Iron-Doped Biochar Regulated Soil Nickel Adsorption, Wheat Growth, Its Physiology and Elemental Concentration under Contrasting Abiotic Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Preparation, Enrichment and Experimental Design
2.2. Shoot and Root Elemental and Biochemical Analysis
2.2.1. Analysis of Sodium (Na), Calcium (Ca), Magnesium (Mg), Potassium (K), Iron (Fe) and Nickel (Ni)
2.2.2. Estimation of Hydrogen Peroxide (H2O2), Melondialdehyde (MDA) and Cell Membrane Permeability (CMP)
2.2.3. Determination of Root and Shoot Phenolics and Protein Concentrations
2.2.4. Analysis of Antioxidant Enzymes
2.2.5. Determination of Chlorophyll Contents
2.3. Post-Harvest Soil Analysis
2.3.1. Study of Ni Sorption through Isotherm Models
- Qe = Adsorbent capacity, expressed as amount of Ni per adsorbent mass unit at equilibrium (mg g−1)
- V = Volume of Ni solution (L)
- Co = Initial Ni concentration in soil solution (mg L−1)
- Ce = Equilibrium Ni concentration in soil solution (mg L−1)
- m = Dry mass of adsorbent
2.3.2. Soil pH, Moisture and Soluble Chlorides
Statistical Analysis
3. Results
3.1. Characterization of Simple Rice Husk Biochar and Enriched (BC) Biochar
3.2. Biomass Accumulation and Elemental Concentration in Wheat Root and Shoot
3.3. Shoot Hydrogen Peroxide (H2O2), Melondialdehyde (MDA), Cell Membrane Permeability (CMP) and Shoot Antioxidant Enzymes
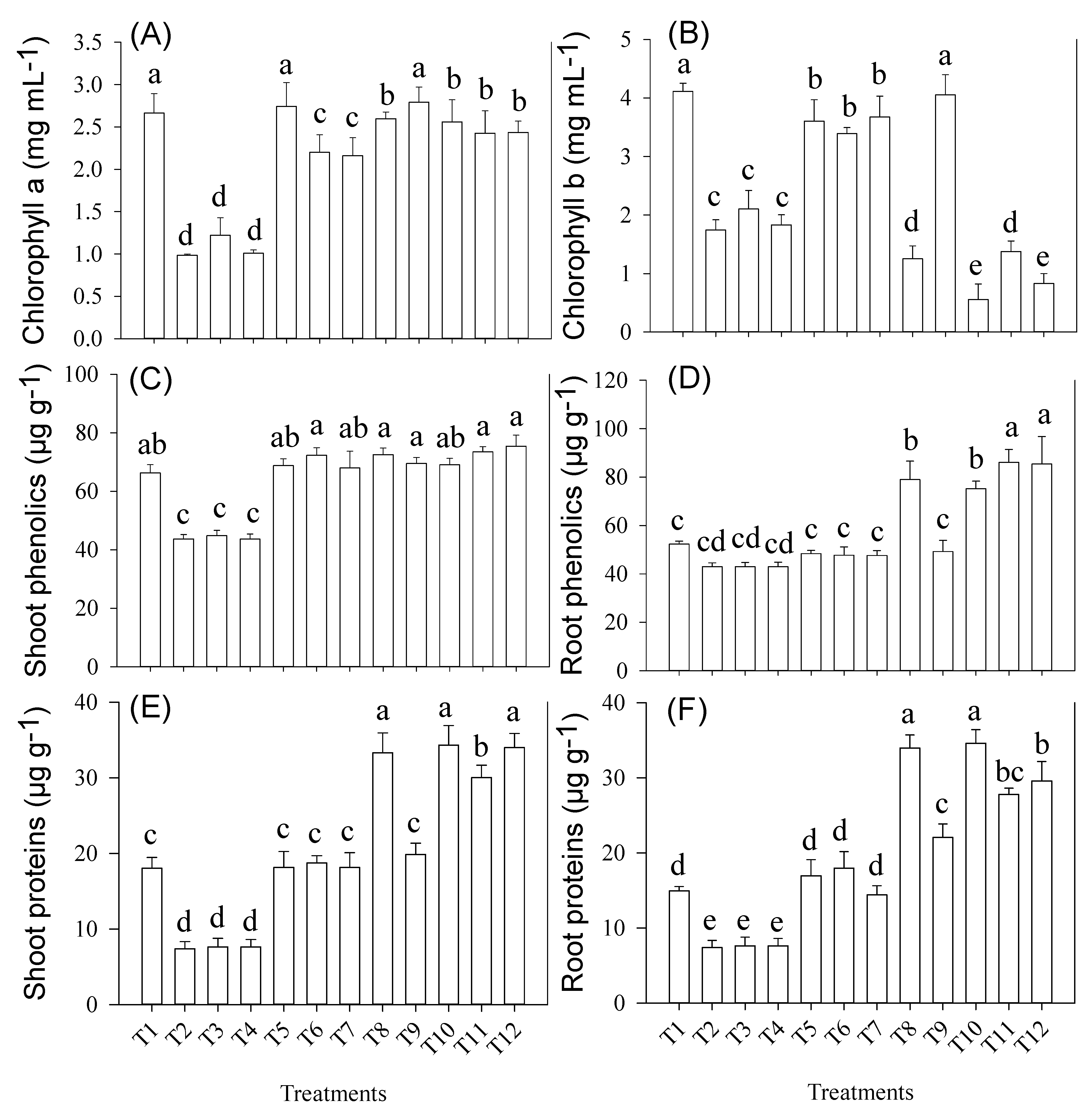
3.4. Root and Shoot Chlorophyll (a & b), Non-Enzymatic Antioxidants (Phenolics) and Protein Concentration
3.5. Sorption Isotherms for Ni
4. Discussion
4.1. Enhanced Ni Adsorption on BC-Soil Interface Led to Better Wheat Growth
4.2. Rice Husk Fe-Biochar Reduced the Toxicity of Soil Chlorides (Cl−) for Wheat Plants
4.3. Nutrients Uptake in Wheat Plant Regulated by BC under Contrasting Abiotic Stresses
4.4. Rice Husk Fe-Enriched Biochar (BC) Maintained Biomass and Cell Membrane Stability by Triggering Antioxidant Defense System under Contrasting Abiotic Stresses
4.5. Phenolics and Protein Accumulation Mitigated Contrasting Abiotic Stresses through BC Application
4.6. Economics of BC and Its Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, W.U.D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z.; Anwar, M.N.; Baqar, M.; Tauqeer, H.M.; et al. Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, H.; Xia, M.; Lei, W.; Wang, F. The single/co-adsorption characteristics and microscopic adsorption mechanism of biochar-montmorillonite composite adsorbent for pharmaceutical emerging organic contaminant atenolol and lead ions. Ecotoxicol. Environ. Saf. 2020, 187, 109763. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Fujita, M. Calcium-mediated growth regulation and abiotic stress tolerance in plants. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 291–331. [Google Scholar]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, A.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Long, J.; Su, M.; Diao, Z.; Chang, X.; Chen, D.; Song, G.; Shih, K. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef]
- Turan, V.; Ramzani, P.M.A.; Ali, Q.; Abbas, F.; Iqbal, M.; Irum, A.; Khan, W.U.D. Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch. Agron. Soil Sci. 2018, 64, 1053–1067. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar composites with nano zerovalent iron and eggshell powder for nitrate removal from aqueous solution with coexisting chloride ions. Environ. Sci. Pollut. Res. 2018, 25, 25757–25771. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhang, C.; Ren, L. Preparation and evaluation of activated carbon-based iron-containing adsorbents for enhanced Cr (VI) removal: Mechanism study. Chem. Eng. Sci. 2012, 189, 295–302. [Google Scholar] [CrossRef]
- Dad, F.P.; Khan, W.U.D.; Tanveer, M.; Ramzani, P.M.A.; Shaukat, R.; Muktadir, A. Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under cadmium toxicity. Agriculture 2020, 11, 1. [Google Scholar] [CrossRef]
- Abid, N.; Maqbool, A.; Malik, K.A. Screening commercial wheat (Triticum aestivum L.) varieties for Agrobacterium mediated transformation ability. Pak. J. Agric. Sci. 2014, 51, 83–89. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis: A manual for the West Asia and North Africa region. Int. Cent. Agric. Res. Dry Areas (ICARDA) 2013, 3, 55–119. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1982, 22, 867–880. [Google Scholar]
- Cakmak, I.; Horst, W.J. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Strain, H.H.; Svec, W.A. Extraction, separation, estimation, and isolation of the chlorophylls. In The Chlorophylls; Academic Press: Cambridge, MA, USA, 1966; pp. 21–66. [Google Scholar]
- Williams, L.J.; Abdi, H. Fisher’s least significant difference (LSD) test. In Encyclopedia of Research Design; Sage: Newcastle upon Tyne, UK, 2010; Volume 218, pp. 840–853. [Google Scholar]
- Dada, A.O.; Ojediran, J.O.; Olalekan, A.P. Sorption of Pb2+ from aqueous solution unto modified rice husk: Isotherms studies. Adv. Phys. Chem. 2013, 2013, 842425. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. pH mediated facile preparation of hydrotalcite based adsorbent for enhanced arsenite and arsenate removal: Insights on physicochemical properties and adsorption mechanism. J. Mol. Liq. 2017, 240, 240–252. [Google Scholar] [CrossRef]
- Diao, Z.H.; Du, J.J.; Jiang, D.; Kong, L.J.; Huo, W.Y.; Liu, C.M.; Wu, Q.H.; Xu, X.R. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism. Sci. Total Environ. 2018, 642, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards biochar and hydrochar engineering—Influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Pianelli, K.; Mari, S.; Marquès, L.; Lebrun, M.; Czernic, P. Nicotianamine over-accumulation confers resistance to nickel in Arabidopsis thaliana. Transgenic Res. 2005, 14, 739–748. [Google Scholar] [CrossRef]
- Callahan, D.L.; Kolev, S.D.; O’Hair, R.A.; Salt, D.E.; Baker, A.J. Relationships of nicotianamine and other amino acids with nickel, zinc and iron in Thlaspi hyperaccumulators. New Phytol. 2007, 176, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Enhancement of nickel and lead accumulation and their toxic growth-inhibitory effects on amaranth seedlings in the presence of calcium. Russ. J. Plant Physiol. 2009, 56, 80–84. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Jia, R.; Li, L.; Qu, D.; Mi, N. Enhanced iron (III) reduction following amendment of paddy soils with biochar and glucose modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 91–103. [Google Scholar] [CrossRef]
- Jiang, S.F.; Ling, L.L.; Chen, W.J.; Liu, W.J.; Li, D.C.; Jiang, H. Highly efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. J. Chem. Eng. Technol. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Holland, J.E.; Cammarano, D.; Fitzgerald, G.J.; Perry, E.M.; Poile, G.; Conyers, M.K. Proximal fluorescence sensing of potassium responsive crops to develop improved predictions of biomass, yield and grain quality of wheat and barley. Precis. Agric. 2019, 20, 379–397. [Google Scholar] [CrossRef]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, K.; Bose, J.; Shabala, L.; Shabala, S. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J. Exp. Bot. 2016, 67, 4611–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S. Signaling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Interactive role of epibrassinolide and hydrogen peroxide in regulating stomatal physiology, root morphology, photosynthetic and growth traits in Solanum lycopersicum L. under nickel stress. Environ. Exp. Bot. 2019, 162, 479–495. [Google Scholar] [CrossRef]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt Stress in Brassica: Effects, Tolerance Mechanisms, and Management. J. Plant Growth Regul. 2022, 41, 781–795. [Google Scholar] [CrossRef]
- Abd-Allah, E.F.; Hashem, A.; Alam, P.; Ahmad, P. Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 2019, 38, 1260–1273. [Google Scholar] [CrossRef]
- Khan, M.T.; Ahmed, S.; Shah, A.A.; Noor Shah, A.; Tanveer, M.; El-Sheikh, M.A.; Siddiqui, M.H. Influence of Zinc Oxide Nanoparticles to Regulate the Antioxidants Enzymes, Some Osmolytes and Agronomic Attributes in Coriandrum sativum L. Grown under Water Stress. Agronomy 2021, 11, 2004. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Pathak, K.; Kataria, S.; Gadre, R. Trending Methods to Enhance Antioxidant Activities in Wheat. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 241–260. [Google Scholar]
- Sharma, S.; Chandra, S.; Kumar, A.; Bindraban, P.; Saxena, A.K.; Pande, V.; Pandey, R. Foliar application of iron fortified bacteriosiderophore improves growth and grain Fe concentration in wheat and soybean. Indian J. Microbiol. 2019, 59, 344–350. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.; Anawar, H.M.; Storer, P.; Blackwell, P.; Chee, C.; Yun, L.; Munroe, P.; Donne, S.; Horvat, J.; Wang, J.; et al. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 2015, 25, 749–760. [Google Scholar] [CrossRef]
- Taqdees, Z.; Khan, J.; Khan, W.U.D.; Kausar, S.; Afzaal, M.; Akhtar, I. Silicon and zinc nanoparticles-enriched miscanthus biochar enhanced seed germination, antioxidant defense system, and nutrient status of radish under NaCl stress. Crop Pasture Sci. 2022, 73, 556–572. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]

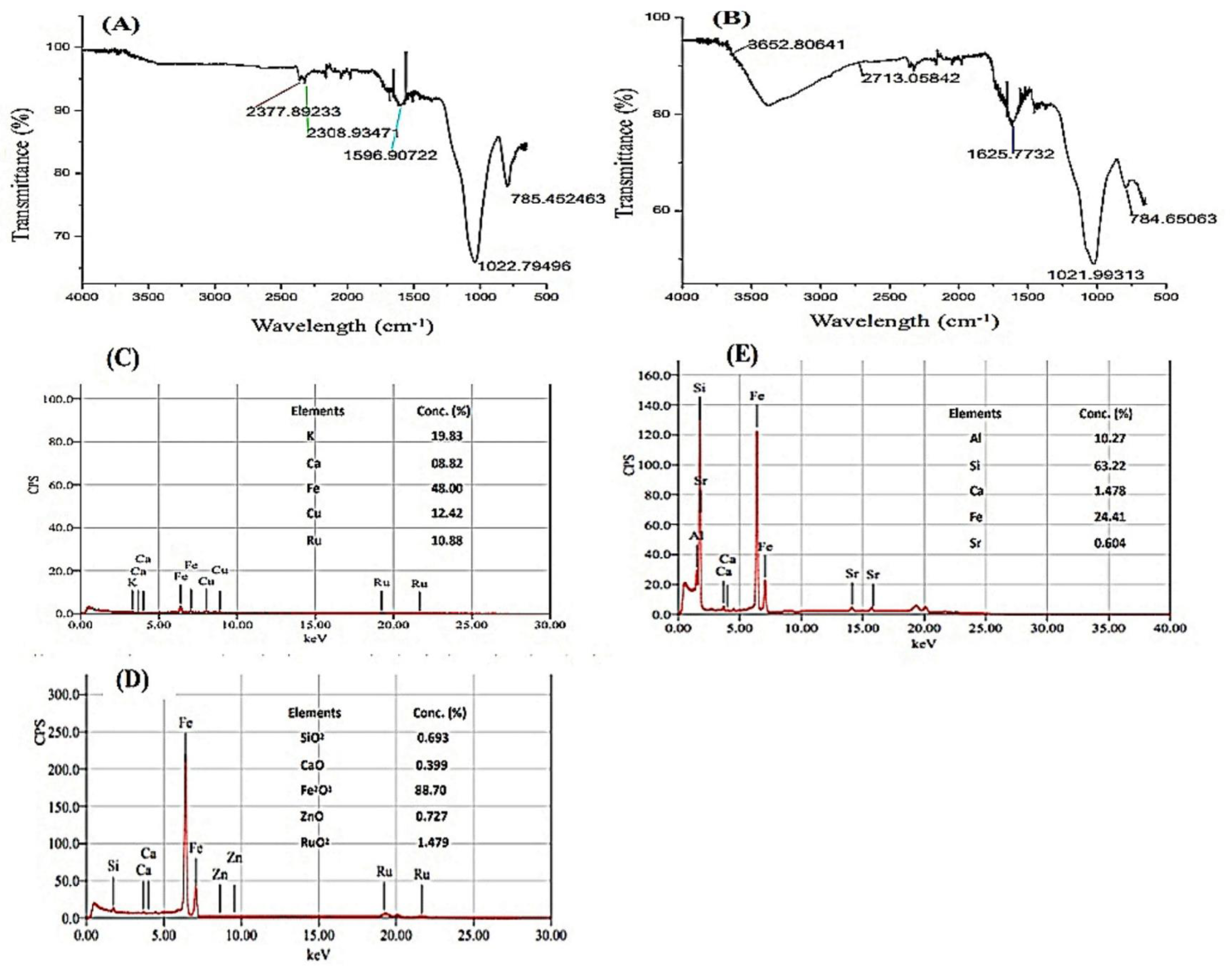

| Treatments | Root Ni | Shoot Ni | Root:Shoot Ni | DTPA-Extractable Ni in Soil |
|---|---|---|---|---|
| mg kg−1 | mmol L−1 | |||
| T1 | * N.D | * N.D | - | 5 ± 0.25 |
| T2 | 4.013 ± 0.437d | 8.013 ± 0.426d | 6.5 | 8.55 ± 0.04 |
| T3 | 0.070.01c | * N.D | - | 5.98 ± 0.30 |
| T4 | 0.0124 ± 0.01c | * N.D | - | 5.87 ± 0.43 |
| T5 | 0.307 ± 0.01c | * N.D | - | 5.83 ± 0.19 |
| T6 | 7.010 ± 0.48a | 1.080 ± 0.20d | 6.4 | 8.33 ± 0.05 |
| T7 | 0.103 ± 0.02c | * N.D | - | 5 ± 0.20 |
| T8 | 0.035 ± 0.96d | 0.013 ± 0.12de | 3 | 5 ± 0.30 |
| T9 | * N.D | 0.077 ± 0.50c | - | 28.67 ± 0.05 |
| T10 | 6.070 ± 0.16a | 17.818 ± 1.45a | 0.34 | 15.83 ± 0.09 |
| T11 | 0.059 ± 0.03d | 0.823 ± 0.25d | 0.07 | 5.83 ± 0.02 |
| T12 | 5.270 ± 1.06a | 13.035 ± 1.19b | 0.4 | 16.66 ± 0.05 |
| F-value | 2.056 | 2.136 | ||
| LSD0.05 | 2.056 | 2.136 | ||
| Treatments | DW | Root | DW | Shoot | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Ca | Mg | Na | K | Fe | S | Ca | Mg | Na | K | Fe | |
| g | mg g−1 | mg kg−1 | g | mg g−1 | mg kg−1 | |||||||
| T1 | 0.125 ± 0.007f | 0.519 ± 0.001c | 0.262 ± 0.001e | 0.491 ± 0.003e | 0.460 ± 0.002c | 24.23 ± 1.62f | 0.139 ± 0.01e | 0.505 ± 0.006h | 0.2105 ± 0.01e | 0.591 ± 0.003e | 1.745 ± 0.01c | 26.13 ± 1.60f |
| T2 | 0.05 ± 0.001c | 0.123 ± 0.001c | 0.022 ± 0.002c | 0.03 ± 0.001c | 0.032 ± 0.002c | 20.21 ± 1.111f | 0.007 ± 0.001c | 0.121 ± 0.001c | 0.023 ± 0.002c | 0.023 ± 0.001c | 0.033 ± 0.002c | 20.23 ± 1.111f |
| T3 | 0.08 ± 0.002b | 1.260 ± 0.002c | 0.042 ± 0.001c | 0.021 ± 0.002b | 0.042 ± 0.001c | 19.86 ± 1.211f | 0.009 ± 0.002c | 1.270 ± 0.001c | 0.052 ± 0.002c | 0.031 ± 0.002b | 0.052 ± 0.001c | 19.82 ± 1.211f |
| T4 | 0.04 ± 0.001e | 0.002 ± 0.001c | 0.011 ± 0.002c | 1.892 ± 0.003b | 0.021 ± 0.002c | 16.62 ± 1.161f | 0.005 ± 0.001c | 0.002 ± 0.001c | 0.021 ± 0.002b | 1.992 ± 0.002c | 0.031 ± 0.001c | 16.52 ± 1.161f |
| T5 | 0.515 ± 0.005a | 0.267 ± 0.001e | 0.506 ± 0.001c | 0.485 ± 0.001e | 0.654 ± 0.001b | 40.56 ± 1.14e | 0.855 ± 0.02a | 0.841 ± 0.006e | 0.229 ± 0.01de | 0.443 ± 0.001f | 2.245 ± 0.04a | 48.86 ± 1.14e |
| T6 | 0.175 ± 0.005e | 0.420 ± 0.01d | 0.541 ± 0.01c | 0.702 ± 0.02d | 0.467 ± 0.02c | 104.54 ± 2.29b | 0.31 ± 0.01b | 0.77 ± 0.001f | 0.207 ± 0.01e | 0.15 ± 0.01h | 1.92 ± 0.02b | 53.40 ± 1.14e |
| T7 | 0.23 ± 0.01d | 0.464 ± 0.01d | 0.956 ± 0.03b | 0.1994 ± 0.01g | 0.450 ± 0.04c | 72.72 ± 2.29c | 0.24 ± 0.01cd | 2.199 ± 0.01a | 0.132 ± 0.01g | 0.319 ± 0.01g | 1.847 ± 0.0003bc | 101.13 ± 1.14c |
| T8 | 0.11 ± 0.01f | 0.001 ± 0.001g | 0.222 ± 0.004e | 0.727 ± 0.01d | 0.228 ± 0.01d | 76.13 ± 3.44c | 0.195 ± 0.01de | 1.140 ± 0.005d | 0.295 ± 0.009c | 2.87 ± 0.01b | 1.293 ± 0.06d | 48.86 ± 1.14e |
| T9 | 0.485 ± 0.005a | 1.68 ± 0.0001a | 1.166 ± 0.01a | 0.374 ± 0.01f | 0.157 ± 0.02e | 98.86 ± 3.44b | 0.395 ± 0.03b | 0.104 ± 0.01i | 0.195 ± 0.002f | 0.659 ± 0.01d | 2.221 ± 0.04a | 397.72 ± 11.47a |
| T10 | 0.435 ± 0.005b | 0.395 ± 0.008d | 0.432 ± 0.01d | 1.082 ± 0.01b | 0.334 ± 0.003d | 73.86 ± 1.14c | 0.375 ± 0.02b | 1.52 ± 0.01c | 0.441 ± 0.05b | 3.228 ± 0.01a | 2.206 ± 0.04a | 73.86 ± 1.14d |
| T11 | 0.265 ± 0.01c | 0.521 ± 0.004c | 0.476 ± 0.002d | 0.9066 ± 0.01c | 0.332 ± 0.005d | 126.13 ± 3.44a | 0.185 ± 0.005de | 1.57 ± 0.01b | 0.283 ± 0.002cd | 2.900 ± 0.026b | 0.953 ± 0.0004e | 155.68 ± 5.73b |
| T12 | 0.28 ± 0.01c | 1.573 ± 0.009b | 0.3594 ± 0.001e | 3.04 ± 0.02a | 1.474 ± 0.004a | 53.409 ± 4.82d | 0.295 ± 0.02bc | 0.515 ± 0.01g | 0.694 ± 0.0001a | 1.67 ± 0.007c | 0.432 ± 0.02f | 77.27 ± 2.29d |
| F-value | 1.83 | 4672 | 298 | 3827 | 358 | 4.78 | 2.34 | 1922 | 72.9 | 8974 | 306 | 99.2 |
| LSD0.05 | 0.025 | 0.0305 | 0.0451 | 0.0421 | 0.0662 | 8.3077 | 0.063 | 0.031 | 0.0644 | 0.0396 | 0.1157 | 14.08 |
| Treatment | Freundlich Isotherm Model | Langmuir Isotherm Model | ||||
|---|---|---|---|---|---|---|
| KF | 1/n | R2 | Xm (qmax) | KL | R2 | |
| mg g−1 | mg g−1 | mg g−1 | ||||
| T1 | 1.55 | 5.017 | 0.899 | 2.57 | 0.155 | 1 |
| T2 | 1.21 | 6.25 | 1 | 26.2 | 0.007 | 1 |
| T3 | 1.738 | 7.276 | 1 | 6.25 | 0.191 | 1 |
| T4 | 1.95 | 9.017 | 1 | 2.87 | 0.176 | 1 |
| T5 | 1.425 | 5.25 | 0.993 | 25.9 | 0.9091 | 0.998 |
| T6 | 0.921 | 0.062 | 0.999 | 2433 | 10.638 | 1 |
| T7 | 0.922 | 2.927 | 0.995 | 143 | 0.9902 | 0.999 |
| T8 | 1.0039 | 3.246 | 0.987 | 110 | 0.2968 | 0.996 |
| T9 | 0.996 | 0.219 | 0.999 | 2304 | 2.9569 | 1 |
| T10 | 0.947 | 0.121 | 0.999 | 2380 | 2.8985 | 1 |
| T11 | 1.4078 | 5.15 | 0.981 | 24.7 | 0.9959 | 0.997 |
| T12 | 0.95 | 0.129 | 0.999 | 2375 | 2.5673 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, W.-u.-D.; Shaukat, R.; Farooq, M.A.; Ashraf, M.N.; Nadeem, F.; Tanveer, M.; Hamid, Y.; Sun, N. Iron-Doped Biochar Regulated Soil Nickel Adsorption, Wheat Growth, Its Physiology and Elemental Concentration under Contrasting Abiotic Stresses. Sustainability 2022, 14, 7852. https://doi.org/10.3390/su14137852
Khan W-u-D, Shaukat R, Farooq MA, Ashraf MN, Nadeem F, Tanveer M, Hamid Y, Sun N. Iron-Doped Biochar Regulated Soil Nickel Adsorption, Wheat Growth, Its Physiology and Elemental Concentration under Contrasting Abiotic Stresses. Sustainability. 2022; 14(13):7852. https://doi.org/10.3390/su14137852
Chicago/Turabian StyleKhan, Waqas-ud-Din, Rabia Shaukat, Muhammad Ansar Farooq, Muhammad Nadeem Ashraf, Faisal Nadeem, Mohsin Tanveer, Yasir Hamid, and Nan Sun. 2022. "Iron-Doped Biochar Regulated Soil Nickel Adsorption, Wheat Growth, Its Physiology and Elemental Concentration under Contrasting Abiotic Stresses" Sustainability 14, no. 13: 7852. https://doi.org/10.3390/su14137852
APA StyleKhan, W.-u.-D., Shaukat, R., Farooq, M. A., Ashraf, M. N., Nadeem, F., Tanveer, M., Hamid, Y., & Sun, N. (2022). Iron-Doped Biochar Regulated Soil Nickel Adsorption, Wheat Growth, Its Physiology and Elemental Concentration under Contrasting Abiotic Stresses. Sustainability, 14(13), 7852. https://doi.org/10.3390/su14137852











