Alloy Profusion, Spice Metals, and Resource Loss by Design
Abstract
:1. Introduction
2. Alloy Profusion
3. Spice Metals
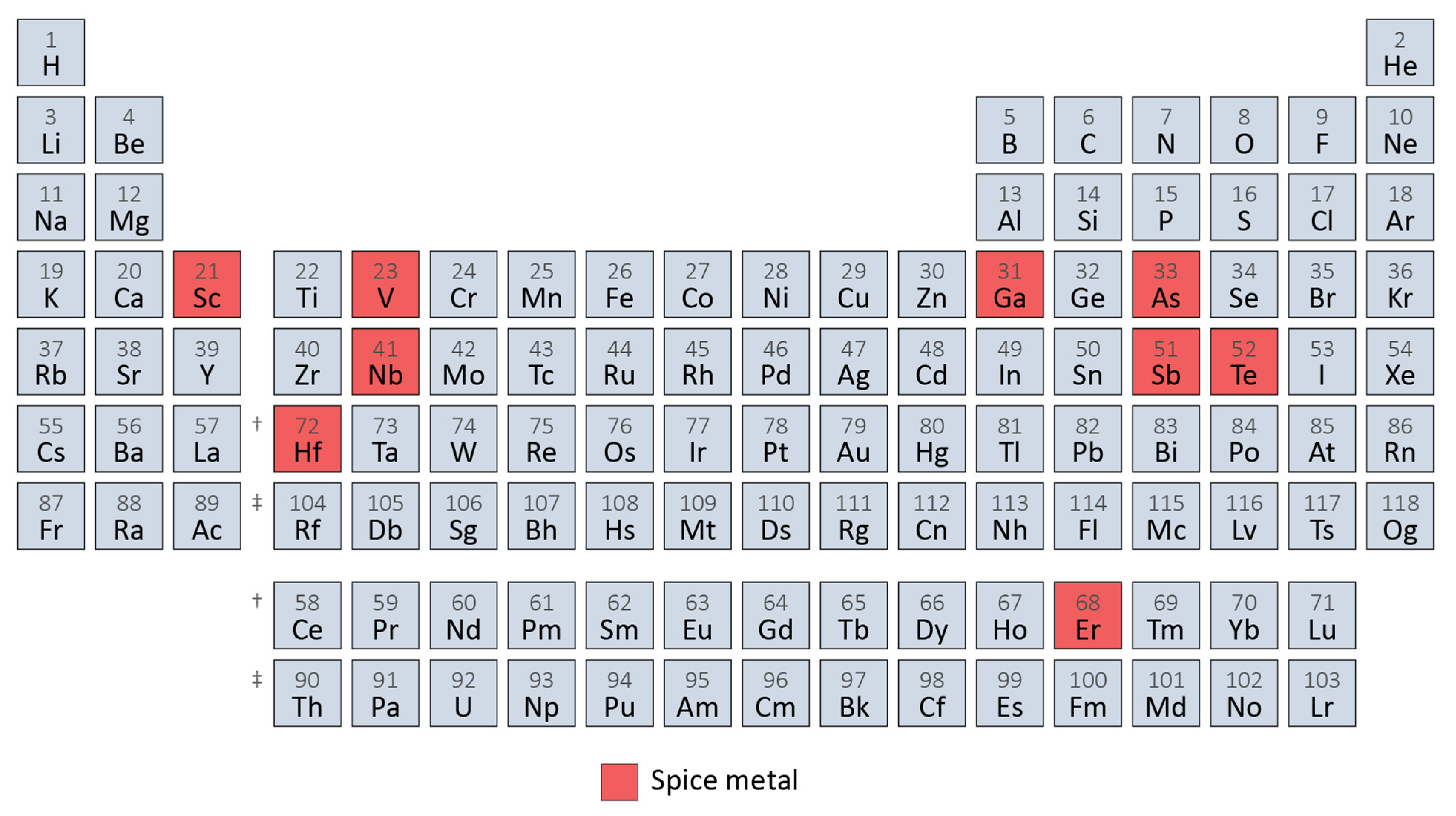
A Formal Definition for Spice Metals
4. Serviceable Resources: Abandonment or Reuse?
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zink, T.; Geyer, R. Material Recycling and the Myth of Landfill Diversion. J. Ind. Ecol. 2019, 23, 541–548. [Google Scholar] [CrossRef]
- Gutowski, T.G. Thermodynamics and recycling, a review. In Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA, 19–22 May 2008; pp. 1–5. [Google Scholar]
- Chen, P.-C.; Chiu, M.-C.; Ma, H.-W. Measuring the reduction limit of repeated recycling—A case study of the paper flow system. J. Clean. Prod. 2016, 132, 98–107. [Google Scholar] [CrossRef]
- Kuczenski, B.; Geyer, R. Material flow analysis of polyethylene terephthalate in the US, 1996–2007. Resour. Conserv. Recycl. 2010, 54, 1161–1169. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daehn, K.E.; Cabrera Serrenho, A.; Allwood, J.M. How Will Copper Contamination Constrain Future Global Steel Recycling? Environ. Sci. Technol. 2017, 51, 6599–6606. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kondo, Y.; Nakajima, K.; Ohno, H.; Pauliuk, S. Quantifying Recycling and Losses of Cr and Ni in Steel Throughout Multiple Life Cycles Using MaTrace-Alloy. Environ. Sci. Technol. 2017, 51, 9469–9476. [Google Scholar] [CrossRef] [Green Version]
- Daehn, K.E.; Serrenho, A.C.; Allwood, J. Finding the Most Efficient Way to Remove Residual Copper from Steel Scrap. Metall. Mater. Trans. B 2019, 50, 1225–1240. [Google Scholar] [CrossRef]
- Daigo, I.; Tajima, K.; Hayashi, H.; Panasiuk, D.; Takeyama, K.; Ono, H.; Kobayashi, Y.; Nakajima, K.; Hoshino, T. Potential Influences of Impurities on Properties of Recycled Carbon Steel. ISIJ Int. 2021, 61, 498–505. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Jaunich, M.K.; Levis, J.W.; DeCarolis, J.F.; Barlaz, M.A.; Ranjithan, S.R. Solid Waste Management Policy Implications on Waste Process Choices and Systemwide Cost and Greenhouse Gas Performance. Environ. Sci. Technol. 2019, 53, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.R.P.; Gomes, M.I.; Barbosa-Póvoa, A.P. Assessing and improving management practices when planning packaging waste collection systems. Resour. Conserv. Recycl. 2014, 85, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Daigo, I.; Nansai, K.; Matsubae, K.; Takayanagi, W.; Tomita, M.; Matsuno, Y. Global distribution of material consumption: Nickel, copper, and iron. Resour. Conserv. Recycl. 2018, 133, 369–374. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Montanelli, L.; Homer, E.R.; Olivetti, E. Factors to Consider When Designing Aluminium Alloys for Increased Scrap Usage. In REWAS 2022: Developing Tomorrow’s Technical Cycles (Volume I); Springer: Cham, Switzerland, 2022; pp. 465–473. [Google Scholar]
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Chancerel, P.; Rotter, V.S.; Ueberschaar, M.; Marwede, M.; Nissen, N.F.; Lang, K.-D. Data availability and the need for research to localize, quantify and recycle critical metals in information technology, telecommunication and consumer equipment. Waste Manag. Res. 2013, 31, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Korf, N.; Løvik, A.N.; Figi, R.; Schreiner, C.; Kuntz, C.; Mählitz, P.M.; Rösslein, M.; Wäger, P.; Rotter, V.S. Multi-element chemical analysis of printed circuit boards—Challenges and pitfalls. Waste Manag. 2019, 92, 124–136. [Google Scholar] [CrossRef]
- European Commission. Critical Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; Publications Office of the European Union: Luxembourg, 2020; p. 100. [Google Scholar]
- Ciacci, L.; Reck, B.K.; Nassar, N.T.; Graedel, T.E. Lost by Design. Environ. Sci. Technol. 2015, 49, 9443–9451. [Google Scholar] [CrossRef]
- AZO Materials. AISI 4140 Alloy Steel (UNS G41400). Available online: https://www.azom.com/article.aspx?ArticleID=6769 (accessed on 13 April 2022).
- AZO Materials. Beryllium Copper UNS C17200. Available online: https://www.azom.com/article.aspx?ArticleID=6326 (accessed on 13 April 2022).
- Economic Times. Alloy Steel: Everything You Need to Know about Alloy Steels and Their Role in Building and Construction Industry. Available online: https://economictimes.indiatimes.com/small-biz/productline/building-materials/alloy-steel-everything-you-need-to-know-about-alloy-steels-and-their-role-in-building-and-construction-industry/articleshow/70344024.cms (accessed on 7 April 2022).
- World Steel Association. About Steel. Available online: https://worldsteel.org/about-steel/about-steel/ (accessed on 7 April 2022).
- Copper Development Association. Copper Alloys Advanced Search. Available online: https://alloys.copper.org/ (accessed on 7 April 2022).
- Sankaran, K.K.; Mishra, R.S. Chapter 4—Aluminum Alloys. In Metallurgy and Design of Alloys with Hierarchical Microstructures; Sankaran, K.K., Mishra, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–176. [Google Scholar]
- Silva, M.S.; Barbosa, C.; Acselrad, O.; Pereira, L.C. Effect of chemical composition variation on microstructure and mechanical properties of a 6060 aluminum alloy. J. Mater. Eng. Perform. 2004, 13, 129–134. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Li, D.; Zhang, Y.; Gao, Y.; Liaw, P.K. Mechanical behavior of high-entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- U.S. Department of the Interior. Final List of Critical Minerals 2018; Federal Register, 2018; pp. 23295–23296. Available online: https://www.federalregister.gov/documents/2018/05/18/2018-10667/final-list-of-critical-minerals-2018 (accessed on 7 April 2022).
- Australian Government. Australian Critical Minerals Prospectus 2020; 2020; p. 172. Available online: https://www.austrade.gov.au/ArticleDocuments/5572/Australian_Critical_Minerals_Prospectus.pdf.aspx (accessed on 7 April 2022).
- Government of Canada. Canada’s Critical Minerals List 2021; 2021. Available online: https://www.nrcan.gc.ca/sites/nrcan/files/mineralsmetals/pdf/Critical_Minerals_List_2021-EN.pdf (accessed on 7 April 2022).
- Nakano, J. The Geopolitics of Critical Minerals Supply Chains; Center for Strategic & International Studies: Washington, DC, USA, 2021. [Google Scholar]
- Ministry of Natural Resources & National Development and Reform Commission of the People’s Republic of China. National Mineral Resources Planning 2016–2020. Available online: https://www.ndrc.gov.cn/fggz/fzzlgh/gjjzxgh/201705/t20170511_1196755.html?code=&state=123 (accessed on 4 May 2022).
- Ministry of Economy, Trade, and Industry (METI) of Japan. New International Resource Strategy Formulated. Available online: https://www.meti.go.jp/english/press/2020/0330_005.html (accessed on 4 May 2022).
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI: Wallingford, UK, 2008. [Google Scholar]
- Reller, A.; Bublies, T.; Staudinger, T.; Oswald, I.; Meißner, S.; Allen, M. The Mobile Phone: Powerful Communicator and Potential Metal Dissipator. GAIA Ecol. Perspect. Sci. Soc. 2009, 18, 127–135. [Google Scholar] [CrossRef]
- Senk, D.; Meyer, F.M.; Pretz, T.; Abrasheva, G. Strategies for fulfilment of critical raw materials demand in Europe. Rev. Métallurgie 2012, 109, 333–339. [Google Scholar] [CrossRef]
- Hieronymi, K. Electronics Industry Competes for Raw Materials: Will the Scarcity of Natural Resources Become a Showstopper for the Information and Communications Industry? In E-Waste Management; Hieronymi, K., Kahhat, R., Williams, E., Eds.; Routledge: London, UK, 2012; pp. 253–266. [Google Scholar]
- Hagelüken, C. Recycling of Technology Metals from Electronics. A Good Opportunity—And a Complex Challenge. Available online: https://www.p-plus.nl/resources/articlefiles/ClosingtheLoopNL2013-10Hagelueken.pdf (accessed on 7 April 2022).
- Ely, J.C.; Neal, C.R.; Kulpa, C.F.; Schneegurt, M.A.; Seidler, J.A.; Jain, J.C. Implications of Platinum-Group Element Accumulation along U.S. Roads from Catalytic-Converter Attrition. Environ. Sci. Technol. 2001, 35, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Artelt, S.; Kock, H.; König, H.P.; Levsen, K.; Rosner, G. Engine dynamometer experiments: Platinum emissions from differently aged three-way catalytic converters. Atmos. Environ. 1999, 33, 3559–3567. [Google Scholar] [CrossRef]
- Oja, O.; Saastamoinen, A.; Patnamsetty, M.; Honkanen, M.; Peura, P.; Jarvenpaa, M. Microstructure and Mechanical Properties of Nb and V Microalloyed TRIP-Assisted Steels. Metals 2019, 9, 887. [Google Scholar] [CrossRef] [Green Version]
- Graedel, T.E.; Reck, B.K.; Miatto, A. Alloy information helps prioritize material criticality lists. Nat. Commun. 2022, 13, 150. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Metal Recycling: Opportunities, Limits, Infrastructure, a Report of the Working Group on the Global Metal Flows to the International Resource Panel; 2013; p. 320. Available online: https://wedocs.unep.org/20.500.11822/8423 (accessed on 7 April 2022).
- AZO Materials. An Overview of Aluminium-Scandium (AlSc). Available online: https://www.azom.com/article.aspx?ArticleID=10670 (accessed on 5 May 2022).
- Carapella, S.C. Arsenic and Arsenic Alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; 2002; Available online: https://doi.org/10.1002/0471238961.0118190503011801.a01.pub2 (accessed on 7 April 2022).
- Prengaman, R.D. Secondary Batteries—Lead–Acid Systems|Lead Alloys. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 648–654. [Google Scholar]
- Yaguchi, H.; Onodera, N. The Effect of Tellurium on the Machinability of AISI 12L14+Te Steel. Trans. Iron Steel Inst. Jpn. 1988, 28, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.X.; Shu, D.; Chen, H.Y.; Li, A.J.; Wang, H.; Xiao, G.M.; Dou, C.L.; Peng, S.G.; Wei, W.W.; Zhang, W.; et al. Study on the structure and property of lead tellurium alloy as the positive grid of lead-acid batteries. J. Alloys Compd. 2009, 475, 102–109. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Xu, G.; Chang, H.; Cui, Y. Effects of Trace Erbium Addition on Microstructure and Mechanical Properties of Ti6Al4V-xEr Alloys. Metals 2019, 9, 628. [Google Scholar] [CrossRef] [Green Version]
- Pollock, T.M.; Tin, S. Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Env. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [Green Version]
- Ku, A.Y. Anticipating critical materials implications from the Internet of Things (IOT): Potential stress on future supply chains from emerging data storage technologies. Sustain. Mater. Technol. 2018, 15, 27–32. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Dariusch Jalalpoor, D.; Korf, N.; Rotter, V.S. Potentials and Barriers for Tantalum Recovery from Waste Electric and Electronic Equipment. J. Ind. Ecol. 2017, 21, 700–714. [Google Scholar] [CrossRef]
- Wagger, D.; (Washington, DC, USA). Institute of Scrap Recycling Industries. 2019. [Google Scholar]
- Lifset, R.J.; Eckelman, M.J.; Harper, E.M.; Hausfather, Z.; Urbina, G. Metal lost and found: Dissipative uses and releases of copper in the United States 1975–2000. Sci. Total Environ. 2012, 417–418, 138–147. [Google Scholar] [CrossRef]
- Helbig, C.; Thorenz, A.; Tuma, A. Quantitative assessment of dissipative losses of 18 metals. Resour. Conserv. Recycl. 2020, 153, 104537. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Recycling Rates of Metals: A Status Report, a Report of the Working Group on the Global Metal Flows to the International Resource Panel; United Nations Environment Programme, 2011; ISBN 978-92-807-3161-3. Available online: https://www.resourcepanel.org/reports/recycling-rates-metals. (accessed on 7 April 2022).
- Henckens, M.L.C.M. The Energy Transition and Energy Equity: A Compatible Combination? Sustainability 2022, 14, 4781. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Cullen, J.M. Toward a sustainable materials system. Science 2018, 360, 1396–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuwalka, K.; Field, F.R.; De Kleine, R.D.; Kim, H.C.; Wallington, T.J.; Kirchain, R.E. Characterizing the Changes in Material Use due to Vehicle Electrification. Environ. Sci. Technol. 2021, 55, 10097–10107. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, R.; Anastas, P.T. Design for degradation or recycling for reuse? Curr. Opin. Green Sustain. Chem. 2021, 31, 100528. [Google Scholar] [CrossRef]
- Curtarolo, S.; Hart, G.L.W.; Nardelli, M.B.; Mingo, N.; Sanvito, S.; Levy, O. The high-throughput highway to computational materials design. Nat. Mater. 2013, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D.P.; Roch, L.M.; Saikin, S.K.; Kreisbeck, C.; Sheberla, D.; Montoya, J.H.; Dwaraknath, S.; Aykol, M.; Ortiz, C.; Tribukait, H.; et al. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat. Rev. Mater. 2018, 3, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Barnett, M.R.; Senadeera, M.; Fabijanic, D.; Shamlaye, K.F.; Joseph, J.; Kada, S.R.; Rana, S.; Gupta, S.; Venkatesh, S. A scrap-tolerant alloying concept based on high entropy alloys. Acta Mater. 2020, 200, 735–744. [Google Scholar] [CrossRef]
- Cann, J.L.; De Luca, A.; Dunand, D.C.; Dye, D.; Miracle, D.B.; Oh, H.S.; Olivetti, E.A.; Pollock, T.M.; Poole, W.J.; Yang, R.; et al. Sustainability through alloy design: Challenges and opportunities. Prog. Mater. Sci. 2021, 117, 100722. [Google Scholar] [CrossRef]
- Li, X.; Lu, K. Improving sustainability with simpler alloys. Science 2019, 364, 733–734. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, Q.; Chai, Z.; Wei, X.; Xiong, X.; Yi, H.; Huang, M.; Xu, W.; Wang, J. Revolutionizing car body manufacturing using a unified steel metallurgy concept. Sci. Adv. 2021, 7, eabk0176. [Google Scholar] [CrossRef]
- Chappuis, L.B. Material Specifications & Recycling for the 2015 Ford F-150; Ford Motor Company, 2019; Available online: https://www.lbcg.com/media/downloads/events/411/day-2-laurent-b-chappuis-technical-expert-lightweight-stampings-vehicle-program-engineering-sbu-ford.6735.pdf. (accessed on 7 April 2022).
- Maitre-Ekern, E. Re-thinking producer responsibility for a sustainable circular economy from extended producer responsibility to pre-market producer responsibility. J. Clean. Prod. 2021, 286, 125454. [Google Scholar] [CrossRef]
- Aposhian, H.V. Dmsa and Dmps—Water-Soluble Antidotes for Heavy-Metal Poisoning. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Ordog, G. 477 Molybdenum Toxicity: Toxic Leukoencephalopathy as a Cause of Autism. J. Investig. Med. 2006, 54, S161. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Omar, N.M.A.; Othman, M.H.D.; Wahab, M.A.; Akhtar Uzzaman, M.; Alharbi, H.M.; Wazeer, I. Recent Progress, Challenges, and Opportunities of Membrane Distillation for Heavy Metals Removal. Chem. Rec. 2022, e202100323. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; He, B.; Lyu, T.; Zou, Y. A Review on Additive Manufacturing of Titanium Alloys for Aerospace Applications: Directed Energy Deposition and Beyond Ti-6Al-4V. JOM 2021, 73, 1804–1818. [Google Scholar] [CrossRef]
- Copper Development Association Grid Infrastructure. Available online: https://www.copper.org/environment/sustainable-energy/grid-infrastructure/ (accessed on 6 May 2022).
- National Material Company Galvanized Steel. Available online: https://www.nationalmaterial.com/steel-processing-capabilities/galvanized-steel/ (accessed on 6 May 2022).
- ASTM International. Handbook of Comparative World Steel Standards, 5th ed.; ASTM International: Mayfield, PA, USA, 2016. [Google Scholar]
- Sanz, J.; Tomasa, O.; Jimenez-Franco, A.; Sidki-Rius, N. Ruthenium (Ru) [Z = 44]. In Elements and Mineral Resources; Springer International Publishing: Cham, Switzerland, 2022; pp. 185–187. [Google Scholar]
- Apergis, N.; Carmona-González, N.; Gil-Alana, L.A. Persistence in silver prices and the influence of solar energy. Resour. Policy 2020, 69, 101857. [Google Scholar] [CrossRef]
- Sanders, M. Lithium Ion Battery Raw Material Supply & Demand 2016–2025. 2017. Available online: http://www.avicenne.com/pdf/Lithium-Ion%20Battery%20Raw%20Material%20Supply%20and%20Demand%202016-2025%20C.%20Pillot%20-%20M.%20Sanders%20Presentation%20at%20AABC-US%20San%20Francisco%20June%202017.pdf (accessed on 12 January 2022).
- Rae, A.; Handwerker, C.A. NEMI’s Lead-Free Alloy. Circuits Assem. 2004, 15. Available online: https://www.nist.gov/publications/nemis-lead-free-alloy (accessed on 6 May 2022).
- Habib, K.; Schibye, P.K.; Vestbø, A.P.; Dall, O.; Wenzel, H. Material Flow Analysis of NdFeB Magnets for Denmark: A Comprehensive Waste Flow Sampling and Analysis Approach. Environ. Sci. Technol. 2014, 48, 12229–12237. [Google Scholar] [CrossRef]
- Pyrhönen, J.; Nerg, J.; Kurronen, P.; Puranen, J.; Haavisto, M. Permanent Magnet Technology in Wind Power Generators. In Proceedings of the XIX International Conference on Electrical Machines—ICEM 2010, Rome, Italy, 6–8 September 2010; pp. 1–6. [Google Scholar]
- Das, B.; Choudhary, R.; Skomski, R.; Balasubramanian, B.; Pathak, A.K.; Paudyal, D.; Sellmyer, D.J. Anisotropy and orbital moment in Sm-Co permanent magnets. Phys. Rev. B 2019, 100, 024419. [Google Scholar] [CrossRef] [Green Version]
- Deng, G. Terbium glows green. Nat. Chem. 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ramakrishnan, T.S.; Elkady, Y.M.; Feng, Y.; Elias, Q.K. Comparative Evaluation of Bismuth-Silver and Bismuth-Tin Alloys for Plug and Abandonment. SPE Drill. Completion 2021, 36, 368–382. [Google Scholar] [CrossRef]
| Element | Total Alloy Use in at Least One Application ≥ 5% a | End-of-Use Functional Recycling Rate < 20% a,b | Concentration ≤ 1% in One Application Whose Total Alloy Use ≥ 5% c | ||
|---|---|---|---|---|---|
| 2 | He | Helium | ✗ | ✓ (3%) | ✗ |
| 3 | Li | Lithium | ✗ | ✓ (3%) | ✗ |
| 4 | Be | Beryllium | ✓ Industrial components, aerospace, automotive, electronics, telecommunications | ✗ (21%) | ✗ |
| 5 | B | Boron | ✗ | ✓ (4%) | ✗ |
| 6 | C | Carbon (graphite) | ✗ | ✓ (10%) | ✗ |
| 9 | F | Fluorine | ✓ Aluminum fluoride | ✓ (0%) | ✗ |
| 12 | Mg | Magnesium (metal) | ✓ Die-castings, aluminum alloys, nodular cast iron | ✗ (39%) | ✗ |
| 13 | Al | Aluminum | ✓ Transportation, packaging, construction, electrical, consumer durables, machinery | ✗ (60%) | ✗ |
| 14 | Si | Silicon (metal) | ✓ Aluminum alloys, solar applications, electronics | ✗ (63%) | ✗ |
| 15 | P | Phosphorus | ✗ | ✓ (0%) | ✗ |
| 19 | K | Potassium | ✗ | ✓ (0%) | ✗ |
| 21 | Sc | Scandium | ✓ Aerospace, sporting goods | ✓ (5%) | ✓ Al alloys (0.3%) d |
| 22 | Ti | Titanium (metal) | ✓ Aerospace, other alloys | ✗ (70%) | ✗ |
| 23 | V | Vanadium | ✓ High-strength low-alloy steel, special steel | ✓ (5%) | ✓ TRIP steel (<0.1%) e |
| 24 | Cr | Chromium | ✓ Stainless steel | ✗ (36%) | ✗ |
| 25 | Mn | Manganese | ✓ Steel alloys, non-steel alloys | ✗ (53%) | ✗ |
| 26 | Fe | Iron | ✓ Steel alloys | ✗ (78%) | ✗ |
| 27 | Co | Cobalt | ✓ Superalloys, cemented carbides, magnets | ✗ (68%) | ✗ |
| 28 | Ni | Nickel | ✓ Stainless steel, alloy steel, Ni-Cu alloys | ✗ (60%) | ✗ |
| 29 | Cu | Copper | ✓ Constructions, transportation, consumer goods | ✗ (48%) | ✗ |
| 30 | Zn | Zinc | ✓ Zinc alloys | ✗ (52%) | ✗ |
| 31 | Ga | Gallium | ✓ Integrated circuits | ✓ (5%) | ✓ Electronics (0.001%) f |
| 32 | Ge | Germanium | ✓ Eletrical, solders | ✗ (30%) | ✗ |
| 33 | As | Arsenic | ✓ Semiconductors, copper alloys | ✓ (1%) | ✓ Arsenical Copper Alloys (<0.5%) g |
| 34 | Se | Selenium | ✓ Semiconductors | ✓ (5%) | ✗ |
| 37 | Rb | Rubidium | ✗ | ✓ (0%) | ✗ |
| 38 | Sr | Strontium | ✗ | ✓ (1%) | ✗ |
| 39 | Y | Yttrium | ✗ | ✓ (1%) | ✗ |
| 40 | Zr | Zirconium | ✗ | ✓ (1%) | ✗ |
| 41 | Nb | Niobium | ✓ Steel alloys, superalloys | ✓ (6%) | ✓ TRIP steel (0.04%) e |
| 42 | Mo | Molybdenum | ✓ Steel alloys, stainless steels, tool steels | ✗ (30%) | ✗ |
| 44 | Ru | Ruthenium | ✓ Electrical components | ✓ (10%) | ✗ |
| 45 | Rh | Rhodium | ✗ | ✗ (60%) | ✗ |
| 46 | Pd | Palladium | ✗ | ✗ (60%) | ✗ |
| 47 | Ag | Silver | ✓ Automotive, industrial machinery, electronics | ✗ (35%) | ✗ |
| 48 | Cd | Cadmium | ✓ Alloys | ✗ (23%) | ✗ |
| 49 | In | Indium | ✓ Alloys, solders | ✓ (5%) | ✗ |
| 50 | Sn | Tin | ✓ Solder, tinplate, lead-acid batteries | ✗ (30%) | ✗ |
| 51 | Sb | Antimony | ✓ Lead alloys | ✓ (5%) | ✓ Lead alloys (1%) h |
| 52 | Te | Tellurium | ✓ Solar power, thermo-electric, metallurgy | ✓ (1%) | ✓ Steel and lead alloys (<1%) i,j |
| 55 | Cs | Cesium | ✗ | ✗ (67%) | ✗ |
| 56 | Ba | Barium | ✗ | ✓ (0%) | ✗ |
| 57 | La | Lanthanum | ✓ Batteries, metal alloys | ✓ (0%) | ✗ |
| 58 | Ce | Cerium | ✓ Metal alloys, batteries | ✓ (0%) | ✗ |
| 59 | Pr | Praseodymium | ✓ Magnets, metal alloys, batteries | ✓ (1%) | ✗ |
| 60 | Nd | Neodymium | ✓ Magnets, metal alloys, batteries | ✓ (1%) | ✗ |
| 62 | Sm | Samarium | ✓ Batteries | ✓ (1%) | ✗ |
| 63 | Eu | Europium | ✗ | ✓ (0%) | ✗ |
| 64 | Gd | Gadolinium | ✗ | ✓ (1%) | ✗ |
| 65 | Tb | Terbium | ✓ Magnets | ✓ (1%) | ✗ |
| 66 | Dy | Dysprosium | ✓ Magnets | ✓ (0%) | ✗ |
| 67 | Ho | Holmium | ✗ | ✓ (1%) | ✗ |
| 68 | Er | Erbium | ✓ Vanadium alloys | ✓ (3%) | ✓ Vanadium alloys ( < 0.6%) k |
| 69 | Tm | Thulium | ✗ | ✓ (1%) | ✗ |
| 70 | Yb | Ytterbium | ✗ | ✓ (0%) | ✗ |
| 71 | Lu | Lutetium | ✗ | ✓ (0%) | ✗ |
| 72 | Hf | Hafnium | ✓ Superalloys, machinery | ✓ (1%) | ✓ Superalloys (0.15%) l |
| 73 | Ta | Tantalum | ✓ Superalloys, mill products, carbides | ✗ (20%) | ✗ |
| 74 | W | Tungsten | ✓ Cemented carbides, steels, mill products | ✗ (25%) | ✗ |
| 75 | Re | Rhenium | ✓ Superalloys | ✗ (52%) | ✗ |
| 76 | Os | Osmium | ✗ | ✓ (0%) | ✗ |
| 77 | Ir | Iridium | ✓ Electrical | ✗ (25%) | ✗ |
| 78 | Pt | Platinum | ✗ | ✗ (65%) | ✗ |
| 79 | Au | Gold | ✓ Jewelry | ✗ (90%) | ✗ |
| 80 | Hg | Mercury | ✓ Dental amalgams, electronics | ✗ (44%) | ✗ |
| 81 | Tl | Thallium | ✗ | ✓ (0%) | ✗ |
| 82 | Pb | Lead | ✗ | ✗ (92%) | ✗ |
| 83 | Bi | Bismuth | ✓ Fusible alloys, metallurgical additives | ✓ (1%) | ✗ |
| 92 | U | Uranium | ✗ | ✓ (0%) | ✗ |
| Atomic Number | 21 | 23 | 31 | 33 | 41 | 51 | 52 | 68 | 72 |
|---|---|---|---|---|---|---|---|---|---|
| Chemical Symbol | Sc | V | Ga | As | Nb | Sb | Te | Er | Hf |
| Upper crustal abundance [ppm] a | 14 | 106 | 18.6 | 5.7 | 11.6 | 0.75 | 0.03 | 2.30 | 5.07 |
| Toxicity concerns b | ✓ | ✓ | ✓ | ||||||
| Significant use in information technology c | ✓ | ✓ | ✓ | ✓ | |||||
| Critical element in Australia d | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Critical element in Canada e | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Critical element in the European Union f | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Critical element in Japan g | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Critical element in the United States h | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
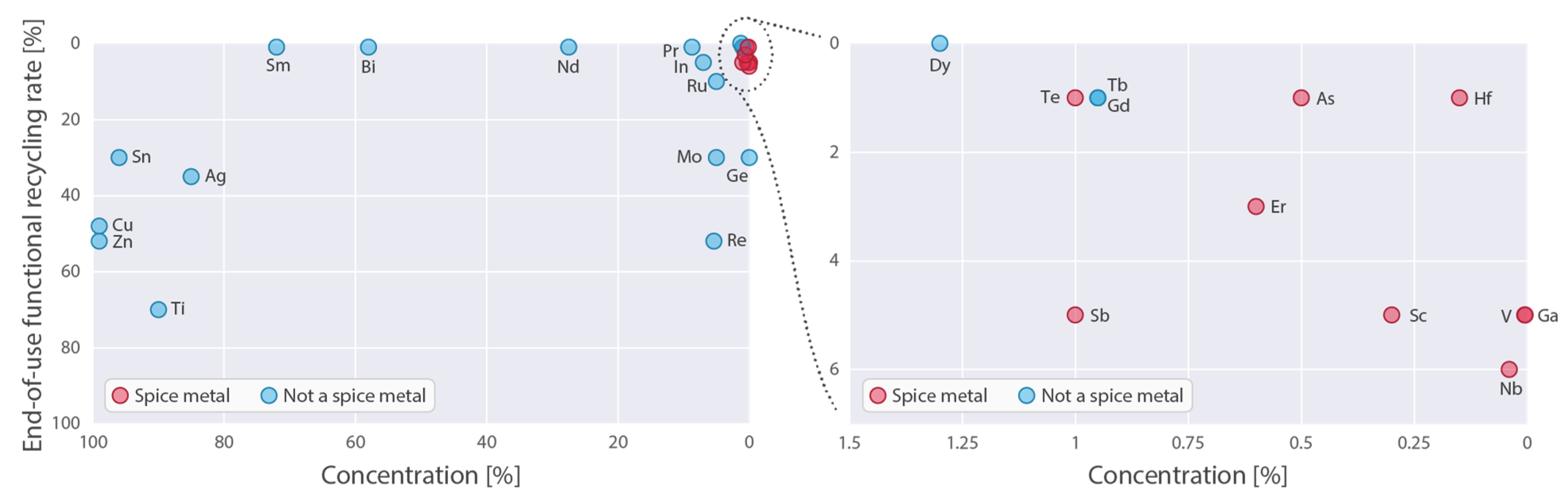
| Element | Potentially Recyclable a | Presently Unrecyclable a | Dissipated During Use a | Unspecified a | Current Recycling b | ||
|---|---|---|---|---|---|---|---|
| 21 | Sc | Scandium | 90% | 0% | 0% | 10% | 5% |
| 23 | V | Vanadium | 97% | 1% | 0% | 2% | 5% |
| 31 | Ga | Gallium | 18% | 76% | 0% | 6% | 5% |
| 33 | As | Arsenic | 14% | 64% | 17% | 5% | 1% |
| 41 | Nb | Niobium | 92% | 0% | 0% | 8% | 6% |
| 51 | Sb | Antimony | 26% | 66% | 4% | 4% | 5% |
| 52 | Te | Tellurium | 85% | 10% | 5% | 0% | 1% |
| 68 | Er | Erbium | 0% | 100% | 0% | 0% | 3% |
| 72 | Hf | Hafnium | 81% | 13% | 0% | 6% | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graedel, T.E.; Miatto, A. Alloy Profusion, Spice Metals, and Resource Loss by Design. Sustainability 2022, 14, 7535. https://doi.org/10.3390/su14137535
Graedel TE, Miatto A. Alloy Profusion, Spice Metals, and Resource Loss by Design. Sustainability. 2022; 14(13):7535. https://doi.org/10.3390/su14137535
Chicago/Turabian StyleGraedel, Thomas E., and Alessio Miatto. 2022. "Alloy Profusion, Spice Metals, and Resource Loss by Design" Sustainability 14, no. 13: 7535. https://doi.org/10.3390/su14137535
APA StyleGraedel, T. E., & Miatto, A. (2022). Alloy Profusion, Spice Metals, and Resource Loss by Design. Sustainability, 14(13), 7535. https://doi.org/10.3390/su14137535






