B.; Park, S.A. Improved cognitive function and emotional condition measured using electroencephalography in the elderly during horticultural activities. HortScience 2021, 1, 985–994. [Google Scholar] [CrossRef]
- Bakhtadze, S.; Beridze, M.; Geladze, N.; Khachapuridze, N.; Bornstein, N. Effect of EEG biofeedback on cognitive flexibility in children with attention deficit hyperactivity disorder with and without epilepsy. Appl. Psychophys. Biof. 2016, 41, 71–79. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Sauseng, P.; Doppelmayr, M.; Schabus, M.; Klimesch, W. Increasing individual upper alpha by neurofeedback improves cognitive performance in human subjects. Appl. Psychophysiol. Biofeedback 2006, 30, 1–10. [Google Scholar] [CrossRef]
- Rescher, B.; Rappelsberger, P. Gender dependent EEG-changes during a mental rotation task. Int. J. Psychophysiol. 1999, 33, 209–222. [Google Scholar] [CrossRef]
- Kimura, D. Are men’s and women’s brains really different? Can. Psychol. 1987, 28, 133. [Google Scholar] [CrossRef]
- Shaywitz, B.A.; Shaywltz, S.E.; Pugh, K.R.; Constable, R.T.; Skudlarski, P.; Fulbright, R.K.; Bronen, R.A.; Fletcher, J.M.; Shankweiler, D.P.; Katz, L.; et al. Sex differences in the functional organization of the brain for language. Nature 1995, 373, 607–609. [Google Scholar] [CrossRef]
- Volf, N.V.; Razumnikova, O.M. Sex differences in EEG coherence during a verbal memory task in normal adults. Int. J. Psychophysiol. 1999, 34, 113–122. [Google Scholar] [CrossRef]
- Zaidel, E.; Aboitiz, F.; Clarke, J. Sexual dimorphism in interhemispheric relations: Anatomical-behavioral convergence. Biol. Res. 1995, 28, 27–43. [Google Scholar]
- Razumnikova, O.M. Gender differences in hemispheric organization during divergent thinking: An EEG investigation in human subjects. Neurosci. Lett. 2004, 362, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Cabrera, M.; Ramos, J.; Guevara, M.A.; Arce, C.; Gutierrez, S. Gender differences in the EEG during cognitive activity. Intl. J. Neurosci. 1993, 72, 257–264. [Google Scholar] [CrossRef]
- Park, K.J.; Jeong, H. Assessing methods of heart rate variability. Kor. J. Clin. Neurophysiol. 2014, 16, 49–54. [Google Scholar] [CrossRef]
- Moore, L.J.; Wilson, M.R.; McGrath, J.S.; Waine, E.; Masters, R.S.W.; Vine, S.J. Surgeons’ display reduced mental effort and workload while performing robotically assisted surgical tasks, when compared to conventional laparoscopy. Surg. Endosc. 2015, 29, 2553–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, J.M.; Jerram, M.; Abbs, B.; Whitfield-Gabrieli, S.; Makris, N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010, 30, 431–438. [Google Scholar] [CrossRef]
- Ives, C.D.; Abson, D.J.; von Wehrden, H.; Dorninger, C.; Klaniecki, K.; Fischer, J. Reconnecting with nature for sustainability. Sustain. Sci. 2018, 13, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Kotozaki, Y. Horticultural activity improves postpartum women’s cognitive function: Preliminary evidence from an exploratory pilot study. Cogent Psychol. 2020, 7, 1851003. [Google Scholar] [CrossRef]
- Masuya, J.; Ota, K. Efficacy of horticultural activity in elderly people with dementia: A pilot study on the influence on vitality and cognitive function. Int. J. Nurs. Clin. Pract. 2014, 1, 2394–4978. [Google Scholar] [CrossRef] [Green Version]

| Activity | Cognitive Demand Levels | Descriptions | Exercise Intensity (METs) 1 |
|---|---|---|---|
| Soil-mixing activity | Low level cognitive demand | Mixing pre-mixed soil (including peat moss, perlite, and water) in a basin evenly with both hands for 2 min. | 3.6 |
| High level cognitive demand | Putting peat moss and perlite in an empty basin in a ratio of 7:3, pouring half of the water (300 mL) in a beaker, and mixing evenly with both hands for 2 min. |
| Analysis Indicator | The Full Name of the EEG Parameters | Indicator Estimate (Ratio) | State |
|---|---|---|---|
| RLB | Relative Low-Beta | Low-Beta (12–15 Hz)/totalfrequency (4–50 Hz) | Attentive status |
| RFA | Relative Fast-Alpha | Fast-Alpha (11–13 Hz)/totalfrequency (4–50 Hz) | Relaxation and stabilization |
| SEF50 | Spectral Edge Frequency 50% of Total Spectrum Band | The lowest frequency below which 50% of the total power in the total frequency band (4–50 Hz) | Awareness |
| ASEF50 | Spectral Edge Frequency 50% of Alpha Spectrum Band | The lowest frequency below which 50% of the total power in the alpha frequency (8–13 Hz) | Adequate awareness withstability and relaxation |
| Variable | Male (n = 30) | Female (n = 30) | Total (N = 60) | Significance 1 |
|---|---|---|---|---|
| M ± SD | ||||
| Age (years) | 25.8 ± 2.4 | 24.5 ± 2.9 | 25.2 ± 2.7 | NS |
| Height 2 (cm) | 176.3 ± 5.7 | 160.9 ± 6.1 | 168.7 ± 9.7 | 0.000 *** |
| Body weight 3 (kg) | 76.2 ± 12.9 | 54.7 ± 8.5 | 65.6 ± 15.3 | 0.000 *** |
| Body mass index 4 (kg∙m−2) | 24.4 ± 3.6 | 21.6 ± 2.3 | 23.0 ± 3.3 | 0.001 ** |
| Variable | Activity | RLB | RFA | ||
|---|---|---|---|---|---|
| F3 | F4 | F3 | F4 | ||
| M ± SD | |||||
| Male (n = 30) | Low level cognitive demand | 0.063 ± 0.016 | 0.066 ± 0.018 | 0.054 ± 0.015 | 0.056 ± 0.016 |
| High level cognitive demand | 0.068 ± 0.020 | 0.072 ± 0.021 | 0.056 ± 0.018 | 0.061 ± 0.023 | |
| Significance | 0.716 NS | 0.323 NS | 0.443 NS | 0.201 NS | |
| Female (n = 30) | Low level cognitive demand | 0.058 ± 0.012 | 0.060 ± 0.011 | 0.049 ± 0.012 | 0.052 ± 0.012 |
| High level cognitive demand | 0.063 ± 0.019 | 0.065 ± 0.019 | 0.053 ± 0.017 | 0.057 ± 0.017 | |
| Significance | 0.002 ** | 0.003 ** | 0.033 * | 0.023 * | |
| Total (N = 60) | Low level cognitive demand | 0.061 ± 0.014 | 0.063 ± 0.015 | 0.052 ± 0.014 | 0.054 ± 0.015 |
| High level cognitive demand | 0.066 ± 0.019 | 0.068 ± 0.020 | 0.054 ± 0.018 | 0.059 ± 0.021 | |
| Significance | 0.025 * | 0.016 * | 0.052 NS | 0.042 * | |
| Variable | Activity | SEF50 | ASEF50 | ||
|---|---|---|---|---|---|
| F3 | F4 | F3 | F4 | ||
| M ± SD | |||||
| Male (n = 30) | Low level cognitive demand | 12.271 ± 5.734 | 10.992 ± 4.559 | 9.986 ± 0.382 | 9.974 ± 0.372 |
| High level cognitive demand | 12.121 ± 6.125 | 10.827 ± 3.468 | 10.094 ± 0.414 | 10.075 ± 0.387 | |
| Significance | 0.583 NS | 0.479 NS | 0.353 NS | 0.476 NS | |
| Female (n = 30) | Low level cognitive demand | 10.023 ± 4.710 | 9.366 ± 2.429 | 9.913 ± 0.264 | 9.899 ± 0.269 |
| High level cognitive demand | 11.289 ± 5.905 | 9.455 ± 3.424 | 10.059 ± 0.353 | 10.038 ± 0.342 | |
| Significance | 0.336 NS | 0.300 NS | 0.045 * | 0.040 * | |
| Total (N = 60) | Low level cognitive demand | 11.147 ± 5.325 | 10.179 ± 3.713 | 9.949 ± 0.328 | 9.937 ± 0.324 |
| High level cognitive demand | 11.705 ± 5.980 | 10.141 ± 3.486 | 10.077 ± 0.382 | 10.057 ± 0.363 | |
| Significance | 0.323 NS | 0.971 NS | 0.050 NS | 0.082 NS | |
| Variable | Activity | Heart Rate | LF | HF | SDNN |
|---|---|---|---|---|---|
| M ± SD | |||||
| Male (n = 30) | Low level cognitive demand | 84.88 ± 9.25 | 0.71 ± 0.14 | 0.29 ± 0.14 | 34.68 ± 9.81 |
| High level cognitive demand | 83.72 ± 9.56 | 0.73 ± 0.14 | 0.27 ± 0.14 | 41.60 ± 15.62 | |
| Significance | 0.969 NS | 0.590 NS | 0.590 NS | 0.028 * | |
| Female (n = 30) | Low level cognitive demand | 84.38 ± 9.76 | 0.66 ± 0.19 | 0.34 ± 0.19 | 36.23 ± 12.60 |
| High level cognitive demand | 84.77 ± 8.93 | 0.72 ± 0.15 | 0.28 ± 0.15 | 39.50 ± 26.59 | |
| Significance | 0.522 NS | 0.070 NS | 0.070 NS | 0.293 NS | |
| Total (N = 60) | Low level cognitive demand | 84.63 ± 9.43 | 0.68 ± 0.17 | 0.32 ± 0.17 | 35.45 ± 11.22 |
| High level cognitive demand | 84.25 ± 9.19 | 0.73 ± 0.15 | 0.27 ± 0.15 | 40.55 ± 21.65 | |
| Significance | 0.690 NS | 0.102 NS | 0.102 NS | 0.057 NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-O.; Kim, Y.-J.; Park, S.-A. Psychophysiological Responses of Adults According to Cognitive Demand Levels for Horticultural Activities. Sustainability 2022, 14, 8252. https://doi.org/10.3390/su14148252
Kim S-O, Kim Y-J, Park S-A. Psychophysiological Responses of Adults According to Cognitive Demand Levels for Horticultural Activities. Sustainability. 2022; 14(14):8252. https://doi.org/10.3390/su14148252
Chicago/Turabian StyleKim, Seon-Ok, Yun-Jin Kim, and Sin-Ae Park. 2022. "Psychophysiological Responses of Adults According to Cognitive Demand Levels for Horticultural Activities" Sustainability 14, no. 14: 8252. https://doi.org/10.3390/su14148252
APA StyleKim, S.-O., Kim, Y.-J., & Park, S.-A. (2022). Psychophysiological Responses of Adults According to Cognitive Demand Levels for Horticultural Activities. Sustainability, 14(14), 8252. https://doi.org/10.3390/su14148252







