Abstract
The silverleaf whitefly (Bemisia tabaci) is one of the most harmful insects attacking several economic plant crops worldwide, and it has developed a resistance toward several conventional insecticides. This study was conducted to estimate the impact of potassium phosphite (PK), effective microorganisms (EMs), and salicylic acid (SA) as plant inducers, and imidacloprid (IMI) as a synthetic insecticide on the systemic acquired resistance of sweet pepper (Capsicum annuum var. annuum) crop, whitefly population, and crop yield under greenhouse conditions. The treatment plots were sprayed with IMI, PK, EMs, SA, and water (control) on the 27th day after planting, and dinotefuran was applied when the whitefly-infestation ratio reached 3.00%. The enzymes responsible for the internal defence system, whitefly population, and crop yield were determined. Our results confirmed the idea that the PK, EMs, and SA may induce the synthesis of plant enzymes responsible for the internal defence system. The IMI, PK, EMs, and SA significantly suppressed the whitefly population compared with the control. Moreover, the reduction percentages of the whitefly population were significantly higher when using IMI and PK than EMs and SA. The IMI, PK, EMs, and SA improved the crop yield. It could be concluded that PK, EMs, and SA enhanced the systemic acquired resistance in sweet pepper crop causing high defence against the population of whitefly and might be a potent alternative to conventional insecticides and compatible with an integrated pest management program.
1. Introduction
Pepper (Capsicum spp.) is an important vegetable and spice crop worldwide owing to its color, taste, pungency, flavor, and aroma [1]. Estimates have shown that up to 36.70 million tons of fresh pepper fruit and 4.16 million tons of dried pods were harvested from 3.76 million hectares worldwide [2]. Pepper production is frequently threatened by many biotic factors, such as diseases, weeds, and pests, including whiteflies, aphids, and thrips [3,4]. Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most harmful and invasive crop pests worldwide [5]; it is widely polyphagous and causes economic damage to plants in several ways [6]. Severe infestation of whitefly adults and progeny can cause seedling death or a reduction in the vigor and yield of older plants [7,8]. Pepper is one of its major plant hosts [9]. The whitefly has evolved resistance to insecticides from most chemical classes [10].
Whitefly is difficult to control with conventional insecticides for two reasons; firstly, adult and immature whiteflies infest the leaves’ lower surfaces, which are difficult to spray with insecticide, and secondly, they have developed resistance to several conventional insecticides. Therefore, the predators have potent importance in terms of biological controls [11,12,13]. Moreover, systemic acquired resistance (SAR) activators at concentrations suitable for different plant-growth stages and applied by the proper method can be included in integrated pest-management programs [14]. The induced resistance is intended to be broad-spectrum and efficient over the long term [15]. Furthermore, Guerra et al. [16] and Liang et al. [17] reported that the high activity of peroxidase (POX) and polyphenol oxidase (PPO) increased the synthesis of phenolic compounds, which improved plant resistance against pests and diseases. Jafarbeigi et al. [18] found that PPO activities increased the formation of phenols, which may have inhibited the growth of B. tabaci.
Phosphite is a trivalent inorganic anion that has been obtained by the removal of all three protons from phosphorous acid. It is a conjugate base of hydrogen phosphite. It is a systemic mobile chemical that has been used in the management of diseases caused by different pests [19]. When the phosphite concentration is low, it induces the synthesis of the host defense enzymes, phytoalexins, and phenolic; when its concentration is high, it inhibits pathogen growth [20].
EMs include lactic acid bacteria (Lactobacillus plantarum, Streptoccus lactis, and Lactobacillus casei), photosynthetic bacteria (Rhodobacter spaeroides and Rhodopseudomonas palustrus), yeasts (Candida utilis and Saccharomyces cerevisiae), actinomycetes (Streptomyces albus and Streptomyces griseus), and fermenting fungi (Aspergillus oryzae and Mucor hiemalis), which had moderate efficacy against B. tabaci; this may be due to the presence of several microbial isolates in the formulation that have entomopathogenic activity through the production of some toxic secondary metabolites [21]. EMs are a mixture of many useful microbes, which are effective in insect control perhaps due to their ability to create esterases compounds that are repellent to insects and secrete hydrolyses acids and defense enzymes [22,23]. The beneficial microorganisms, plant-growth-promoting fungi, plant-growth-promoting rhizobacteria, and arbuscular mycorrhizal fungi, can promote plant growth (e.g., shoot length, nitrogen content, leaf area, chlorophyll content, and yield) and increase tolerance to biotic and abiotic stressors [24].
Salicylic acid (SA) is a phenolic compound of hormones, naturally produced by plants, and plays an important role in responses to several abiotic stresses and pests attacks [25]. War et al. [26] showed that the exogenous application of SA protected plants against biotic and abiotic stresses. Most of its effects on various physiological processes have been related to the growth and development of plants [27]. Furthermore, SA has promoted the stimulation of plant defense genes, resulting in the creation and accumulation of antioxidant enzymes, steroid glycoalkaloids, and the release of volatile organic compounds that attracted natural enemies of insect herbivores [28,29]. El-Sherbeni et al. [30] indicated that when SA was added to insecticides used to control whiteflies on cotton plants, the process was more efficient, and pesticide use was reduced by 25%; this was attributed to increased phenolic chemical concentration in the leaves. Moreover, the use of exogenous SA reduced caterpillars’ capacity to eat the plant and delayed the development of insect resistance [31,32].
IMI belongs to the chloronicotinyl nitroguanidine chemical family of neonicotinoid insecticides [33]. IMI is a systemic insecticide that translocates rapidly through plant tissues following application and has been used to control sucking insects; it may be applied as a foliar spray and as a seed treatment [34], which acts as an acetylcholine agonist on a certain receptor located on postsynaptic nicotinic acetylcholine receptors [35]. IMI foliar sprays have been used by farmers to suppress sucking pests such as whitefly on cotton crops [36]. Oosterhuis et al. [37] stated that a multiple-application of a spray with IMI starting early to mid-season resulted in improved plant growth and yield, even in low-insect populations. These results could be attributed in part to increased plant physiological functioning via the creation of SAR, according to the researchers [38,39]. The possibility that IMI possesses antioxidant effects that help plants to cope with stress has been noted [40,41]. Moreover, dinotefuran is a third-generation nicotine insecticide that has the characteristics of a highly quick-acting effect, high activity, long-lasting effect, a wide insecticidal spectrum, and does not cause phytotoxicity [42]. The whitefly may build resistance toward IMI, while dinotefuran can control the pest population of the whitefly in the long term [43].
We hypothesize that certain microbial and chemical agents increase plant enzyme production, which can enhance the plant’s internal defense system, reducing pest populations and increasing sweet-pepper yield. To test such a hypothesis, we measured the activity of enzymes known to be involved in the plant’s internal defense system. In addition, we investigated if the treatments have the potential to reduce silver-whitefly populations while increasing sweet-pepper productivity.
2. Materials and Methods
2.1. Study Site
This research was carried out under greenhouse circumstances in Kafr Al Battikh city, Damietta province (31°25′0″ N, 31°49′17″ E), Egypt (Figure 1) across two growing seasons in 2019 and 2020.
Figure 1.
A map with the location of the experimental area.
2.2. The Tested Formulations
The effective microorganisms (EMs) were obtained from the Ministry of Agriculture, Cairo, Egypt. The formulation of EMs contained some different beneficial microorganisms that were reared in particular media, produced in Egypt under the supervision of the Japanese EMs Research Organization [44]. The microorganisms included photosynthetic bacteria (Rhodobacter sphaeroides and Rhodopseudomonas palustris), lactic acid bacteria (Lactobacillus casei, Lactobacillus plantarum, and Streptoccus lactis), Actinomycetes (Streptomyces griseus and Streptomyces albus), yeasts (Candida utilis and Saccharomyces cerevisiae), and fermenting fungi (Mucor hiemalis and Aspergillus oryzae). The formulation pH was 3.00, and they were stored in a refrigerator at zero °C. They were diluted in water at 5 mL/L.
Neonicotinoids included: 1. Imidacloprid (IMI) (Admire 20% SL) was purchased from BAYER and was used at a dosage of 119.05 mL/ha, 2. Dinotefuran (Oshin 20% SG) was purchased from the Mitsui Chemical Company and was used at a dosage of 476.19 mL/ha.
Potassium phosphite (PK), with the trade name Naturephos (58% P2O5 and 32% K2O), was purchased from Daymasa Company, Zaragoza, Spain and was used at a dosage of 1 g/L. Salicylic acid (SA) (phenolic acid), an inducer compound with a purity of 99.99%, was purchased from the Al-Gomhoreya Chemical Company, El Geish St., Tanta, Egypt and used at a dosage of 2 mM/L.
2.3. The Experimental Procedure
The experimental area of the greenhouse was approximately 4200 m2. The pepper seedlings var. TOP STAR (Takii & Co., Ltd., Kyoto, Japan) were cultivated on 15 September 2019 and 20 September 2020. All standard agricultural practices were followed, and treatments were set up in the experimental area using a randomised complete block design including five treatments: IMI as a synthetic insecticide in comparison with PK, EMs, and SA as plant inducers, water (positive control) and water (negative control). The treatment plots were sprayed with IMI, PK, EMs, SA, water (positive control) and water (negative control) treatment on the 27th day after planting. Additionally, when the infestation ratio of B. tabaci reached 3.00% (45 days after planting), the dinotefuran at the recommended rate was applied in the all treatments except for the negative control. Each treatment and the positive control and negative control had four replicates, and each replicate contained 150 plants in 75 m2 (each treatment 300 m2). All agricultural practices were followed to imitate commercial plantations.
2.4. Field and Laboratory Inspection of B. tabaci
Twenty-four plant leaves were randomly inspected at three levels of the plant (8 leaves from the upper, 8 leaves from the middle, and 8 leaves from the lower canopy) per each replicate. Then, the whitefly adults were counted and recorded in the field at 6.00 am, one day before treatment and 1, 3, and 7 days after the application of dinotefuran.
For determination of the numbers of whitefly nymphs, twenty-four leaves were randomly collected at three levels of the plant (8 leaves from the upper, 8 leaves from the middle, and 8 leaves from the lower canopy) in each replicate one day before treatment and 1, 3, and 7 days after the application of dinotefuran. The leaves from each replicate were inserted individually into a plastic bag and transferred directly to the laboratory. The leaves were checked in the laboratory under a binocular microscope, and the numbers of whitefly nymphs were counted and recorded.
The reduction percentage in the insect population (nymphs and adults) due to the treatments was calculated based on the negative control (plots not treated with dinotefuran) using the equation of Henderson and Teliton [45] as follows:
where: B = No. individuals in the treated sample after spraying, B′ = No. individuals in the negative control sample before spraying, and A = No. individuals in the negative control sample after spraying. A′= No. individuals in the control sample before spraying.
2.5. Morphological Identification of B. tabaci
After field inspection, some samples of B. tabaci were collected and inserted into a plastic bag for identification in the laboratory by examining the pupal and adult stages under a binocular microscope [46].
2.6. Enzymes’ Assay
Pepper leaves were collected 4 days after treating the plots, 3.00 g of leaves were taken, mixed, and chopped [47]. A sample of 1.00 g of this chopped mixture was ground in a mortar with liquid nitrogen and homogenized in 6.00 mL of cold homogenizing buffer. The suspension was centrifuged at 7000 rpm for 20 min at 4 °C. The supernatant was used for enzyme assays as in War et al. [47]. Peroxidase (POX), polyphenol oxidase (PPO), catalase (CAT), chitinase (CHI), glutathione peroxidase (GSHpx), glutathione transferase (GSHtf), and glutathione reductase (GSHrd) activities were determined calorimetrically according to the method described by Moran and Cipolin [48], Mayer and Harel [49], Aebi [50], Harman et al. [51], Floh’e and Gunzler [52], Warholm et al. [53], and Goldberg and Spooner [54], respectively. The enzymes’ specific activities were determined in the Biotechnology and Pathogens Lab, Botany Department, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt.
2.7. The Economic Efficiency
The pepper yield from the different treatments was weighted and recorded during the two tested seasons. Moreover, the commercial prices of the yield were calculated. The cost of the insect pest control was estimated according to the number of sprays during the season and price of the used material.
2.8. Statistical Analysis
The experimental data were analyzed using the one-way analysis of variance. The normality in data was tested by the Shapiro–Wilk normality test, which indicated the normal distribution of the data; therefore, the original data were analyzed. Tukey’s HSD post-hoc test was used for comparison among treatments via the Costat system for windows (Costat, 2006) [55].
3. Results
The impact of microbial and chemical agents on the enzymes responsible for the internal defence system was assessed.
The data in Table 1 and Table 2 indicate that the application of IMI, PK, SA, and EMs significantly enhanced the enzyme activities of POX, PPO, and CAT compared to the control treatment. Moreover, the results indicated that IMI was the most significantly effective treatment on POX and CAT activities compared with other treatments. The highest significant increase in GSHs enzymes activities was obtained from the SA treatment, while the control did not affect the activities of the GSHs enzymes.

Table 1.
The effect of microbial and chemical agents on the activity of the enzymes responsible for the internal defence system of sweet pepper.

Table 2.
Analysis of variance of different enzyme activities in sweet pepper.
Based on the data illustrated in Figure 2 and Figure 3 and Table 3, the microbial and chemical agents significantly reduced the whitefly population on sweet pepper. Based on the average of the three inspection times, the plots treated previously with IMI, PK, SA, and EMs gave a high reduction percentage of the adult B. tabaci population compared with the positive control treatment. Moreover, IMI and PK showed the highest potent reduction during the 2019 and 2020 seasons compared with EMs and SA. The reduction percentages did not differ between the treatments of IMI and PK. Moreover, the reduction percentages of whitefly nymphs based on the average of the three inspection times during the 2019 and 2020 seasons were significantly higher with the treatments of IMI, PK, SA, and EMs than the positive control treatment Figure 4 and Figure 5.
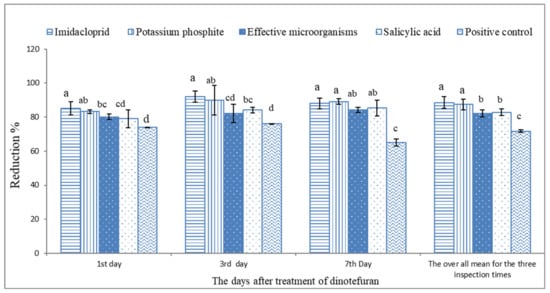
Figure 2.
The reduction percentage of adult silverleaf whitefly population on sweet pepper based on the non-treated plants during 2019. Each column shows reduction values of silverleaf whitefly adults with the standard error. Different letters above the bars of the same day indicate a significant (p < 0.05) difference as determined by Tukey’s test.
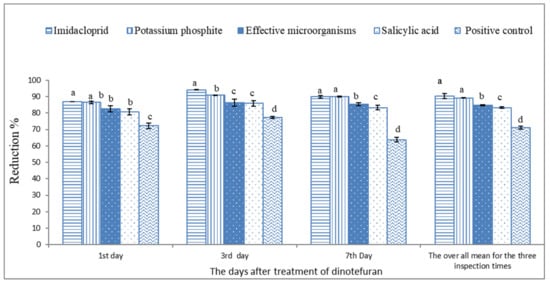
Figure 3.
The reduction percentage of adult silverleaf whitefly population on sweet pepper based on the non-treated plants during 2020. Each column shows reduction values of silverleaf whitefly adults with the standard error. Different letters above the bars of the same day indicate a significant (p < 0.05) difference as determined by Tukey’s test.

Table 3.
Analysis of variance of the reduction percentages of B. tabaci on sweet pepper.
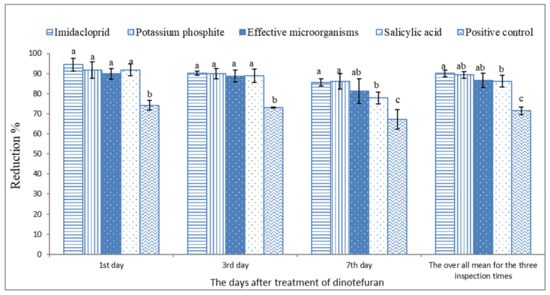
Figure 4.
The reduction percentage of silverleaf whitefly nymph population on sweet pepper based on the non-treated plants during 2019. Each column means reduction values of silverleaf whitefly nymphs with the standard error. Different letters above the bars of the same day indicate a significant (p < 0.05) difference as determined by Tukey’s test.
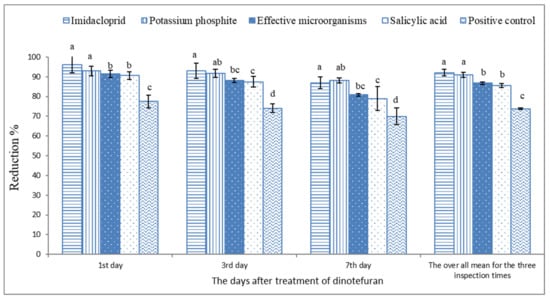
Figure 5.
The reduction percentage of silverleaf whitefly nymph population on sweet pepper based on the non-treated plants during 2020. Each column shows reduction values of silverleaf whitefly nymphs with the standard error. Different letters above the bars of the same day indicate a significant (p < 0.05) difference as determined by Tukey’s test.
Data shown in Table 4 and Table 5 cover some of the economic efficiency (quality and quantity requirements) of the tested treatments applied on the sweet pepper crop. The plots treated with IMI, PK, SA, and EMs achieved a higher price return (price L.E/kg) than the positive control. The IMI, PK, SA, and EMs enhanced the crop yield compared with the positive control. The PK treatment showed the best economic efficiency; the productivity yield/ha was 101,102.70 L.E, with the lowest control cost with 4478.41 L.E/ha, and the spray number during the season was 7.23.

Table 4.
The economic efficiency of microbial and chemical agents on sweet pepper.

Table 5.
Analysis of variance of the economic efficiency of microbial and chemical agents on sweet pepper.
4. Discussion
Pretreating plants with various chemical inducers results in plant resistance, which may protect the plants from insect attacks. In response to herbivore attacks, plants produce a variety of inducible defence mechanisms [56]. The results of the current study indicated that the foliar application of IMI, PK, EMs, and SA on sweet-pepper plants on the 27th day after plantation resulted in a large rise in crop productivity and a high reduction in the silverleaf whitefly infestation compared with the control treatment. The use of PK, EMs, and SA may induce the synthesis of plant enzymes (POX, PPO, and CAT) responsible for the internal defence system, which may be the reason for the reduction in the whitefly population. Moreover, a previous study reported that treatment of the plants with some hormones, especially SA, jasmonates, and ethylene, resulted in the reprogramming of the host metabolism gene expression and modulation of plant defence responses [57]. Some antioxidant enzymes that play a role in plant resistance or defence mechanisms against pest attacks were determined for this study. The increase in the activities of POX, PPO, and CAT are the most common efficient defence mechanism activated after the recognition of pests by their host [58]. The same hypothesis agrees with Khan et al. [59], who found that the biological processor resulted in induced resistance to multiple reactions involving several different compounds and enzymes. The induced resistance was associated with POX and CHI [60]. The high activity of POX and PPO is one of the most effective defense mechanisms activated after a pathogen is recognized by its host [58]. Guerra et al. [16] and Liang et al. [17] found that increasing the activity of POX and PPO, which enhanced the formation of phenolic compounds, improved cotton and cucumber resistance to pests and diseases. The PPO activity facilitated the accumulation of phenols, which may have affected the growth of B. tabaci [18]. The PPO catalyzed the oxidation of monophenols or o-diphenols to o-diquinones in the presence of oxygen, which may decrease plant digestibility for insects [61]. We suppose that the suppression of the silverleaf whitefly population was due to the antifeedant activity of plant enzymes and a reduction in food digestion caused by the phenolic compounds’ harmful effects [12].
The glutathione S-transferase (GST) is a phase II enzyme that aids in conjugating pollutants or/and their metabolites with glutathione favoring their further excretion [62]. High activities of GST are often linked to the presence of organic contaminants or pro-oxidant circumstances [63]. Glutathione reductase is one of the potential enzymes of the enzymatic antioxidant system, which sustains the reduced status of GSH via the ascorbate–glutathione pathway, plays a vital role in the maintenance of the sulfhydryl group, and acts as a substrate for GST [64]. In our research, in the plants treated with SA, GSH activity and total GSH content increased considerably compared to the control (Table 1). This may be expressed by the fact that GSH enzymes formed were being used as an antioxidant during the detoxification reactions [27]. Gonias et al. [46] recorded a reduction in GSHrd in the IMI-treated cotton plants in the growth chamber, indicating that the untreated plants were experiencing more stress, necessitating the activation of this defence mechanism.
IMI is one of the most successfully commercialized synthetic insecticides that has shown negative effects against many organisms [65,66]. The IMI is a neonicotinoid that breaks down in a plant to 6- chloronicotinic acid, which is closely related to the SAR inducer isonicotinic acid [37]. The antioxidant enzyme activity measured after foliar applications has shown that the IMI molecule possibly has antioxidant properties, enabling plants to better tolerate pest infestation [38]. Additionally, a multiple application spray with IMI resulted in improved plant growth and yield [37]. In the current work, a considerable reduction in adult (16.67 and 19.20%) and nymph populations (18.60 and 18.27%) due to IMI application in 2019 and 2020, respectively, occurred. These improvements could be attributed in part to improved plant physiological functioning via the establishment of SAR [38,39]. Moreover, the IMI insecticide may have antioxidant properties that assist plants to cope with stress [38,39]. The potential impact of IMI on whitefly eggs was explored in a greenhouse test that showed egg mortality occurring in both early (one-day-old) and late (three-day-old) eggs on cotton leaves systemically treated with IMI [6].
For SA, our results agreed with the findings of Zinovieva et al. [67], who reported that SA increased the activity of antioxidant enzymes. In the present study, a moderate reduction in adult (11.06 and 12.23%) and nymph populations (14.74 and 11.84%) due to SA application in 2019 and 2020, respectively, occurred. The SA stimulated plant defence genes, which produced antioxidant enzymes, steroid glycoalkaloids, and the secretion of volatile chemical compounds that attracted insects’ natural enemies. Exogenous SA inhibits caterpillars’ ability to consume plants and inhibits insect resistance development [31,32]. Moreover, Miura et al. [25] revealed that SA is a phenolic component of the hormone naturally created by plants and plays an essential role in responses to pest attacks.
For the EMs, we suppose that some microbes can secrete a variety of volatile and nonvolatile metabolites that promote plant growth via different pathways or maybe secrete some toxins, which decrease the numbers of B. tabaci. In the current work, a moderate reduction in adult (10.37 and 13.64%) and nymph populations (15.14 and 12.97%%) due to EMs application in 2019 and 2020, respectively, occurred. Our results agreed with Fincheira and Quiroz [68], who revealed that the volatile organic compounds (VOCs) generated by microorganisms linked with root plants could be a unique way to encourage growth in agricultural species. Some of these compounds were 2,3 butanediol, 3-hydroxy-2-butanone (acetoin), 2-pentylfuran, or dimethylhexadecylmine, and they induced root or leaf growth. It has been demonstrated that several VOCs (2-octanone, 3-octanol, and 2–5-dimethylfuran), produced primarily by the fungus, caused Drosophila melanogaster mortality [69]. The microorganisms, including plant-growth-promoting fungi, plant-growth-promoting rhizobacteria, and arbuscular mycorrhizal fungi, improved plant growth (e.g., shoot length, leaf area, nitrogen content, chlorophyll content, and yield) and tolerance to biotic and abiotic stressors [24]. Moreover, B. tabaci may be affected by toxic compounds produced by microorganisms’ colonization on plants, and our results are in harmony with those indicated by Monnerat et al. [70].
Phosphite concentration is very important because when its concentration is high, it may decrease pathogen growth [20]. Ávila et al. [71] stated that phosphite improved nutrient absorption and assimilation, as well as product quality and biotic and abiotic stress tolerance, as a biostimulator. In the current work, a considerable reduction in adult (15.62 and 18.07%) and nymph populations (17.83 and 17.10%) due to PK application in 2019 and 2020, respectively, occurred. Plant growth, nutritional value, and productivity were all improved by phosphite.
The treatments of IMI, PK, EMs, and SA achieved higher price returns (price L.E/1 kg) than the control, which may be due to the low population of whitefly, which resulted in high crop yield and quality. These results agree with those found by Kumar et al. [7] and Horowitz et al. [8], who showed that high infestation with whitefly can cause seedling death or a reduction in the vigor and yield of older plants. Additionally, the increase in crop yield and fruit quality may be due to factors in phosphite availability [72,73]. The total crop yield (L.E)/ha was significantly higher with the PK treatment than the other treatments. Based on the positive control, an increase in price return (26.79, 34.56, 28.10, and 10.54%) occurred in plots treated with IMI, PK, EMs, and SA, respectively. It is known that the supply of phosphorus is usually associated with a significant increase in the number and mass of roots, which results in the absorption of higher concentrations of mineral element nutrients from the soil, including nitrogen, resulting in increased growth and total chlorophyll. In this respect, PK, which is characterized by high phosphorus content and high water solubility, caused the highest contents of plant pigments, nutrients, and protein. Moreover, Araujo et al. [19] stated that spraying mango plants with Phi prevented mango wilt and induced microscopic defence responses to Caprinia fimbriata infection, including the accumulation of phenolic-like compounds, the formation of an antifungal barrier, and the rapid deposition of phenolic-impregnated tyloses. These findings could be explained by potassium phosphite’s participation in plant metabolism and numerous essential regulatory mechanisms. Moreover, it increased mineral element uptake by plants [74].
5. Conclusions
To summarize, our findings revealed that IMI, PK, EMs, and SA had a significant effect on the population of B. tabaci compared to the control. The IMI and PK were the most effective compounds that decreased the population of B. tabaci followed by EMs and SA against the adults and nymphs of B. tabaci during two successive 2019 and 2020 seasons. Finally, this study suggests that using microbial and chemical agents as inducer compounds for enhancing systemic acquired resistance, such as PK, EMs, and SA, may be useful for managing silverleaf whitefly adults and nymphs and increasing sweet-pepper yield, C. annuum L. var. annuum. These promising agents may have the potential to become alternatives for silverleaf-whitefly control instead of some harmful conventional insecticides.
Author Contributions
Conceptualization, M.S.Z., E.-S.M.E. and E.-K.A.T.; methodology, M.S.Z., E.-S.M.E. and E.-K.A.T.; validation, M.S.Z., E.-S.M.E., E.-K.A.T. and M.M.H.; formal analysis, M.S.Z., E.-S.M.E., E.-K.A.T. and M.M.H.; investigation, M.S.Z., E.-S.M.E., E.-K.A.T. and M.M.H.; resources, M.S.Z., E.-S.M.E., E.-K.A.T. and M.M.H.; data curation, M.S.Z., E.-S.M.E., E.-K.A.T. and M.M.H.; writing—original draft preparation, M.S.Z., E.-S.M.E. and E.-K.A.T.; writing—review and editing, M.S.Z., E.-S.M.E. and E.-K.A.T.; visualization, M.S.Z., E.-S.M.E. and M.M.H.; supervision, E.-K.A.T. and E.-S.M.E.; project administration, M.S.Z. and E.-S.M.E.; funding acquisition, M.S.Z. and M.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University Researchers Supporting Project number (TURSP-2020/119), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting this study’s findings are available upon fair request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rohini, N.; Lakshmanan, V. Evaluation studies of hot pepper hybrids (Capsicum annuum L.) for yield and quality characters. J. Electron. J. Plant Breed. 2017, 8, 643–651. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization (FAO) of the United Nation Crops and Livestock Products Department. 2018. Available online: https://www.fao.org/statistics/en (accessed on 2 May 2022).
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Yusoff, M.M.; Miah, G.; Usman, M.G. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Zhang, B. Market demand and breeding trend of pepper varieties in China. J. China Veg. 2019, 8, 1–4. [Google Scholar]
- Lapidot, M.; Legg, J.P.; Wintermantel, W.M.; Polston, J.E. Management of whitefly-transmitted viruses in open-field production systems. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2014; Volume 90, pp. 147–206. [Google Scholar]
- Prabhaker, N.; Castle, S.J.; Merten, P. Comparative susceptibility of Bemisia tabaci to imidacloprid in field- and laboratory-based bioassays. J. Pest Manag. Sci. 2014, 70, 1538–1546. [Google Scholar]
- Kumar, P.; Naqvi, A.R.; Meena, R.S.; Mahendra, M. Seasonal incidence of whitefly, Bemisia tabaci (Gennadius) in tomato (Solanum lycopersicum Mill). Int. J. Chem. Stud. 2019, 7, 185–188. [Google Scholar]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E.; Webb, S.E. Suppression of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), and incidence of cucurbit leaf crumple virus, a whitefly-transmitted virus of zucchini squash new to Florida, with mulches and imidacloprid. Fla. Entomol. 2008, 91, 460–465. [Google Scholar] [CrossRef]
- De Marchi, B.R.; Smith, H.; Turechek, W.; Riley, D. A Maximum Dose Bioassay to Assess Efficacy of Key Insecticides against Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2021, 13, 914–921. [Google Scholar] [CrossRef]
- El-Nabawy, E.-S.M.; Tsuda, K.; Sakamaki, Y. Attractiveness of spiders and insect predators and parasitoids to flowering plants. Egypt. J. Biol. Pest Cont. 2015, 25, 245–250. [Google Scholar]
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 2015, 88, 65–73. [Google Scholar] [CrossRef]
- El-Nabawy, E.S.M.; Tsuda, K.; Sakamaki, Y.; Oda, A.; Ushijima, Y. The effect of organic fertilizers and flowering plants on sheet-web and wolf spider populations (Araneae: Lycosidae and Linyphiidae) and its importance for pest control. J. Insect Sci. 2016, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA signaling pathway and ethylene biosynthesis in Trichoderma harzianum treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. J. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.M.N.M.; Rodrigues, F.A.; Berger, P.G.; Barros, A.F.; Silva, Y.C.R.; Lima, T.C. Aspectos bioquimicos da resistencia do algodoeiro a ramulose potencializada pelo silicio. Bragantia 2013, 72, 292–303. [Google Scholar] [CrossRef][Green Version]
- Liang, Y.C.; Sun, W.C.; Si, J.; Romheld, V. Effects of foliar-and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- Jafarbeigi, F.; Samih, M.A.; Alaei, H.; Shirani, H. Induced tomato resistance against Bemisia tabaci Triggered by Salicylic Acid, β-Aminobutyric Acid, and Trichoderma. Neotrop Entomol. 2020, 49, 456–467. [Google Scholar] [CrossRef]
- Araujo, L.; Bispo, W.M.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the phenylpropanoid pathway by acibenzolar-s-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Fleischmann, F.; Humez, M.; Osswald, W. Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 2014, 9, e87860. [Google Scholar] [CrossRef]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fannin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3316. [Google Scholar] [CrossRef]
- Ndona, R. Einfluss von Behandlungen Mit EMs. Effektiven Mikroorganismen auf Tomato in Geschützten Anbau. Ph.D. Thesis, Universität für Bodenkultur, Vienna, Austria, 2008. [Google Scholar]
- Filipp, M.; Spornberger, A.; Keppel, H.; Brunmayer, R. Influence of effective microorganisms (EM) on yield and quality in organic apple production. Univ. Nat. Res. Appl. Life Sci. J. Crop Prod. 2009, 281–284. Available online: https://www.researchgate.net/profile/Andreas-Spornberger/publication/268299040_Influence_of_effective_microorganisms_EM_on_yield_and_quality_in_organic_apple_production/links/551937c20cf2d241f355d0ea/Influence-of-effective-microorganisms-EM-on-yield-and-quality-in-organic-apple-production.pdf (accessed on 2 May 2022).
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Akbulut, G.B.; Yigit, E.; Bayram, D. Investigation of the effects of salicylic acid on some biochemical parameters in Zea mays to glyphosate herbicide. J. Environ. Anal. Toxicol. 2015, 5, 271. [Google Scholar] [CrossRef]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochem. Biophys. Acta 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Chehab, E.W.; Kaspi, R.; Savchenko, T.; Rowe, H.; Negre-Zakarov, N.; Kliebenstein, D.; Dehesh, K. Distinct roles of jasmonates and aldehydes in plantdefense responses. PLoS ONE 2008, 3, e1904. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeni, A.E.; Khaleid, M.S.; AbdAllah, S.A.; Ali, O.S.M. Effect of some insecticides alone and in combination with salicylic acid against aphid, Aphis gossypii, and whitefly Bemisia tabaci on the cotton field. Bull. Nat. Res. Cen. 2019, 43, 57. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plants responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Iverson, A.L.; Iverson, L.R.; Eshita, S. The effects of surface-applied jasmonic and salicylic acids on caterpillar growth and damage to tomato plants. Ohio J. Sci. 2001, 101, 90–94. [Google Scholar]
- Tomlin, C.D.S. The Pesticide Manual, a World Compendium, 14th ed.; British Crop Protection Council: Surry, UK, 2006; pp. 598–599. [Google Scholar]
- Talcott, M.S. Small Animal Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780323241984. [Google Scholar]
- Dalefield, R. Veterinary Toxicology for Australia and New Zealand, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780127999128. [Google Scholar]
- Kranthi, K.R. Bt-Cotton Question and Answer; Indian Society for Cotton Improvement: Mumbai, India, 2012. [Google Scholar]
- Oosterhuis, D.M.; Brown, R.S.; Gonias, E.D. Effects of Trimax™ insecticide application under water-deficit stress conditions on the lint yield and physiology of field-grown cotton. In Summaries of Arkansas Cotton Research 2003; Series 521; Arkansas Agricultural Experiment Station: Fayetteville, AR, USA, 2003. [Google Scholar]
- Francis, M.I.; Redondo, A.; Burns, J.K.; Graham, J.H. Soil application of imidacloprid and related SAR-inducing compounds produce effective and persistent control of citrus canker. Eur. J. Plant Pathol. 2009, 124, 283–292. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E.; Chandran, D.; Gulevich, A.G.; Okrent, R.A.; Durkin, K.A.; Sarpong, R.; Bunnelle, E.M.; Wildermuth, M.C. Neonicotinoid insecticides induce salicylate- associated plant defense responses. Proc. Nat. Acad. Sci. USA 2010, 107, 17527–17532. [Google Scholar] [CrossRef]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. How the insecticide TRIMAXTM improves growth and yield of cotton. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 3–6 January 2006. [Google Scholar]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. Physiologic response of cotton to the insecticide imidacloprid under high-temperature stress. J. Plant Growth Regul. 2008, 27, 77–82. [Google Scholar] [CrossRef]
- Kumar, V.; Garima, K.; Cindy, L.M.; Lance, S.O. Effect of Dinotefuran on Bemisia tabaci and Amblyseius swirskii, SALVIA: Salvia nemorosa (L.), ‘New Dimension Blue. Arthropod Manag. Tests 2016, 41, tsw100. [Google Scholar] [CrossRef][Green Version]
- Bin Hamid, M.S. Assessment of Imidacloprid and Dinotefuran on Whitefly Bemisia tabaci of Chilli. Bachelor‘s Thesis, Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, Shah Alam, Malaysia, 2015; p. 48. [Google Scholar]
- Derbalah, A.S.; Khidr, A.A.; Moustafa, H.Z.; Taman, A. Laboratory evaluation of some non-conventional pest control agents against the pink bollworm Pectinophora gossypiella (Saunders). Egypt. Biol. Pest Control 2014, 24, 363–368. [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Test with acaricides against the brown wheat mite in Gossypium hirsutum and isolation of mutants with improved yield and fiber characters. Actainduction of mutation in cotton. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Bink-Moenen, R.M. Revision of the African Whiteflies (Aleyrodidae) Mainly Bases on a Collection from Tchad; CABI: Wallingford, UK, 1983; p. 201. [Google Scholar]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of salicylic acid in induction of plant defence system in chickpea (Cicer arientum L.). Plant Signal. Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef]
- Moran, P.J.; Cipolini, D.F. Effect of fungal infection and mechanical stress on peroxidase activity and resistance to pests in cucumber. J. Phytopathol. 1998, 147, 313–316. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E. Polyphenol oxidases in plant. J. Phytochem. 1979, 18, 193–215. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. J. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Harman, G.E.; Hayes, C.K.; Lorito, M.; Broadway, R.M.; Di Pietro, A.; Peterbauer, C.; Tronsmo, A. Chitinolytic enzymes of Trichoderma harzianum, purification of chitobiosidase and endo chitinases. Phytopathology 1993, 83, 313–318. [Google Scholar] [CrossRef]
- Flohé, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1985, 105, 114–121. [Google Scholar]
- Warholm, M.; Guthenberg, C.; von Bahr, C.; Mannervik, B. Glutathione transferases from human liver. J. Methods Enzymol. 1985, 113, 449–503. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Assay of Glutathione Reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyen, H.V., Ed.; Verlog Chemie: Weinheim, Germany, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Costat. Costat Statistical Software: Micro Computer Program Analysis Version 4.20; Cohort Software: Berkeley, CA, USA, 2006. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. J. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defence-related proteins in infected plants. J. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid induced a biotic stress tolerance and underlying mechanisms in plants. J. Plant Sci. 2015, 6, 462. [Google Scholar]
- Bargaus-Lars, N.; Rebecca, L.; Parry, J. Systemic resistance in sugar beet is SA independent and NPR1 dependent. J. Sugar Beet Res. 2007, 44, 2–17. [Google Scholar]
- Ramiro, D.A.; Guerreiro-Filho, O.; Mazzafera, P. Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J. Chem. Ecol. 2006, 32, 1977–1988. [Google Scholar] [CrossRef]
- Mena, F.; Fernandez, S.J.M.; Campos, B.; Sánchez-Ávila, J.; Faria, M. Pesticide residue analyses and biomarker responses of native Costa Rican fish of the Poeciliidae and Cichlidae families to assess environmental impacts of pesticides in Palo Verde National Park. J. Environ. Biol. 2014, 35, 19–27. [Google Scholar]
- Livingstone, D.R. Contaminated-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress BT-Abiotic Stress Responses. In Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Tisler, T.; Jemec, A.; Mozetic, B.; Trebse, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immune competence of honey bees (Apis mellifera L.). J. Insect. Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Zinovieva, S.V.; Vasyukova, N.I.; Udalova, Z.V.; Gerasimova, N.G. The Participation of salicylic and jasmonic acids in genetic and induced resistance of tomato to Meloidogyne incognita (Kofoid and White, 1919). Biol. Bull. 2013, 40, 297–303. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The Effect of Volatile Organic Compounds on Different Organisms: Agrobacteria, Plants and Insects. Microorganisms 2022, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, R.G.; Soares, C.M.; Capdeville, G.; Jones, G.; Martins, E.S.; Praça, L.; Cordeiro, B.A.; Braz, S.V.; dos Santos, R.C.; Berry, C. Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microb. Biotechnol. 2009, 2, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.W.; Faquin, V.; Da Silva Lobato, A.K.; Ávila, P.A.; Marques, D.J.; Guedes, E.M.S.; Tan, D.K.Y. Effect of phosphite supply in nutrient solution on yield, phosphorus nutrition and enzymatic behavior in common bean (Phaseolus vulgaris L.) plants. Aust. J. Crop Sci. 2013, 7, 713–722. [Google Scholar]
- Eltelib, H.A.; Hamad, M.A.; Ali, E. The effect of nitrogen and phosphorus fertilization on growth, yield and quality of forage maize (Zea mays L.). J. Agron. 2006, 5, 515–518. [Google Scholar]
- Al-Kahtani, S.N.; Taha, E.K.A.; Al-Abdulsalam, M. Alfalfa (Medicago sativa L.) seed yield in relation to phosphorus fertilization and honeybee pollination. Saudi J. Biol. Sci. 2017, 24, 1051–1055. [Google Scholar] [CrossRef]
- El-Bassiouny, A.M.; Ghoname, A.A.; El-Awadi, M.E.; Fawzy, Z.F.; Gruda, N. Ameliorative Effects of Brassinosteroids on Growth and Productivity of Snap Beans Grown Under High Temperature. Gesunde Pflanzen 2012, 64, 175–182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).