Climate Change and Seed System Interventions Impact on Food Security and Incomes in East Africa
Abstract
:1. Introduction
1.1. Genetic Diversity in Seed Systems and Its Link to Food Security
1.2. Seed Systems for Food Security
2. Materials and Methods
2.1. Definition of Measurement Variables
2.2. Data Analysis
2.3. Theoretical Framework for Propensity Score Matching
3. Results
3.1. Descriptive Statistics of the Respondents
3.2. Interventions on Crop Production
3.3. Impact of the Intervention on Income from Seed Sales
3.4. Impact of the S4N Intervention on Food Security, Household Consumption, Livestock Feeding and Seed Storage
3.5. Econometric Model Estimated Result
Estimated Overall Impact Results of PSM
3.6. Summary of Results from the Four-Cell Analysis, Indicating Increased Use of Seed Crop Varieties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Total Observation N = 294 | 1 S4N N = 207 | 2 Non-S4N N = 87 | t-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | 1 vs. 2 | |
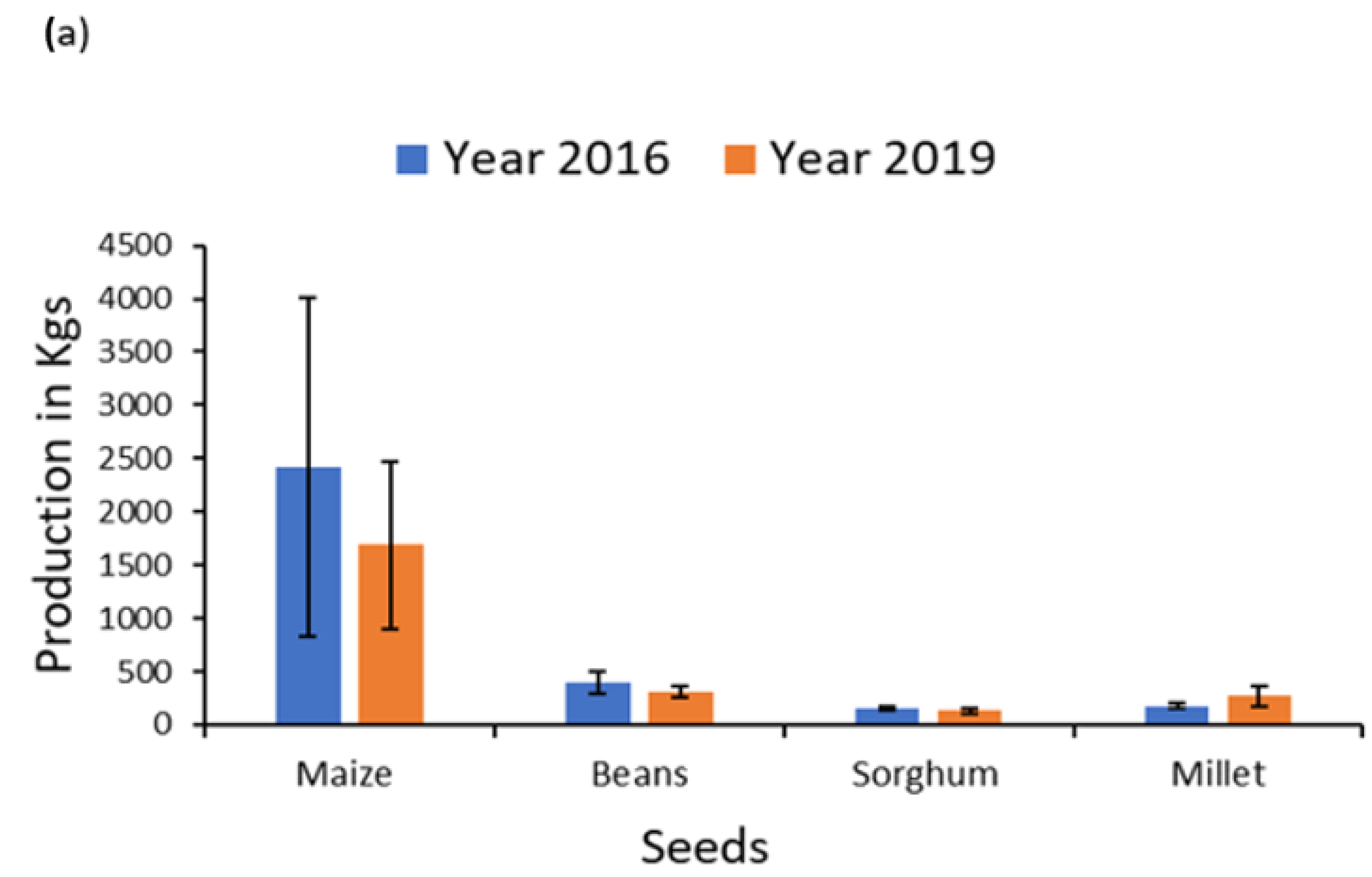
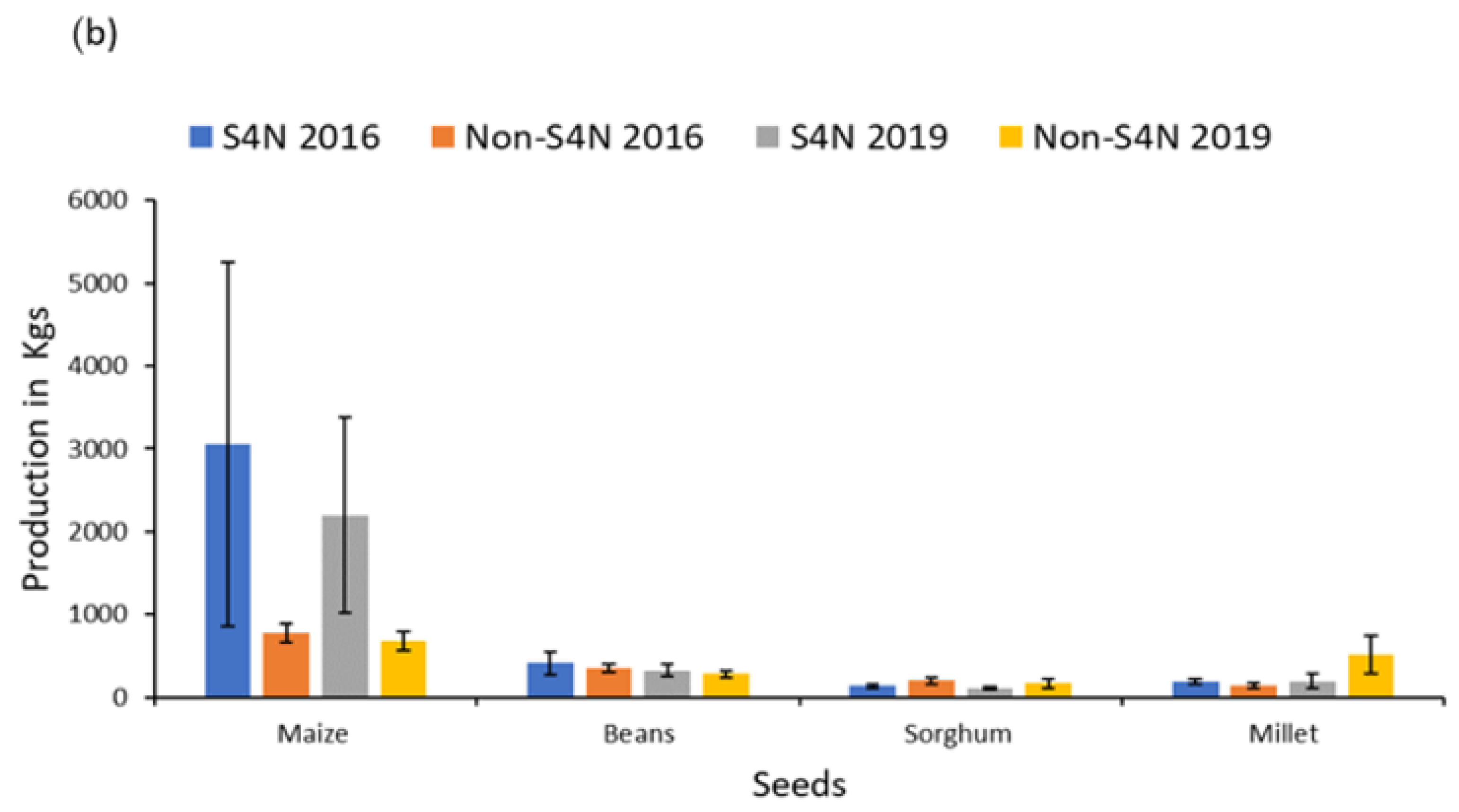
| Maize production 2019 | 1686.97 | 787.53 | 2200.39 | 1186.41 | 679.88 | 111.10 | −0.91 |
| Bean production 2019 | 313.44 | 56.25 | 327.50 | 79.51 | 282.60 | 43.83 | −0.37 |
| Sorghum production 2019 | 129.46 | 21.72 | 111.72 | 20.50 | 172.17 | 55.11 | 1.27 |
| Millet production 2019 | 270.41 | 90.51 | 195.92 | 91.27 | 512.50 | 227.65 | 1.55 |
| Maize production 2016 | 2425.64 | 1589.02 | 3055.81 | 2195.83 | 777.52 | 112.53 | −0.64 |
| Bean production 2016 | 398.63 | 100.82 | 416.23 | 138.39 | 353.09 | 54.39 | −0.28 |
| Sorghum production 2016 | 154.66 | 23.37 | 136.40 | 27.63 | 200.32 | 42.88 | 1.24 |
| Millet production 2016 | 173.87 | 26.55 | 188.50 | 36.69 | 147.27 | 34.59 | −0.74 |
| Variables | Total Observations N = 294 | 1 S4N N = 207 | 2 Non-S4N N = 87 | t-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | 1 vs. 2 | |
| Maize sales | 1898.92 | 779.56 | 2378.02 | 1100.82 | 752.50 | 145.72 | −0.95 |
| Bean sales | 3195.57 | 2883.88 | 331.08 | 93.80 | 9712.28 | 9443.39 | 1.51 |
| Sorghum sales | 166.51 | 29.55 | 165.04 | 35.31 | 168.81 | 53.37 | 0.06 |
| Millet sales | 129.39 | 18.02 | 124.35 | 24.90 | 141.00 | 17.41 | 0.42 |
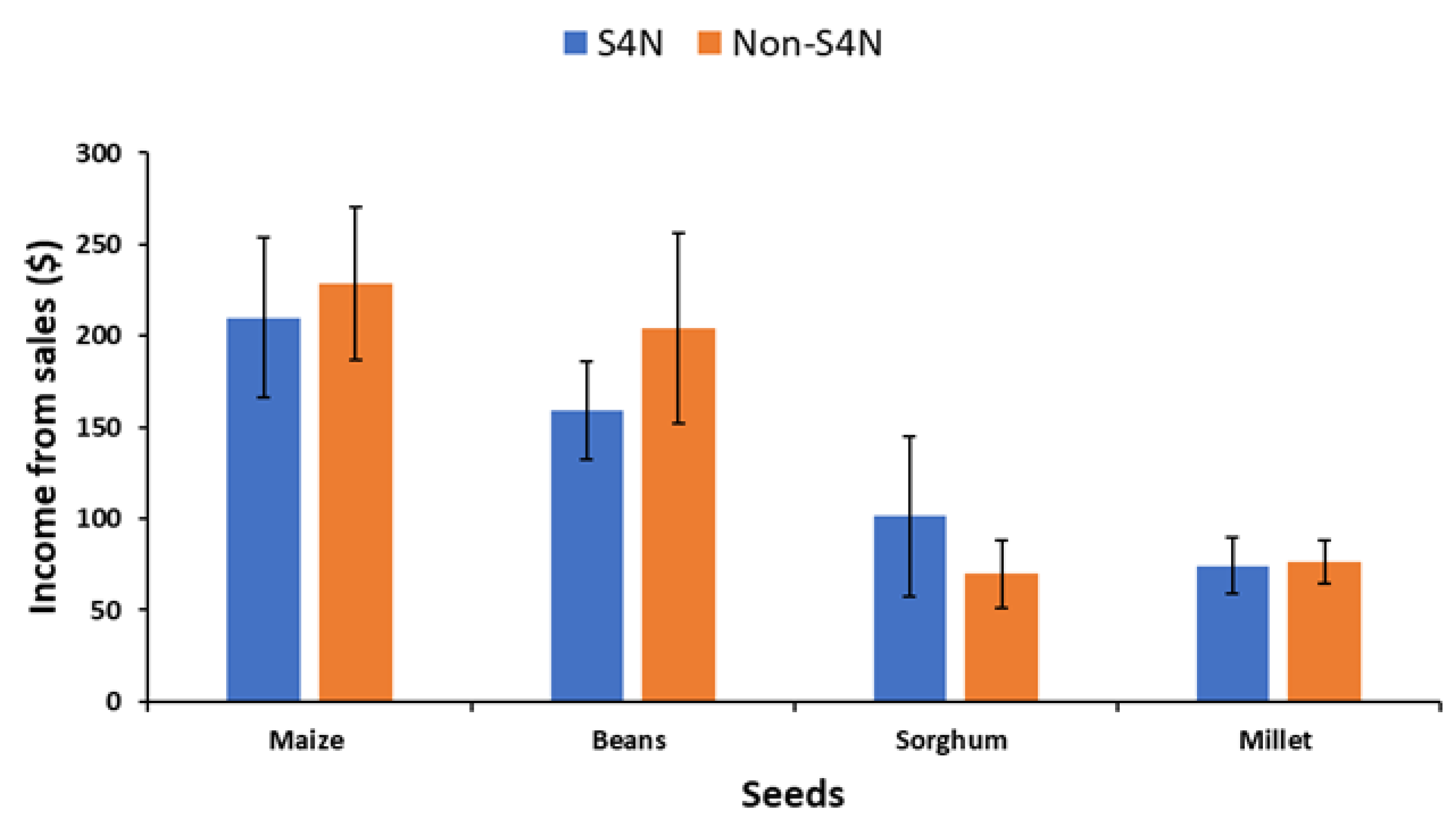
| Maize income | 215 | 32.8 | 209.8 | 43.6 | 228.4 | 42.02 | 0.26 |
| Bean income | 173.04 | 24.49 | 159.3 | 26.97 | 204.3 | 51.9 | 0.846 |
| Sorghum income | 89.02 | 27.4 | 101.39 | 43.6 | 69.69 | 18.7 | −0.5591 |
| Millet income | 75.0 | 10.9 | 74.4 | 15.1 | 76.4 | 11.81 | 0.085 |
| Variables | Total Observations N = 294 | 1 S4N N = 207 | 2 Non S4N N = 87 | t-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | 1 vs. 2 | |
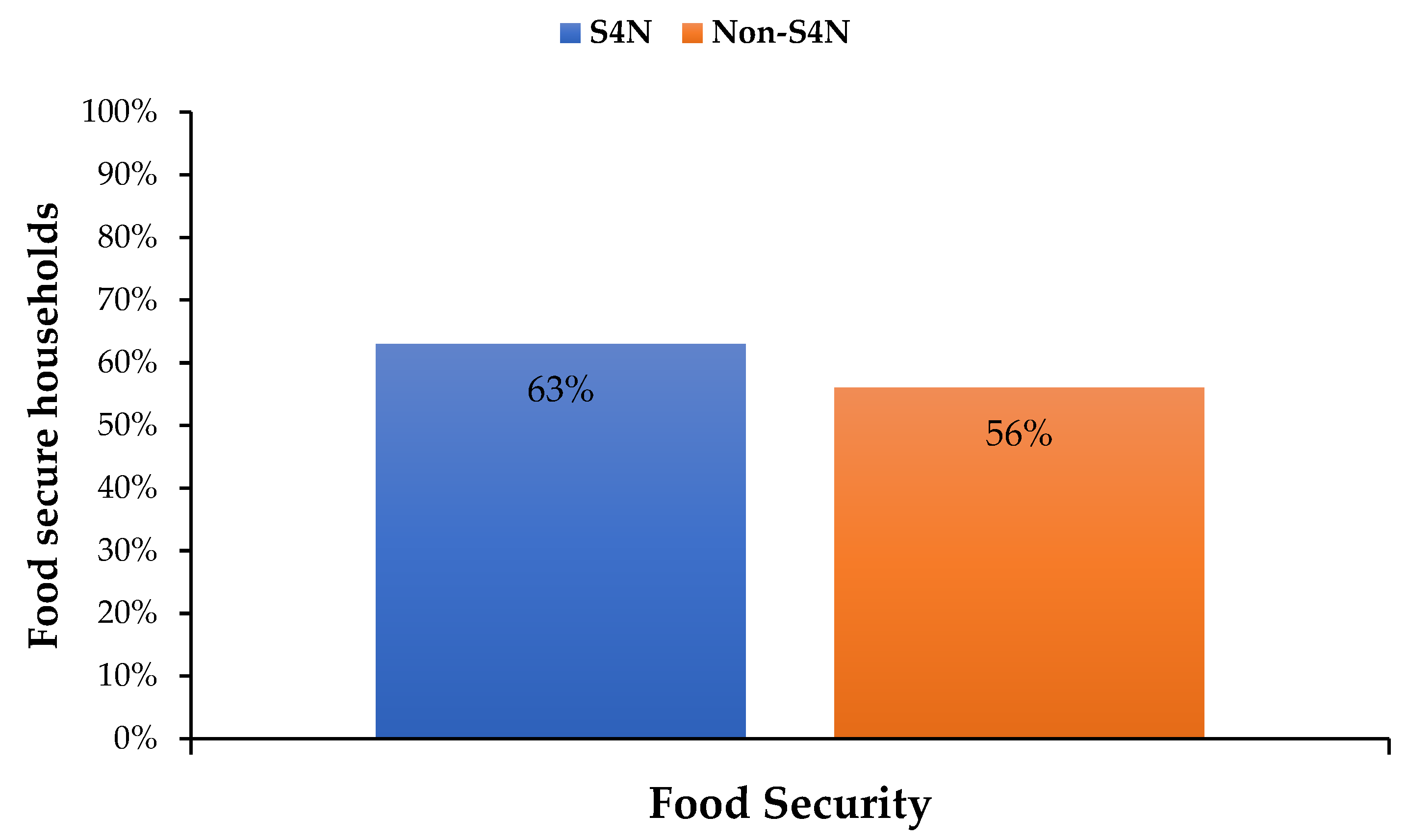
| Food security | 0.61 | 0.03 | 0.63 | 0.04 | 0.56 | 0.07 | −1.00 |
| Maize for human consumption | 187.41 | 20.05 | 180.58 | 26.77 | 200.81 | 27.96 | 0.48 |
| Beans for human consumption | 83.49 | 12.44 | 85.30 | 17.49 | 79.58 | 11.21 | −0.21 |
| Sorghum for human consumption | 48.30 | 5.28 | 45.80 | 5.79 | 54.20 | 11.42 | 0.73 |
| Millet for human consumption | 40.10 | 5.31 | 38.97 | 7.10 | 42.86 | 6.15 | 0.33 |
| Maize for livestock feeding | 808.77 | 108.74 | 660.89 | 117.34 | 1043.65 | 202.73 | 1.75 ** |
| Beans for livestock feeding | 376.94 | 53.56 | 357.35 | 65.95 | 422.00 | 94.20 | 0.55 |
| Sorghum for livestock feeding | 352.34 | 66.86 | 336.17 | 86.44 | 383.33 | 107.37 | 0.33 |
| Millet for livestock feeding | 280.00 | 33.35 | 270.71 | 43.38 | 294.44 | 54.93 | 0.34 |
| Maize seed stored | 98.00 | 17.77 | 72.76 | 14.13 | 148.48 | 44.01 | 2.06 * |
| Bean seed stored | 43.21 | 3.83 | 42.13 | 3.79 | 45.49 | 8.88 | 0.41 |
| Sorghum seed stored | 13.76 | 2.85 | 11.52 | 2.11 | 19.29 | 8.43 | 1.24 |
| Millet seed stored | 16.61 | 2.88 | 18.11 | 3.88 | 12.91 | 2.85 | −0.82 |
| 2016 Four-Cell Analysis in Hoima for Finger Millet | 2021 Four-Cell Analysis in Hoima for Finger Millet | ||||
|---|---|---|---|---|---|
| Many Farming Households | Few Farming Households | Many Farming Households | Few Farming Households | ||
| Large Area | A. | C. | Large Area | A. | C. |
| Local = 1 | Local = 0 | Local = 1 | Local = 0 | ||
| improved = 0 | improved = 3 | improved = 0 | Improved = 0 | ||
| Intervention-0 | Intervention-0 | Intervention-0 | Intervention-0 | ||
| Small Area | B. | D. | Small Area | B. | D. |
| Local = 3 | Local varieties = 1 | Local = 3 | Local = 1 | ||
| Improved = 0 | Improved = 3 | Improved = 0 | Improved = 3 | ||
| Intervention-0 | Intervention-0 | Intervention-3 | Intervention-3 | ||
| 2016 Four-Cell Analysis in Nyando for Finger Millet | 2021 Four-Cell Analysis in Nyando for Finger Millet | ||||
|---|---|---|---|---|---|
| Many Farming Households | Few Farming Households | Many Farming Households | Few Farming Households | ||
| Large Area | A. | C. | Large Area | A. | C. |
| Local varieties = 3 | Local varieties = 0 | Local varieties = 3 | Local varieties = 0 | ||
| improved = 0 | improved =0 | Improved = 0 | Improved = 0 | ||
| Intervention-0 | Intervention-0 | Intervention-4 | Intervention-0 | ||
| Small Area | B. | D. | Small Area | B. | D. |
| Local = 0 | Local = 2 | Local = 3 | Local = 2 | ||
| Improved = 0 | Improve = 1 | Improve =0 | Improved =1 | ||
| Intervention-0 | Intervention-0 | Intervention-0 | Intervention-4 | ||
| 2016 Four-Cell Analysis in Nyando for Sorghum Adoption | 2021 Four-Cell Analysis in Nyando for Sorghum Adoption | ||||
|---|---|---|---|---|---|
| Many Farming Households | Few Farming Households | Many Farming Households | Few Farming Households | ||
| Large Area | A. | C. | Large Area | A. | C. |
| Local = 3 | Local = 0 | Local = 3 | Local = 0 | ||
| improved = 0 | improved = 1 | improved = 0 | improved = 1 | ||
| Intervention-0 | Intervention-0 | Intervention-0 | Intervention-3 | ||
| Small Area | B. | D. | Small Area | B. | D. |
| Local = 0 | Local = 11 | Local = 0 | Local = 11 | ||
| improved = 1 | improved = 5 | improved = 0 | Improve = 5 | ||
| Intervention-0 | Intervention-0 | Intervention-4 | Intervention-0 | ||
| 2016 Four-Cell Analysis in Hoima for Bean Adoption | 2021 Four-Cell Analysis in Hoima for Bean Adoption | ||||
|---|---|---|---|---|---|
| Many Farming Households | Few Farming Households | Many Farming Households | Few Farming Households | ||
| Large Area | A. | C. | Large Area | A. | C. |
| Local = 3 | Local varieties = 0 | Local = 2 | Local varieties = 1 | ||
| improved = 2 | Improved = 0 | improved = 2 | improved = 2 | ||
| Intervention-0 | Intervention-0 | Intervention-1 | Intervention-0 | ||
| Small Area | B. | D. | Small Area | B. | D. |
| Local varieties = 1 | Local varieties = 22 | Local = 0 | Local = 17 | ||
| improved = 2 | Improved = 7 | improved = 1 | Improved = 7 | ||
| Intervention-0 | Intervention-3 | Intervention-0 | Intervention-17 new varieties introduced | ||
| Variable | VIF | 1/VIF |
|---|---|---|
| Household size | 1.26 | 0.7965 |
| Household sex | 1.25 | 0.8007 |
| Land size | 1.17 | 0.8537 |
| Farmer group | 1.13 | 0.8812 |
| Distance to market | 1.13 | 0.8848 |
| Land ownership | 1.13 | 0.8855 |
| Plots_count | 1.09 | 0.9175 |
| Household head age | 1.08 | 0.9250 |
| Household education level | 1.06 | 0.9390 |
| Employment income | 1.05 | 0.9544 |
| Source of labour | 1.04 | 0.9626 |
| Business income | 1.01 | 0.9864 |
| Mean VIF | 1.12 |
References
- UNFPA. United Nations Population Fund. 2021. Available online: https://www.unfpa.org/data/world-population-dashboard (accessed on 24 March 2022).
- UNDPI. United Nations Department of Public Information 405 East 42. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100—Says UN. 2017, pp. 1–4. Available online: https://www.un.org/en/development/desa/population/events/pdf/other/21/21June_FINAL%20PRESS%20RELEASE_WPP17.pdf (accessed on 22 May 2022).
- Mbow, Y.X.; Rosenzweig, C.C.; Barioni, L.G.; Benton, T.G.; Herrero, M.; Krishnapillai, M.; Liwenga, E.; Pradhan, P.; Rivera-Ferre, M.G.; Sapkota, T.; et al. Food Security. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystem; IPCC: Geneva, Switzerland, 2019; pp. 437–550. Available online: https://www.ipcc.ch/site/assets/uploads/2019/11/08_Chapter-5.pdf (accessed on 22 May 2022).
- Otieno, G.; Westphal, I. Building Resilience through “Open Source Seed Systems” for Climate Change Adaptation in Kenya, Uganda, and Tanzania: What Are the Options for Policy? CGIAR: Montpellier, France, 2018; pp. 1–8. Available online: https://hdl.handle.net/10568/100157 (accessed on 22 May 2022).
- Global Panel on Agriculture and Food Systems for Nutrition. Food Systems and Diets: Facing the Challenges of the 21st Century; Global Panel on Agriculture and Food Systems for Nutrition: London, UK, 2016; pp. 1–133. Available online: https://glopan.org/sites/default/files/ForesightReport.pdf (accessed on 14 January 2022).
- FAO; IFAD; UNICEF; WFP. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021; pp. 1–240. Available online: https://www.fao.org/3/cb4474en/cb4474en.pdf (accessed on 12 March 2022).
- Fraval, S.; Hammond, J.; Bogard, J.R.; Kanui, M.N. Food Access Deficiencies in Sub-saharan Africa: Prevalence and Implications for Agricultural Interventions. Front. Sustain. Food Syst. 2019, 3, 104. [Google Scholar] [CrossRef] [Green Version]
- FAO. COVID-19 and malnutrition: Situation analysis and options in Africa. COVID-19 Malnutrition Situat. Anal. Options Africa 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S. Climate change and eastern Africa: A review of impact on major crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Mastenbroek, A. Research Report on Climate Resilient Local Seed Businesses: Integrated Seed Sector Development Programme in Uganda; Centre for Development Innovation: Wageningen, The Netherlands, 2015; pp. 1–84. Available online: https://edepot.wur.nl/407778 (accessed on 15 March 2022).
- Kimenye, L. (Ed.) Best-Bet Technologies for Addressing Climate Change and Variability in Eastern and Central Africa; ASARECA (Association for Strengthening Agricultural Research in Eastern and Central Africa): Entebbe, Uganda, 2014; pp. 1–234. Available online: https://www.asareca.org/sites/default/files/publications/ASARECA%20Best%20Bet%20Technologies%204%20Climate%20change%20and%20variability.pdf (accessed on 22 May 2022).
- WFP. East Africa Regional Food Security & Nutrition Update. WFP Report. 2019. Available online: https://reliefweb.int/report/ethiopia/east-africa-regional-food-security-nutrition-update-november-2019 (accessed on 22 May 2022).
- Engels, J.; Diulgheroff, S.; Alvarez, S.J. Management of Crop Diversity; FAO: Rome, Italy, 2014; pp. 1–48. Available online: https://www.fao.org/3/i3767e/i3767e.pdf (accessed on 22 May 2022).
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Change 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, G.; Li, X.; Zhang, H.; Wang, C.; Liu, Q.; Chen, X. Closing yield gaps in China by empowering smallholder farmers. Nature 2016, 537, 671–674. [Google Scholar] [CrossRef]
- Seed Availability and Access. Available online: https://ciat.cgiar.org/what-we-do/seed-availability-and-access/ (accessed on 24 March 2022).
- Quarshie, P.T.; Abdulai, A.-R.; Fraser, E.D.G. Africa′s “Seed” Revolution and Value Chain Constraints to Early Generation Seeds Commercialization and Adoption in Ghana. Front. Sustain. Food Syst. 2021, 5, 665297. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; p. 4. Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 22 May 2022).
- Macauley, H. Cereal Crops: Rice, Maize, Millet, Sorghum, Wheat. Chem. Eng. News 2015, 86, 74. [Google Scholar] [CrossRef]
- Seeds for Needs’ Is a Bioversity International Participatory Research–Farmer/Citizen Scientist Initiative. Available online: https://www.bioversityinternational.org/seeds-for-needs/ (accessed on 24 March 2022).
- Louwaars, N.P. Policies and strategies for seed system development. In Proceedings of the Conclusions of the International Workshop on Integrated Seed Systems for Low-Input Agriculture, RILET, Malang, Indonesia, 24–27 October 1995; pp. 5–15. Available online: https://repository.unescap.org/bitstream/handle/20.500.12870/4059/ESCAP-1996-RP-CGPRT-Monograph-No32.pdf?sequence=1#page=24 (accessed on 14 May 2022).
- Jones, S.K. Quality Declared Seed System: FAO Plant Production and Protection Paper 185; Anonymous. Rome: Food and Agriculture Organization of the United Nations (2006), pp. 243, $40.00. ISBN 92-5-105510-6. Exp. Agric. 2007, 43, 261. [Google Scholar] [CrossRef]
- Otieno, G. Accessing genetic diversity for food security and climate change adaptation in select communities in Africa. In Food Security and Climate Change; Yadav, S.S., Ed.; John Wiley: Hoboken, NJ, USA, 2019; pp. 499–522. ISBN 9781119180647. [Google Scholar] [CrossRef]
- Kidane, Y.G.; Gesesse, C.A.; Hailemariam, B.N.; Desta, E.A.; Mengistu, D.K.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. A large nested association mapping population for breeding and quantitative trait locus mapping in Ethiopian durum wheat. Plant Biotechnol. J. 2019, 17, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 22 May 2022).
- Rao, V.R.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult. (PCTOC) 2002, 68, 1–19. [Google Scholar] [CrossRef]
- Li, X.; Siddique, K.H.M. Future Smart Food. Rediscovering Hidden Treasures of Neglected and Underutilized Species for Zero Hunger in Asia; FAO: Bangkok, Thailand, 2018; pp. 1–40. Available online: https://www.fao.org/3/I8907EN/i8907en.pdf (accessed on 22 May 2022).
- Otieno, G.; Zebrowski, W.; Recha, J.; Reynolds, T. Gender and Social Seed Networks for Climate Change Adaptation: Evidence from Bean, Finger Millet, and Sorghum Seed Systems in East Africa. Sustainability 2021, 13, 2074. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, L.; Mcguire, S. Understanding and Strengthening Informal Seed Markets. Exp. Agric. 2010, 46, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Otieno, G.; Lacasse, H.; Fadda, C.; Reynolds, T.W.; Recha, J.W. Social Seed Networks for Climate Change Adaptation in Western Kenya Results from a Study to Better Understand Farmers’ Primary Sources of Seed Information in the Nyando Climate-Smart Villages; CCAFS Info note; CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS): Wageningen, The Netherlands, 2018; pp. 1–4. Available online: https://hdl.handle.net/10568/93210 (accessed on 22 May 2022).
- FAO. Coping with Climate Change—The Roles of Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2015; pp. 1–130. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/a-i3866e.pdf (accessed on 22 May 2022).
- FAO; IFAD; UNICEF. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018; pp. 1–202. Available online: https://docs.wfp.org/api/documents/WFP-0000074343/download/?_ga=2.249465860.2088145878.1647943563-1543092150.1647943563 (accessed on 22 May 2022).
- Jarvis, D.I.; Fadda, C.; De Santis, P.; Thompson, J. Damage, Diversity and Genetic Vulnerability: The Role of Crop Genetic Diversity in the Agricultural Production System to Reduce Pest and Disease Damage. In Proceedings of the International Symposium, Rabat, Morocco, 15–17 February 2011; Jarvis, D.I., Fadda, C., De Santis, P., Thompson, J., Eds.; Bioversity International: Rome, Italy, 2011. [Google Scholar]
- McGuire, S.; Sperling, L. Making seed systems more resilient to stress. Glob. Environ. Change 2013, 23, 644–653. [Google Scholar] [CrossRef]
- Vernooy, R.; Sthapit, B.; Otieno, G.; Shrestha, P.; Gupta, A. The roles of community seed banks in climate change adaption. Dev. Pr. 2017, 27, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Vernooy, R.; Jai, R.; Ahlawat, S.P.; Malik, S.K.; Mbozie, H.; Mugisha, J.; Nyabasha, S.; Otieno, G.; Patil, S.; Roy, S.; et al. Community Seed Banks as Seed Producers: Cases from India, Nepal, Uganda and Zimbabwe; Working Paper Series No. 2; CGIAR Research Program on Grain Legumes and Dryland Cereals: Hyderabad, India, 2020; p. 55. ISBN 978-93-86527-05-9. Available online: https://hdl.handle.net/10568/111420 (accessed on 22 May 2022).
- Van Eerdewijk, A.; Danielsen, K. Gender Matters in Farm Power. 2015, pp. 1–73. Available online: https://www.kit.nl/wp-content/uploads/2018/08/551bcea41f1f2_Gender-Matters-in-Farm-Power-final-150227-AE-KD.pdf (accessed on 22 May 2022).
- Mendola, M. Agricultural technology adoption and poverty reduction: A propensity-score matching analysis for rural Bangladesh. Food Policy 2006, 32, 372–393. [Google Scholar] [CrossRef]
- Becker, S.O.; Caliendo, M. Sensitivity Analysis for Average Treatment Effects. Stata J. Promot. Commun. Stat. Stata 2007, 7, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.O.; Ichino, A. Estimation of Average Treatment Effects Based on Propensity Scores. Stata J. Promot. Commun. Stat. Stata 2002, 2, 358–377. [Google Scholar] [CrossRef] [Green Version]
- Caliendo, M.; Kopeinig, S. Some Practical Guidance for the Implementation of Propensity Score Matching. J. Econ. Surv. 2008, 22, 31–72. [Google Scholar] [CrossRef] [Green Version]
- Maddala, G.S. Introduction to Econometrics, 2nd ed. 2000. Available online: https://jigjids.files.wordpress.com/2011/05/introduction-to-econometric-2nd.pdf (accessed on 24 March 2022).
- Jarvis, D.I.; Sevilla-Panizo, R.; Chavez-Servia, J.-L.; Hodgkin, T. (Eds.) Seed Systems and Crop Genetic Diversity On-Farm. In Proceedings of the a Workshop, Pucallpa, Peru, 16–20 September 2003; International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Symposium on “Food Technology for Better Nutrition”. Compr. Rev. Food Sci. Food Saf. 2008, 7, 320–396. [CrossRef] [Green Version]
- Dabija, A.; Ciocan, M.; Chetrariu, A.; Codină, G. Maize and Sorghum as Raw Materials for Brewing, A Review. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Orr, A.; Mwema, C.; Mulinge, W. The Value Chain for Sorghum Beer in Kenya; Socioeconomics Discussion Paper Series 16; ICRISAT: Nairobi, Kenya, 2014; Available online: https://www.researchgate.net/publication/285512393_A_Orr_C_Mwema_W_Mulinge_The_value_chain_for_sorghum_beer_in_Kenya_Socioeconomics_Discussion_Paper_Series_Number_16_International_Crops_Research_Institute_for_the_Semi-Arid_Tropics (accessed on 22 May 2022).
- Walker, T.S.; Alwang, J. Crop Improvement, Adoption, and Impact of Improved Varieties in Food Crops in Sub-Saharan Africa. CABI, 2015. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=T.+S.+Walker+and+J.+Alwang%2C+Crop+improvement%2C+adoption%2C+and+impact+of+improved+varieties+in+food+crops+in+sub-Saharan+Africa&btnG= (accessed on 22 May 2022).
- Cacho, O.J.; Moss, J.; Thornton, P.K.; Herrero, M.; Henderson, B.; Bodirsky, B.L.; Humpenöder, F.; Popp, A.; Lipper, L. The value of climate-resilient seeds for smallholder adaptation in sub-Saharan Africa. Clim. Change 2020, 162, 1–17. [Google Scholar] [CrossRef]
- Nasidi, M.; Agu, R.; Walker, G.; Deeni, Y. Sweet sorghum: Agronomic practice for food, animal feed and fuel production in Sub-Saharan Africa. In Sweet Sorghum: Characteristics, Cultivation and Uses; Agriculture Issues and Policies; Rogers, L., Willis, M., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; pp. 1–67. Available online: https://novapublishers.com/shop/sweet-sorghum-characteristics-cultivation-and-uses/ (accessed on 22 May 2022).
- Orr, A.; Mwema, C.; Gierend, A.; Nedumaran, S. Sorghum and Millets in Eastern and Southern Africa: Facts, Trends and Outlook; Working Paper. no. 62; ICRISAT: Patancheru, India, 2016; Available online: http://oar.icrisat.org/9441/1/2016-062%20WPS%2062%20S%26M%20ESA.pdf (accessed on 22 May 2022).
- FAO. Youth and Agriculture; FAO: Rome, Italy, 2014. [Google Scholar]
- Martey, E.; Etwire, P.M.; Wiredu, A.N.; Dogbe, W. Factors influencing willingness to participate in multi-stakeholder platform by smallholder farmers in Northern Ghana: Implication for research and development. Agric. Food Econ. 2014, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Hlatshwayo, S.; Ngidi, M.; Ojo, T.; Modi, A.; Mabhaudhi, T.; Slotow, R. A Typology of the Level of Market Participation among Smallholder Farmers in South Africa: Limpopo and Mpumalanga Provinces. Sustainability 2021, 13, 7699. [Google Scholar] [CrossRef]
- Fischer, E.; Qaim, M. Smallholder Farmers and Collective Action: What Determines the Intensity of Participation? J. Agric. Econ. 2014, 65, 683–702. [Google Scholar] [CrossRef]
- Wang, X.; Sarkar, A.; Wang, H.; Zhang, F. Does Participation in Agricultural Value Chain Activities Influence Smallholder Fruit Grower Production Performance? A Cross-Sectional Study of Apple Farmers in Shandong, China. Horticulturae 2021, 7, 153. [Google Scholar] [CrossRef]
- Snel, H. Income Intervention Quick Scan: Productivity Enhancement; Report WCDI-18-036; WCDI: Wageningen, The Netherlands, 2018; pp. 1–24. Available online: https://edepot.wur.nl/460687 (accessed on 22 March 2022).
- Food and Agriculture Organization of the United Nations (FAO). An Introduction to the Basic Concepts of Food Security. EC—FAO Food Security Programme. 2008, pp. 1–3. Available online: https://www.fao.org/3/al936e/al936e.pdf (accessed on 22 May 2022).
- Mujeeb-Kazi, A.; Dundas, I.; Rasheed, A.; Ogbonnaya, F.; Kishii, M.; Bonnett, D.; Wang, R.R.-C.; Xu, S.; Chen, P.; Mahmood, T.; et al. Genetic Diversity for Wheat Improvement as a Conduit to Food Security. Adv. Agron. 2013, 122, 179–257. [Google Scholar] [CrossRef]
- Muñoz-Amatriaín, M.; Mirebrahim, H.; Xu, P.; Wanamaker, S.I.; Luo, M.; AlHakami, H.; Alpert, M.; Atokple, I.; Batieno, B.J.; Boukar, O.; et al. Genome resources for climate-resilient cowpea, an essential crop for food security. Plant J. 2017, 89, 1042–1054. [Google Scholar] [CrossRef] [Green Version]
- Kallow, S.; Mertens, A.; Janssens, S.B.; Vandelook, F.; Dickie, J.; Swennen, R.; Panis, B. Banana seed genetic resources for food security: Status, constraints, and future priorities. Food Energy Secur. 2021, 11, e345. [Google Scholar] [CrossRef]
- Khadka, R.; Gartaula, K.; Shrestha, H.; Upadhay, A.; Chaudhary, D.; Patel, P.; Devkota, K. Farmers’ Seed Networks and Agrobiodiversity Conservation for Sustainable Food Security: A Case from the Mid-Hills of Nepal. 2018, pp. 1–32. Available online: https://idl-bnc-idrc.dspacedirect.org/bitstream/handle/10625/57417/IDL-57417.pdf?sequence=2&isAllowed=y (accessed on 22 May 2022).
- Ma, X.; Mau, M.; Sharbel, T.F. Genome Editing for Global Food Security. Trends Biotechnol. 2018, 36, 123–127. [Google Scholar] [CrossRef]
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A Regional Comparison of Factors Affecting Global Sorghum Production: The Case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef] [Green Version]
- Mijatović, D.; Van Oudenhoven, F.; Eyzaguirre, P.; Hodgkin, T. The role of agricultural biodiversity in strengthening resilience to climate change: Towards an analytical framework. Int. J. Agric. Sustain. 2012, 11, 95–107. [Google Scholar] [CrossRef]
- López-Noriega, I.; Galluzzi, G.; Halewood, M.; Vernooy, R.; Bertacchini, E.; Gauchan, D.; Welch, E. Flows under Stress: Availability of Plant Genetic Resources in Times of Climate and Policy Change; Working Paper 18; CCAFS: Copenhagen, Denmark, 2012; Available online: https://hdl.handle.net/10568/21225 (accessed on 22 May 2022).
- McGuire, S.; Sperling, L. Seed systems smallholder farmers use. Food Secur. 2016, 8, 179–195. [Google Scholar] [CrossRef] [Green Version]
- Sperling, L.; Boettiger, S.; Barker, I. Integrating Seed Systems; AgpatnersXechange: Goodhue, MN, USA, 2013; pp. 1–32. Available online: https://seedsystem.org/wp-content/uploads/2014/03/Integrating-Seed-Systems-.pdf (accessed on 22 May 2022).
- Westengen, O.T.; Brysting, A.K. Crop adaptation to climate change in the semi-arid zone in Tanzania: The role of genetic resources and seed systems. Agric. Food Secur. 2014, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Westengen, O.T.; Berg, T. Crop Adaptation to Climate Change in SSA: The Role of Genetic Resources and Seed Systems. In Climate Change and Multi-Dimensional Sustainability in African Agriculture; Springer: Cham, Switzerland, 2016; pp. 327–343. [Google Scholar] [CrossRef]
- Fenzi, M.; Rogé, P.; Cruz-Estrada, A.; Tuxill, J.; Jarvis, D. Community seed network in an era of climate change: Dynamics of maize diversity in Yucatán, Mexico. Agric. Hum. Values 2021, 39, 339–356. [Google Scholar] [CrossRef]
- Jackson, L.; Pascual, U.; Hodgkin, T. Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 121, 196–210. [Google Scholar] [CrossRef]
- Di Falco, S.; Perrings, C. Crop biodiversity, risk management and the implications of agricultural assistance. Ecol. Econ. 2005, 55, 459–466. [Google Scholar] [CrossRef] [Green Version]



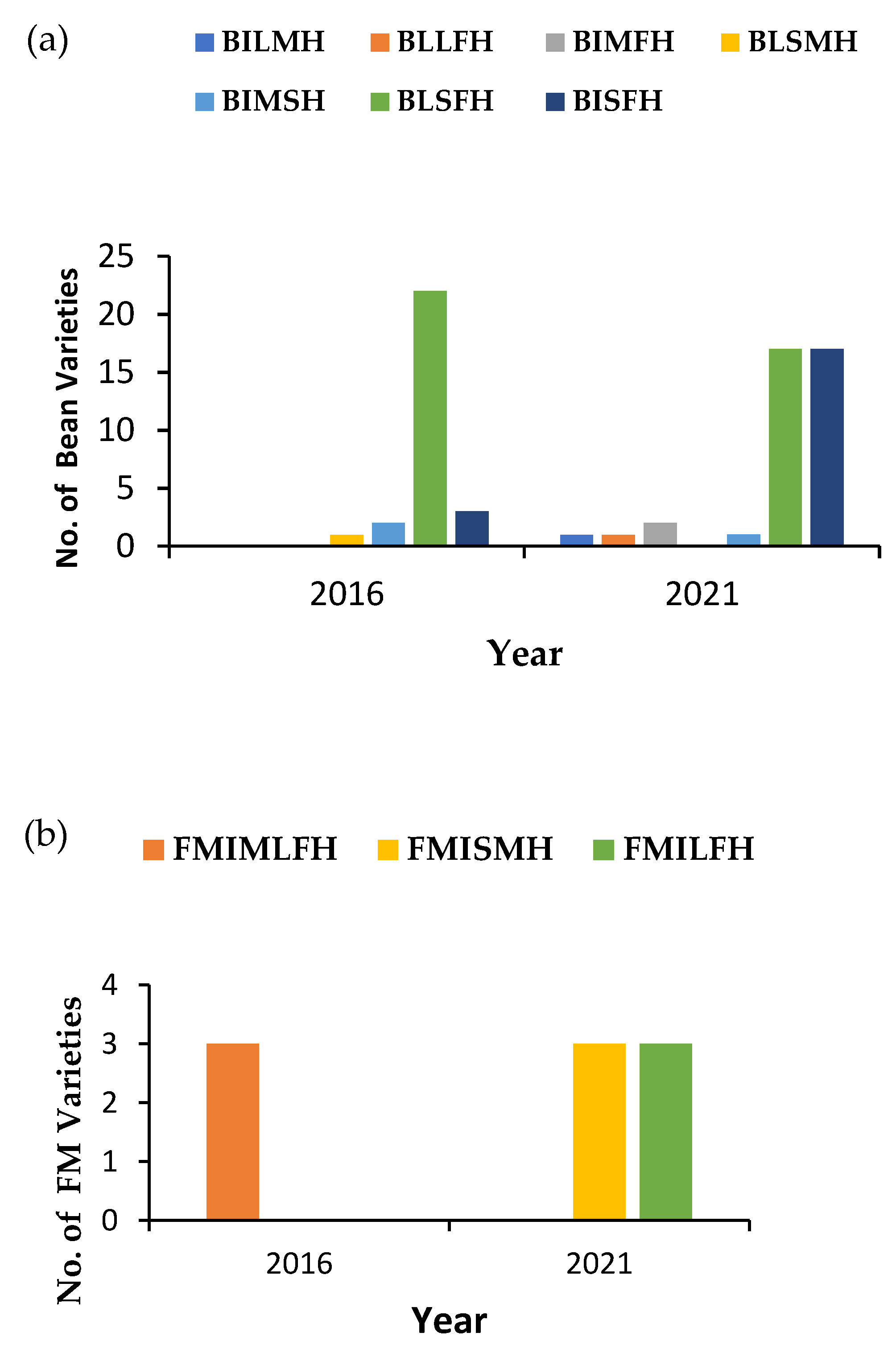

| Variable | Type and Definition | Measurement |
|---|---|---|
| Dependent Variable | ||
| Treatment | Dummy variable representing participation in the Seeds forNeeds (S4N) project | 1 if in S4N, 0 if not in S4N |
| Covariates | ||
| Sex of household head | Dummy, sex of household head | 1 if male, 0 if female |
| Age of household head | Continuous, age of household head | Years completed |
| Education of household head | Dummy, Level of education of household head | 1 basic education, 0 for no education |
| Household size | Discrete, household size | Number of household members |
| Land size | Continuous, size of land holding | The size of land owned by household in hectares |
| Land ownership | Dummy, whether owned or shared | 1 for owned, 0 for shared |
| Plots | Discrete, number of plots | Number of plots |
| Labour | Dummy, source of labour—either unpaid family or hired | 1 for unpaid family labour, 0 for hired labour |
| Employment income | Dummy, whether the household receives additional income from employment (both farm and non-farm) | 1 for employment income, 0 for no employment income |
| Business income | Dummy, whether the household receives additional income from business | 1 for business income, 0 for no business income |
| Distance | Discrete, distance to the nearest market | Distance to the nearest market in kilometers |
| Farmers’ group | Dummy, whether the household head is a member of a farmers’ group | 1 for membership in a farmer’s group, 0 for no membership |
| Livestock (e.g., cattle, goat, sheep & poultry) | Discrete, number of livestock | Number of livestock |
| Outcome indicator | ||
| Crop production | Continuous, total amount of output for each crop—beans, maize, millet and sorghum | Number of bags (for maize) and kilograms for beans, millet and sorghum harvested |
| Crop sales | Continuous, total amount of output sold for each crop—beans, maize, millet and sorghum | Number of bags of maize and kilograms of beans, millet and sorghum harvested sold |
| Crop income | Continuous, amount of income earned in USD | Amount of income earned in USD (exchange rates used are 100Kshs/USD for Kenya and 3600Ugx/USD for \Uganda |
| Human consumption | Continuous, amount of output that is consumed in bags/kilograms | Amount of output that is consumed in bags/kilograms |
| Livestock feeding | Continuous, amount of output that is used for livestock feeding in bags/kilograms | Amount of output that is used for livestock feeding in bags/kilograms |
| Seeds stored | Continuous, amount of output that is stored as seeds in kilograms | Continuous, amount of output that is stored as seeds in kilograms |
| Food security | Dummy, whether household has ever gone without food | 1 for never slept hungry, 0 for has slept hungry more than once |
| Many Households in large areas Common varieties for household food security and for market | Many households in small areas Rare varieties grown for their special traits or characteristics or newly introduced varieties with specific adaptable traits (unique) |
| Few households in large areas Varieties cultivated for home use or related to cultural or religious traditions and rituals (unique or under threat) | Few households in small areas Varieties with low use value or specific use values to particular families (rare and under threat) |
| Variables | Total Observation N = 294 | (1) S4N N = 207 | (2) Non S4N N = 87 | t-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | 1 vs. 2 | |
| Sex of HH | 0.754 | 0.023 | 0.767 | 0.028 | 0.727 | 0.043 | −0.797 |
| Age of the HH | 49.722 | 0.764 | 50.705 | 0.949 | 47.560 | 1.254 | −1.915 ** |
| Education of the HH | 0.678 | 0.026 | 0.652 | 0.033 | 0.747 | 0.045 | 1.595 |
| Household size | 5.455 | 0.132 | 5.454 | 0.170 | 5.456 | 0.201 | 0.008 |
| Land size | 5.066 | 0.497 | 5.030 | 0.575 | 5.143 | 0.960 | 0.107 |
| Land ownership | 0.913 | 0.015 | 0.911 | 0.019 | 0.918 | 0.026 | 0.231 |
| Number of plots | 1.750 | 0.104 | 1.769 | 0.116 | 1.711 | 0.214 | −0.262 |
| Employment income | 0.145 | 0.019 | 0.143 | 0.023 | 0.149 | 0.034 | 0.156 |
| Business income | 0.014 | 0.006 | 0.008 | 0.006 | 0.026 | 0.015 | 1.329 |
| Source of labour | 0.983 | 0.007 | 0.987 | 0.007 | 0.974 | 0.015 | −0.923 |
| Farmers’ groups | 0.733 | 0.024 | 0.874 | 0.022 | 0.439 | 0.047 | −9.705 * |
| Distance to market | 48.897 | 2.017 | 47.684 | 2.503 | 51.491 | 3.380 | 0.879 |
| Project Variables Evaluated | Impact | z-Value | p-Value |
|---|---|---|---|
| Maize production 2019 | 1448.52 | 1.52 | 0.13 |
| Beans production 2019 | 63.96 | 0.81 | 0.42 |
| Sorghum production 2019 | 2.82 | 0.10 | 0.92 |
| Millet production 2019 | 5.52 | 0.09 | 0.93 |
| Maize sales | 1227.98 | 1.47 | 0.14 |
| Bean sales | −30,820.90 | −1.40 | 0.16 |
| Sorghum sales | 17.48 | 0.44 | 0.66 |
| Millet sales | −1.52 | −0.03 | 0.97 |
| Maize income | −35.04 | −0.47 | 0.637 |
| Bean income | −104.2 ** | −1.64 | 0.10 |
| Sorghum income | 47.93 | 1.06 | 0.29 |
| Millet income | −3.04 | −0.11 | 0.916 |
| Food security | −0.005 | −0.08 | 0.94 |
| Maize for human consumption | −32.98 | −0.85 | 0.40 |
| Bean for human consumption | 17.08 | 0.95 | 0.34 |
| Sorghum for human consumption | 8.09 * | 4.93 | 0.00 |
| Millet for human consumption | −7.50 * | −2.67 | 0.01 |
| Maize for livestock feeding | −329.11 | −1.04 | 0.30 |
| Bean for livestock feeding | −73.46 * | −4.37 | 0.00 |
| Sorghum for livestock feeding | 19.18 | 0.20 | 0.84 |
| Maize seed stored | −84.20 * | −5.54 | 0.00 |
| Bean seed stored | 7.05 | 0.87 | 0.38 |
| Sorghum seed stored | −6.84 | −1.51 | 0.13 |
| Millet seed stored | 10.44 * | 3.13 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the CGIAR. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otieno, G.; Ogola, R.J.O.; Recha, T.; Mohammed, J.N.; Fadda, C. Climate Change and Seed System Interventions Impact on Food Security and Incomes in East Africa. Sustainability 2022, 14, 6519. https://doi.org/10.3390/su14116519
Otieno G, Ogola RJO, Recha T, Mohammed JN, Fadda C. Climate Change and Seed System Interventions Impact on Food Security and Incomes in East Africa. Sustainability. 2022; 14(11):6519. https://doi.org/10.3390/su14116519
Chicago/Turabian StyleOtieno, Gloria, Robert John O Ogola, Tobias Recha, Jemal Nurhisen Mohammed, and Carlo Fadda. 2022. "Climate Change and Seed System Interventions Impact on Food Security and Incomes in East Africa" Sustainability 14, no. 11: 6519. https://doi.org/10.3390/su14116519
APA StyleOtieno, G., Ogola, R. J. O., Recha, T., Mohammed, J. N., & Fadda, C. (2022). Climate Change and Seed System Interventions Impact on Food Security and Incomes in East Africa. Sustainability, 14(11), 6519. https://doi.org/10.3390/su14116519








