The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Potential Risks of Introduction of Exotic Insect Species Being Used for Livestock Feed and Exotic Pets’ Food in Europe
3.1.1. Hermetia illucens Linnaeus, 1758 (Diptera, Stratiomyidae)
3.1.2. Musca domestica Linnaeus, 1758 (Diptera, Muscidae)
3.1.3. Drosophila melanogaster Meigen, 1830 (Diptera, Drosophilidae)
3.1.4. Drosophila hydei Stutervant, 1921 (Diptera, Drosophilidae)
3.1.5. Gryllus bimaculatus De Geer, 1773 (Orthoptera, Gryllidae)
3.1.6. Gryllodes sigillatus (Walker, 1869) (Orthoptera, Gryllidae)
3.1.7. Gryllus assimilis (Fabricius, 1775) (Orthoptera, Gryllidae)
3.1.8. Acheta domesticus Linnaeus, 1758 (Orthoptera, Gryllidae)
3.1.9. Tenebrio molitor Linnaeus, 1758 (Coleoptera, Tenebrionidae)
3.1.10. Zophobas morio (Fabricius, 1776) (Coleoptera, Tenebrionidae)
3.1.11. Callosobruchus maculatus (Fabricius, 1775) (Coleoptera, Chrysomelidae)
3.1.12. Pachnoda marginata (Drury, 1773) (Coleoptera, Scarabaeidae)
3.1.13. Alphitobius diaperinus (Panzer, 1797) (Coleoptera, Tenebrionidae)
3.1.14. Locusta migratoria (Linnaeus, 1758) (Orthoptera, Acrididae)
3.1.15. Schistocerca gregaria (Forskal, 1775) (Orthoptera, Acrididae)
3.1.16. Galleria mellonella (Linnaeus, 1758) (Lepidoptera, Pyralidae)
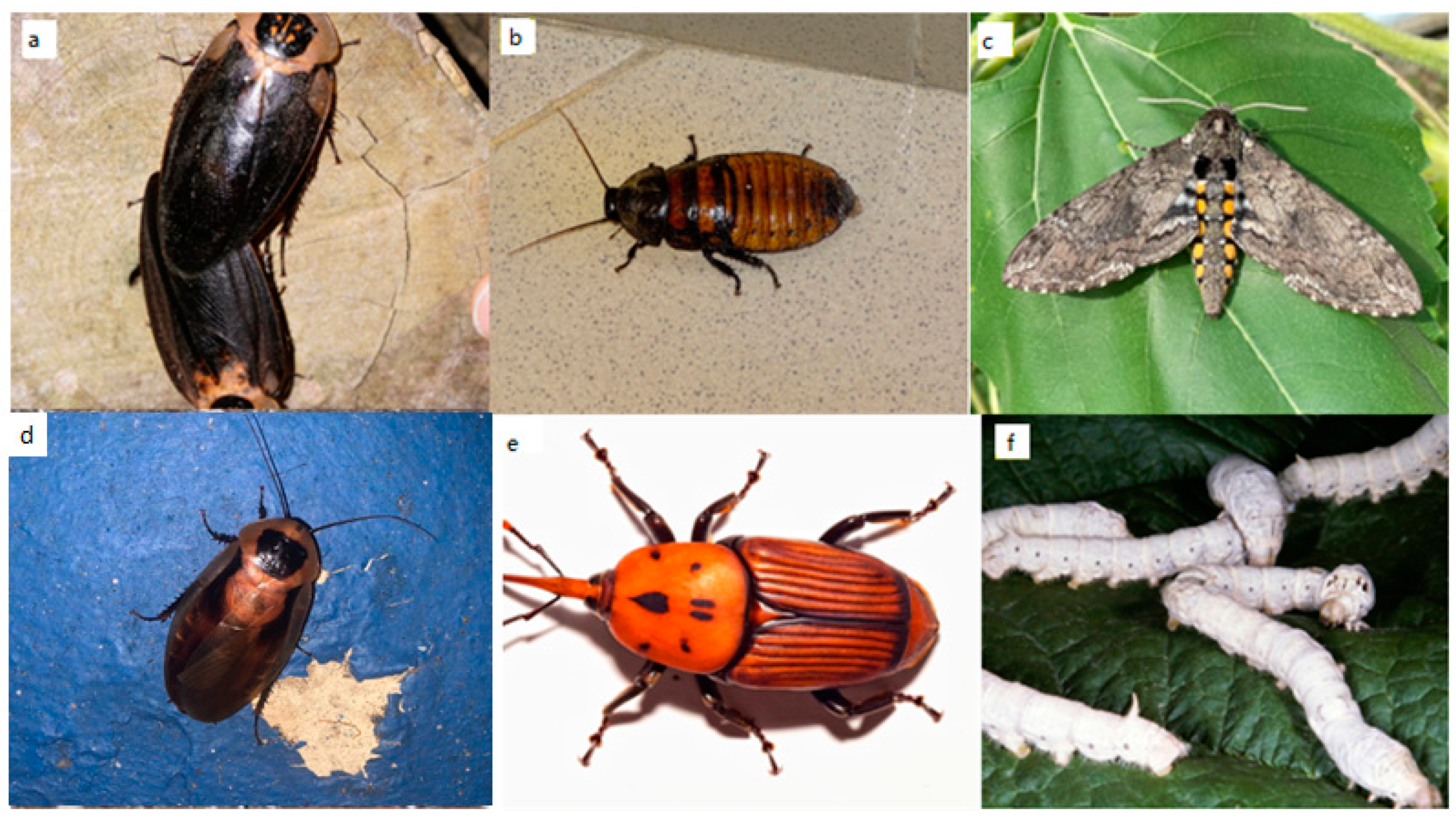
3.1.17. Shelfordella lateralis (Walker, 1868) (Blattodea, Blattidae)
3.1.18. Blaptica dubia (Serville, 1839) (Blattodea, Blaberidae)
3.1.19. Blaberus craniifer Burmeister, 1838 (Blattodea, Blaberidae)
3.2. Potential Risks of Non-Native Species Introduction for Livestock Feed and Exotic Pets’ Food in Other Regions
3.2.1. Africa and Middle East
Gromphadorhina portentosa (Schaum, 1853) (Blattodea, Blaberidae)
3.2.2. North America
Manduca sexta (Linnaeus, 1763) (Lepidoptera, Sphingidae)
Blaberus discoidalis Serville, 1838 (Blattodea, Blaberidae)
3.2.3. Asia and Oceania
Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera, Curculionidae)
Bombyx mori (Linnaeus, 1958) (Lepidoptera, Bombycidae)
Teleogryllus emma (Ohmachi & Matsuura 1951) (Orthoptera, Gryllidae)
3.2.4. South and Central America
Nauphoeta cinerea (Olivier, 1789) (Blattodea, Blaberidae)
Oxyhaloa deusta (Thunberg, 1784) (Blattodea, Blaberidae)
Ulomoides dermestoides (Chevrolat, 1878) (Coleoptera, Tenebrionidae)
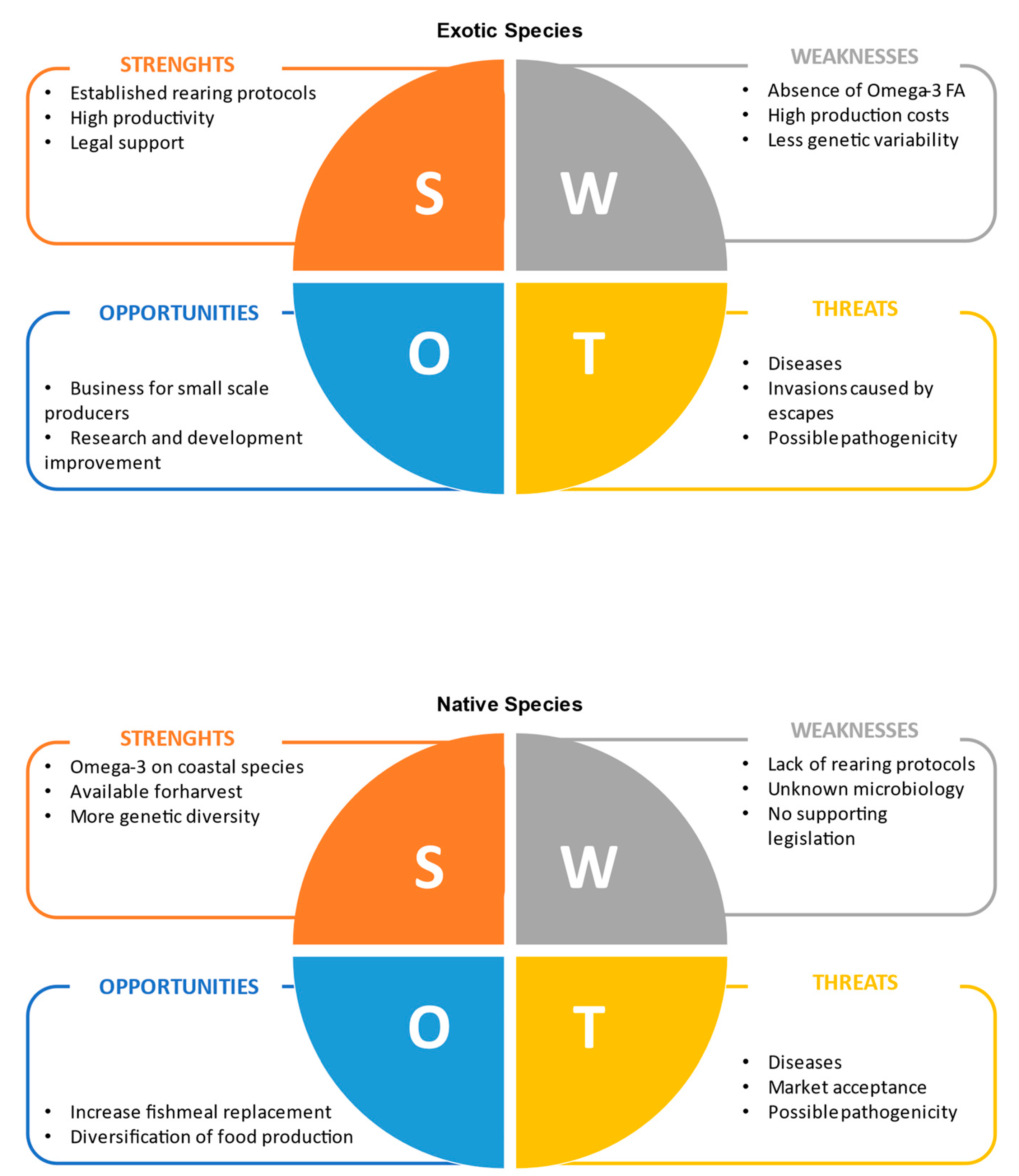
3.3. The Potential of Native Species
4. Native vs. Non-Native Species
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A




References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050 the 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012; Available online: https://ageconsearch.umn.edu/record/288998 (accessed on 9 May 2022).
- Food and Agriculture Organization of the United Nations. The State of the World’s Land and Water Resources for Food and Agriculture: Managing Systems at Risk; FAO: Rome, Italy; London, UK, 2011; Available online: https://www.fao.org/land-water/solaw2021/en/ (accessed on 9 May 2022).
- Lehuger, S.; Gabrielle, B.; Gagnaire, N. Environmental impact of the substitution of imported soybean meal with locally-produced rapeseed meal in dairy cow feed. J. Clean. Prod. 2009, 17, 616–624. [Google Scholar] [CrossRef]
- Prudêncio da Silva, V.; van der Werf, H.M.G.; Spies, A.; Soares, S.R. Variability in environmental impacts of Brazilian soybean according to crop production and transport scenarios. J. Environ. Manag. 2010, 91, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2016; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i5555e.pdf (accessed on 9 May 2022).
- Basset-Mens, C.; Van Der Werf, H.M.G. Scenario-based environmental assessment of farming systems: The case of pig production in France. Agric. Ecosyst. Environ. 2005, 105, 127–144. [Google Scholar] [CrossRef]
- Boggia, A.; Paolotti, L.; Castellini, C. Environmental impact evaluation of conventional, organic and organic-plus poultry production systems using life cycle assessment. World’s Poult. Sci. J. 2010, 66, 95–114. [Google Scholar] [CrossRef]
- Dourmad, J.Y.; Ryschawy, J.; Trousson, T.; Bonneau, M.; Gonzàlez, J.; Houwers, H.W.J.; Hviid, M.; Zimmer, C.; Nguyen, T.L.T.; Morgensen, L. Evaluating environmental impacts of contrasting pig farming systems with life cycle assessment. Animal 2014, 8, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Biermann, G.; Geist, J. Life cycle assessment of common carp (Cyprinus carpio L.)—A comparison of the environmental impacts of conventional and organic carp aquaculture in Germany. Aquaculture 2019, 501, 404–415. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 9789251075951. [Google Scholar]
- Roques, A.; Rabitsch, W.; Rasplus, J.-Y.; Lopez-Vaamonde, C.; Nentwig, W.; Kenis, M. Alien Terrestrial Invertebrates of Europe. In Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; Volume 569, pp. 63–79. ISBN 978-1-4020-8279-5. [Google Scholar]
- Kenis, M. Insects-Insecta. In An Inventory of Alien Species and their Threat to Biodiversity and Economy in Switzerland; Wittenberg, R., Ed.; Report to the Swiss Agency for Environment, Forests and Landscape; CABI Bioscience Switzerland Centre: Delémont, Switzerland, 2005; Volume 29, pp. 131–212. [Google Scholar]
- Cox, G.W. Alien Species and Evolution: The Evolutionary Ecology of Exotic Plants, Animals, Microbes, and Interacting Native Species; Island Press: Washington, DC, USA, 2004; ISBN 1-55963-008-6. [Google Scholar]
- Thompson, J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998, 13, 329–332. [Google Scholar] [CrossRef]
- Nayak, S.B.; Rao, K.S.; Ramalakshmi, V. Impact of Climate Change on Insect Pests and their Natural Enemies. Int. J. Ecol. Environ. Sci. 2020, 2, 579–584. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Ewel, J.J.; O’Dowd, D.J.; Bergelson, J.; Daehler, C.C.; D’Antonio, C.M.; Gómez, L.D.; Gordon, D.R.; Hobbs, R.J.; Holt, A.; Hopper, K.R.; et al. Deliberate Introductions of Species: Research Needs. Bioscience 1999, 49, 619–630. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Hauser, M. The historical spread of the Black Soldier Fly, Hermetia illucens (L.) (Diptera, Stratiomyidae, Hermetiinae), and its establishment in Canada. J. Entomol. Soc. Ontario 2015, 146, 51–54. [Google Scholar]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens Larvae Meal as a Supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- González, O.F.; Oliva, G.R. First report of intestinal myiasis caused by por Hermetia illucens (Diptera: Stratiomyidae)|Primer reporte en Cuba de miasis intestinal por Hermetia illucens (Diptera: Stratiomyidae). Rev. Cubana Med. Trop. 2009, 61, 97–99. [Google Scholar]
- Calderón-Arguedas, O.; Murillo Barrantes, J.; Solano, M.E. Miasis entérica por Hermetia illucens (Diptera: Stratiomyidae) en una paciente geriátrica de Costa Rica. Parasitol. Latinoam. 2005, 60, 162–164. [Google Scholar] [CrossRef]
- Lee, H.L.; Chandrawathani, P.; Wong, W.Y.; Tharam, S.; Lim, W.Y. A case of human enteric myiasis due to larvae of Hermetia illucens (Family: Stratiomyiadae): First report in Malaysia. Malays. J. Pathol. 1995, 17, 109–111. [Google Scholar]
- Mulieri, P.R.; Patitucci, L.D.; Scolaro, A. A Rare Case of Pseudomyiasis in a Dog by Hermetia illucens (Diptera: Stratiomyidae). J. Med. Entomol. 2019, 56, 1726–1728. [Google Scholar] [CrossRef]
- Hora, M. A northernmost European record of the alien black soldier fly Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae)/Nejsevernější evropský výskyt nepůvodní bráněnky Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae). Acta Musei Sil. Sci. Nat. 2013, 62, 101–106. [Google Scholar] [CrossRef]
- De Groot, M.; Veenvliet, P. Hermetia illucens L. (Diptera, Stratiomyidae), a new alien invasive species in Slovenia. Acta Entomol. Slov. 2011, 19, 195–198. [Google Scholar]
- Tsagkarakis, A.E.; Arapostathi, E.I.; Strouvalis, G.I. First record of the black soldier fly, Hermetia illucens, in Greece. Entomol. Hell. 2015, 24, 27–30. [Google Scholar] [CrossRef][Green Version]
- Gladun, V.V. The First Record of Hermetia illucens (DIPTERA, STRATIOMYIDAE) from Russia. Nat. Conserv. Res. 2019, 4, 111–113. [Google Scholar] [CrossRef]
- Hewitt, C.G. The House-Fly, Musca domestica Linn: Its Structure, Habits, Development, Relation to Disease and Control; Cambridge University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Sánchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Čičková, H.; Pastor, B.; Kozánek, M.; Martínez-Sánchez, A.; Rojo, S.; Takáč, P. Biodegradation of pig manure by the housefly, musca domestica: A viable ecological strategy for pig manure management. PLoS ONE 2012, 7, e32798. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 2009, 30, 609–614. Available online: http://www.jeb.co.in/index.php?page=issue_toc&issue=200907_jul09 (accessed on 6 May 2022). [PubMed]
- Ostrolenk, M.; Welch, H. The House Fly as a Vector of Food Poisoning Organisms in Food Producing Establishments. Am. J. Public Health Nations Health 1942, 32, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Szalanski, A.L.; Owens, C.B.; McKay, T.; Steelman, C.D. Detection of Campylobacter and Escherichia coli O157:H7 from filth flies by polymerase chain reaction. Med. Vet. Entomol. 2004, 18, 241–246. [Google Scholar] [CrossRef]
- Nazni, W.A.; Luke, H.; Rozita, W.M.W.; Abdullah, A.G.; Sa’Diyah, I.; Azahari, A.H.; Zamree, I.; Tan, S.B.; Lee, H.L.; Sofian-Azirun, M. Determination of the flight range and dispersal of the house fly, Musca domestica (L.) using mark release. Trop. Biomed. 2005, 22, 53–61. Available online: https://eprints.um.edu.my/5793/ (accessed on 6 May 2022).
- Baudry, E.; Viginier, B.; Veuille, M. Non-African populations of Drosophila melanogaster have a unique origin. Mol. Biol. Evol. 2004, 21, 1482–1491. [Google Scholar] [CrossRef][Green Version]
- Markow, T.A. The Natural History of Model Organisms: The secret lives of Drosophila flies. eLife Sci 2015, 4, e06793. [Google Scholar] [CrossRef]
- Fruit Flies Wingless Small. Available online: https://www.livefoodsdirect.co.uk/products/FFS1TUB/fruit-flies-wingless-small (accessed on 24 March 2022).
- Atkinson, W.; Shorrocks, B. Breeding site specificity in domestic species of Drosophila. Oecologia 1979, 29, 223–232. [Google Scholar] [CrossRef]
- Large Fruit Fly (D. hydei) Go-Large. Available online: https://www.advancedhusbandry.co.uk/drop-shipping/7-fruit-fly-drosophila-hydei-flightless.html (accessed on 24 March 2022).
- Harrison, R.G.; Bogdanowicz, S.M. Mitochondrial DNA phylogeny of North American field crickets: Perspectives on the evolution of life cycles, songs, and habitat associations. J. Evol. Biol. 1995, 8, 209–232. [Google Scholar] [CrossRef]
- Ferreira, M.; Ferguson, J.W.H. Do Mediterranean crickets Gryllus bimaculatus de Geer (Orthoptera: Gryllidae) come from the Mediterranean? Largescale phylogeography and regional gene flow. Bull. Entomol. Res. 2010, 100, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M. Evolutionary Implications of Variation in the Calling Song of the Cricket Gryllus bimaculatus De Geer (Orthoptera: Gryllidae). Ph.D. Thesis, University of Pretoria, Hatfield, South Africa, 2006. [Google Scholar]
- Exotic-pets.co.uk. Black Crickets. Available online: https://www.exotic-pets.co.uk/black-crickets.html (accessed on 24 March 2022).
- Taxonomy, G.B. Gryllus bimaculatus De Geer, 1773 in GBIF Secretariat. 2021. Available online: GBIF.org (accessed on 9 September 2021).
- Ahmad, D. Bionomics and Control of Gryllodes sigillatus Walker. Ph.D. Thesis, Aligarh Muslim University, Aligarh, India, 1965. [Google Scholar]
- Banded Brown Crickets. Available online: https://www.thepetexpress.co.uk/live-reptile-food/banded-brown-crickets-gryllodes-sigillatus-live-food/ (accessed on 24 March 2022).
- Hanson, P. Los insectos invasores de Costa Rica. Rev. Biocenosis 2009, 22, 1–2. Available online: https://revistas.uned.ac.cr/index.php/biocenosis/article/view/1255 (accessed on 8 May 2022).
- Taxonomy, G.B. Gryllodes sigillatus (Walker, 1869) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/1722299 (accessed on 4 May 2022).
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial dynamics during industrial rearing, processing, and storage of tropical house crickets (Gryllodes sigillatus) for human consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef]
- Weissman, D.B.; Walker, T.J.; Gray, D.A. The Field Cricket Gryllus assimilis and Two New Sister Species (Orthoptera: Gryllidae). Ann. Entomol. Soc. Am. 2009, 102, 367–380. [Google Scholar] [CrossRef]
- Weissman, D.B.; Gray, D.A.; Thi Pham, H.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88. [Google Scholar] [CrossRef]
- Alexander, R.D.; Walker, T.J. Two Introduced Field Crickets New to Eastern United States (Orthoptera: Gryllidae). Ann. Entomol. Soc. Am. 1962, 55, 90–94. [Google Scholar] [CrossRef]
- Barranco, P. El grillo de campo jamaicano, Gryllus assimilis (Fabricius, 1775), posible especie invasora para España (Orthoptera, Gryllidae). Boletín La SEA 2012, 55, 537–538. [Google Scholar]
- Tilami, S.K.; Turek, J.; Červený, D.; Lepič, P.; Kozák, P.; Burkina, V.; Sakalli, S.; Tomčala, A.; Sampels, S.; Mráz, J. Insect meal as a partial replacement for fish meal in a formulated diet for perch perca fluviatilis. Turkish J. Fish. Aquat. Sci. 2020, 20, 867–878. [Google Scholar] [CrossRef]
- Labbouz, Y. Use of House Cricket (Acheta domesticus) Meal as a New Source of Protein for Ruminants. Effects on Ruminal Fermentation, Degradation and Biohydrogenation. Master’s Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2021. [Google Scholar]
- Weissman, D.B.; Rentz, D.C.F.; Alexander, R.D.; Loher, W. Field crickets (Gryllus and Acheta) of California and Baja California, Mexico (Orthoptera: Gryllidae: Gryllinae). Trans. Am. Entomol. Soc. 1980, 106, 327–356. Available online: https://www.jstor.org/stable/25078267 (accessed on 8 May 2022).
- Bousquet, Y. Beetles Associated with Stored Products in Canada: An Identification Guide; Publication-Agriculture Canada: Ottawa, ON, Canada, 1990; ISBN 0660132664. Available online: http://esc-sec.ca/wp/wp-content/uploads/2017/03/AAFC_bousquet1990.pdf (accessed on 8 May 2022).
- Richards, O.W.; Davies, R.G. Imms’ General Textbook of Entomology: Volume 2: Classification and Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-94-017-0472-4. [Google Scholar]
- Mewis, I.; Ulrichs, C. Action of amorphous diatomaceous earth against different stages of the stored product pests Tribolium confusum, Tenebrio molitor, Sitophilus granarius and Plodia interpunctella. J. Stored Prod. Res. 2001, 37, 153–164. [Google Scholar] [CrossRef]
- Goptar, I.A.; Semashko, T.A.; Danilenko, S.A.; Lysogorskaya, E.N.; Oksenoit, E.S.; Zhuzhikov, D.P.; Belozersky, M.A.; Dunaevsky, Y.E.; Oppert, B.; Filippova, I.Y.; et al. Cysteine digestive peptidases function as post-glutamine cleaving enzymes in tenebrionid stored-product pests. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 148–154. [Google Scholar] [CrossRef]
- Guo, Z.; Pfohl, K.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Dissemination of Fusarium proliferatum by mealworm beetle Tenebrio molitor. PLoS ONE 2018, 13, e0204602. [Google Scholar] [CrossRef] [PubMed]
- Timuș, A. The invasive coleopterofauna for Republic of Moldova. Curr. Trends Nat. Sci. 2015, 4, 41–49. [Google Scholar]
- Tortuga-Petshop. Zophoba. Available online: https://tortuga-petshop.com/index.php?id_product=42&id_product_attribute=0&rewrite=zophoba&controller=product (accessed on 4 May 2022).
- WaxwormsUK. Morio Worms. Available online: https://www.waxwormsuk.com/morio-worms (accessed on 4 May 2022).
- Leung, D.; Yang, D.; Li, Z.; Zhao, Z.; Chen, J.; Zhu, L. Biodiesel from Zophobas morio larva oil: Process optimization and FAME characterization. Ind. Eng. Chem. Res. 2012, 51, 1036–1040. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. The superworm, Zophobas morio (Coleoptera:Tenebrionidae): A ‘sleeping giant’ in nutrient sources. J. Insect Sci. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Urbanek, A.; Pietrzak, A.; Naczk, A.M.; Bojke, A.; Tkaczuk, C.; Stepnowski, P. Effects of the entomopathogenic fungus Metarhizium flavoviride on the fat body lipid composition of Zophobas morio larvae (Coleoptera: Tenebrionidae). Sci. Nat. 2020, 107, 1–11. [Google Scholar] [CrossRef]
- Tran, B.M.D.; Credland, P.F. Consequences of inbreeding for the cowpea seed beetle, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Biol. J. Linn. Soc. 1995, 56, 483–503. [Google Scholar] [CrossRef]
- Beck, C.W.; Blumer, L.S. A Handbook on Bean Beetles, Callosobruchus Maculatus; National Science Foundation: Virginia, VA, USA, 2011; Available online: https://www.beanbeetle.org/handbook/ (accessed on 6 May 2022).
- Bean Weevil Cultures. Available online: https://www.exotic-pets.co.uk/bean-weevil.html (accessed on 24 March 2022).
- Kalpna; Hajam, Y.A.; Kumar, R. Management of stored grain pest with special reference to Callosobruchus maculatus, a major pest of cowpea: A review. Heliyon 2022, 8, e08703. [Google Scholar] [CrossRef]
- Loganathan, M.; Jayas, D.S.; Fields, P.G.; White, N.D.G. Low and high temperatures for the control of cowpea beetle, callosobruchus maculatus (F.) (coleoptera: Bruchidae) in chickpeas. J. Stored Prod. Res. 2011, 47, 244–248. [Google Scholar] [CrossRef]
- Desroches, P.; Mandon, N.; Baehr, J.C.; Huignard, J. Mediation of host-plant use by a glucoside in Callosobruchus maculatus F. (Coleoptera: Bruchidae). J. Insect Physiol. 1997, 43, 439–446. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Callosobruchus maculatus (Fabricius, 1775) in GBIF Secretariat (2021). Available online: https://www.gbif.org/species/1047343 (accessed on 9 September 2021).
- Zampetti, M.F. Fauna Europaea: Chrysomelidae. In Fauna Europaea: Coleoptera, Bettles; Audisio, O., Ed.; Fauna Europaea version 2017.06; Museum für Naturkunde: Berlin, Germany, 2013; Available online: https://fauna-eu.org (accessed on 4 May 2022).
- Rigout, J. The Beetles of the World. Volume 9: The Cetoniini; Science Nat: Venette, France, 1989. [Google Scholar]
- Cazemier, A.E.; Den Camp, H.J.M.O.; Hackstein, J.H.P.; Vogels, G.D. Fibre digestion in arthropods. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 101–109. [Google Scholar] [CrossRef]
- Pachnoda marginata Marginata (RCA). Available online: https://www.bughouse.be/Pachnoda-marginata-marginata-(RCA)-# (accessed on 24 March 2022).
- EvolutionReptiles. Sun Bettle. Available online: https://www.evolutionreptiles.co.uk/animals/invertebrates/beetles/sun-beetle/ (accessed on 3 May 2022).
- Mohemed Osman, S.E.; Hamid, H.A.; Ahmed Ishag, A.E.S.; Salim Eisa, M.A. Survey and level of infestation of Pachnoda interrupta (Oliver) (Coleoptera: Scarabaeidae) in Gedarif State. Int. J. Eng. Appl. Sci. Technol. 2020, 4, 73–77. [Google Scholar] [CrossRef]
- Wolde-Hawariat, Y.; Seyoum, E.; Jembere, B.; Negash, M.; Hansson, B.S.; Hillbur, Y. Behavioural and electrophysiological response of sorghum chafer Pachnoda interrupta (Coleoptera: Scarabaeidae) to plant compounds. Int. J. Trop. Insect Sci. 2007, 27, 53–61. [Google Scholar] [CrossRef]
- Geden, C.J.; Hogsette, J. Research and Extension Needs for Integrated Pest Management for Arthropods of Veterinary Importance. In Proceedings of the Workshop in Lincoln, Lincoln, NE, USA, 12–14 April 1994; Available online: https://digitalcommons.unl.edu/usdaarsfacpub/1039/ (accessed on 6 May 2022).
- Buffalo Worms. Available online: https://www.exotic-pets.co.uk/buffalo-worms.html (accessed on 24 March 2022).
- Vaughan, J.A.; Turner, E.C.; Ruszler, P.L. Infestation and Damage of Poultry House Insulation by the Lesser Mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 1984, 63, 1094–1100. [Google Scholar] [CrossRef]
- Geden, C.J.; Axtell, R.C. Factors affecting climbing and tunneling behavior of the lesser mealworm (Coleoptera: Tenebrionidae). J. Econ. Entomol. 1987, 80, 1197–1204. [Google Scholar] [CrossRef]
- McAllister, J.C.; Steelman, C.D.; Newberry, L.A.; Skeeles, J.K. Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 1995, 74, 45–49. [Google Scholar] [CrossRef]
- Despins, J.L.; Axtell, R.C.; Rives, D.V.; Guy, J.S.; Ficken, M.D. Transmission of Enteric Pathogens of Turkeys by Darkling Beetle Larva (Alphitobius diaperinus). J. Appl. Poult. Res. 1994, 3, 61–65. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Waltman, W.D. Transmission of Eimeria, viruses, and bacteria to chicks: Darkling beetles (Alphitobius diaperinus) as vectors of pathogens. J. Appl. Poult. Res. 1996, 5, 51–55. [Google Scholar] [CrossRef]
- Watson, D.W.; Guy, J.S.; Stringham, S.M. Limited transmission of turkey coronavirus in young turkeys by adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 2000, 37, 480–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McAllister, J.C.; Steelman, C.D.; Skeeles, J.K. Reservoir competence of the lesser mealworm (Coleoptera: Tenebrionidae) for Salmonella typhimurium (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 1994, 31, 369–372. [Google Scholar] [CrossRef] [PubMed]
- McAllister, J.C.; Steelman, C.D.; Skeeles, J.K.; Newberry, L.A.; Gbur, E.E. Reservoir Competence of Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 1996, 33, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, J.C.; Grant, W.E.; Hunter, D.M.; Milner, R.J. Habitat and environmental factors influencing the control of migratory locusts (Locusta migratoria) with an entomopathogenic fungus (Metarhizium anisopliae). Ecol. Modell. 2001, 136, 223–236. [Google Scholar] [CrossRef]
- Tokuda, M.; Tanaka, S.; Zhu, D.H. Multiple origins of Locusta migratoria (Orthoptera: Acrididae) in the Japanese Archipelago and the presence of two major clades in the world: Evidence from a molecular approach. Biol. J. Linn. Soc. 2010, 99, 570–581. [Google Scholar] [CrossRef]
- Werning, H. Egyptian Walking Grasshoppers (Locusta migratoria). Available online: https://www.bugs-international.com/en-insekten-wanderheuschrecken.html (accessed on 24 March 2022).
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Song, H. On the origin of the desert locust Schistocerca gregaria (Forskål) (Orthoptera: Acrididae: Cyrtacanthacridinae). Proc. R. Soc. B Biol. Sci. 2004, 271, 1641–1648. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- FAO. Desert Locust. Available online: www.fao.org/locusts/en (accessed on 20 January 2022).
- Editorial. A lack of locust preparedness will cost lives. Nature 2020, 579, 174. [Google Scholar] [CrossRef]
- Roussi, A. The battle to contain gigantic locust swarms. Nature 2020, 579, 330. [Google Scholar] [CrossRef]
- Green, J. The Unique Challenges of Responding to Desert Locust Outbreaks. Available online: https://entomologytoday.org/2022/01/11/unique-challenges-integrated-pest-management-desert-locust-outbreaks/ (accessed on 20 January 2022).
- Paddock, F.B. The Chronological Distribution of the Beemoth. J. Econ. Entomol. 1926, 19, 136–141. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’Amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The biology and control of the greater wax moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.; Edwards, J.; Brown, J.; Dixon, R. Galleria mellonella infection model identifies both high and low lethality of clostridium perfringens toxigenic strains and their response to antimicrobials. Front. Microbiol. 2019, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Live Wax Moth Larvae WaxWorms. Available online: https://www.livefoods.co.uk/live-wax-moth-larvae-waxworms-15g-galleria-mellonella-p-50.html (accessed on 24 March 2022).
- Réjasse, A.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Crappart, N.; Sandt, C. Plastic biodegradation: Do Galleria mellonella larvae—Bio-assimilate polyethylene? A spectral histology approach using isotopic labelling and infrared microspectroscopy. Environ. Sci. Technol. 2021, 56, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Shelfordella lateralis “Turkestan Cockroach”. Available online: https://insektenliebe.com/en/shop/shelfordella-lateralis-turkestan-cockroach/ (accessed on 24 March 2022).
- Red Runner Roaches. Available online: https://www.tcinsects.com/product/1-4-live-red-runner-roaches-for-sale/ (accessed on 24 March 2022).
- Alesho, N.A. Synathropic cockroaches of Russia. In International Colloquia on Social Insects: Volume 3–4.; Socium, St.: Petersburg, Russia, 1997; pp. 45–50. [Google Scholar]
- Memona, H.; Manzoor, F.; Riaz, S. Species diversity and distributional pattern of cockroaches in Lahore, Pakistan. J. Arthropod. Borne. Dis. 2017, 11, 249–259. Available online: https://pubmed.ncbi.nlm.nih.gov/29062850/ (accessed on 8 May 2022).
- Davranoglou, L.; Hadjiconstantis, M. First record of the Turkestan cockroach (Shelfordella lateralis) from Cyprus and Turkey (Dictyoptera: Blattidae) First record of the Turkestan cockroach (Shelfordella lateralis) from Cyprus and Turkey (Dictyoptera: Blattidae). Isr. J. Entomol. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- Alamer, A.H. Endocrine Control of Fat Body Composition and Effects of the Insect Growth Regulators Methoprene and Pyriproxyfen on the Development and Reproduction of the Argentinian Cockroach, Blaptica dubia Serville (Blattaria: Blaberidae). Ph.D. Thesis, Universität Bayreuth, Bayreuth, Germany, 2013. [Google Scholar]
- Wu, H.; Hu, X.P.; Appel, A.G. Temperature-Dependent Development and Thermal Sensitivity of Blaptica dubia (Blattodea: Blaberidae). J. Econ. Entomol. 2017, 110, 546–551. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Blaberus Craniifer Burmeister, 1838 in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/5150233 (accessed on 9 September 2021).
- Beccaloni, G.W. Cockroach Species File Online. Version 5.0/5.0. Available online: http://blattodea.speciesfile.org (accessed on 9 September 2021).
- “Death’s Head” Roaches: 50 Mixed Nymphs (No Adults). Available online: https://royalroaches.com/shop-1/ols/products/deaths-head-roaches-10-mixed-nymphs-no-adults (accessed on 24 March 2022).
- Deaths Head Cockroach. Available online: https://www.exotic-pets.co.uk/deaths-head-cockroach.html (accessed on 24 March 2022).
- Kulma, M.; Kouřimská, L.; Homolková, D.; Božik, M.; Plachý, V.; Vrabec, V. Effect of developmental stage on the nutritional value of edible insects. A case study with Blaberus craniifer and Zophobas morio. J. Food Compos. Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Babarinde, S.A.; Mvumi, B.M.; Babarinde, G.O.; Manditsera, F.A.; Akande, T.O.; Adepoju, A.A. Insects in food and feed systems in sub-Saharan Africa: The untapped potentials. Int. J. Trop. Insect Sci. 2021, 41, 1923–1951. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Namulawa, V.; Mbabazi, D.; Mugerwa, S.; Fuuna, P.; Nampijja, Z.; Ekesi, S.; Fiaboe, K.K.M.; Nakimbugwe, D. Use of insects for fish and poultry compound feed in sub-Saharan Africa—A systematic review. J. Insects Food Feed 2017, 3, 289–302. [Google Scholar] [CrossRef]
- Ostadi, Y.; Yavari, G.; Fadaei, M.S.; Ahmadian, M.; Imani, S. Challenges in breeding and consumption of insects as feed and food in Iran. J. Human Health Halal Metrics 2020, 1, 37–46. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Gromphadorhina portentosa (Schaum, 1853) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/1994576 (accessed on 9 September 2021).
- Nelson, M.C. Sound production in the cockroach, Gromphadorhina portentosa: The sound-producing apparatus. J. Comp. Physiol. 1979, 132, 27–38. [Google Scholar] [CrossRef]
- Mulder, P.; Shufran, A. Madagascar Hissing Cockroaches: Information and Care. Available online: https://extension.okstate.edu/fact-sheets/madagascar-hissing-cockroaches-information-and-care.html (accessed on 24 March 2022).
- Work, T.T.; McCullough, D.G.; Cavey, J.F.; Komsa, R. Arrival rate of nonindigenous insect species into the United States through foreign trade. Biol. Invasions 2005, 7, 323–332. [Google Scholar] [CrossRef]
- Haack, R.A. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Dunford, J.C.; Young, D.K. An Annotated Checklist of Wisconsin Darkling Beetles (Coleoptera: Tenebrionidae) with Comparisons to the Western Great Lakes Fauna. Trans. Am. Entomol. Soc. 2004, 130, 57–76. Available online: https://www.jstor.org/stable/25078836 (accessed on 6 May 2022).
- Olson, C.A. Blatta (Shelfordella) lateralis, the Turkestan Cockroach (Blattoidea: Blattidae) Recorded from Arizona. Bull. Entomol. Soc. Am. 1985, 31, 30. [Google Scholar] [CrossRef]
- Del Campo, C.M.L.; Renwick, J.A.A. Dependence on host constituents controlling food acceptance by Manduca sexta larvae. Entomol. Exp. Appl. 1999, 93, 209–215. [Google Scholar] [CrossRef]
- Whitney Cranshaw Hornworms and Sphinx Moths. In Garden Insects of North America: The Ultimate Guide to Backyard Bugs; Princeton University Press: Princeton, NJ, USA, 2004; pp. 146–149.
- Hornworms for Sale. Available online: https://reptilesupply.com/collections/hornworms-for-sale (accessed on 24 March 2022).
- Kingsolver, J.G.; Nagle, A. Evolutionary divergence in thermal sensitivity and diapause of field and laboratory populations of Manduca sexta. Physiol. Biochem. Zool. 2007, 80, 473–479. [Google Scholar] [CrossRef]
- Beccaloni, G.W. Species Blaberus discoidalis Serville. 1838. Available online: http://cockroach.speciesfile.org/Common/basic/Taxa.aspx?TaxonNameID=1174169 (accessed on 2 February 2022).
- Florida State. Introduction, Possession or Movement of Arthropods, Biological Control Agents, Plant Pests, Noxious Weeds, and Invasive Plants, Regulated by the Department, United States. 2020. Available online: https://www.flrules.org/gateway/ChapterHome.asp?Chapter=5b-57 (accessed on 6 May 2022).
- Steltz, E.E. Redesign of the Micromechanical Flying Insect in a Power Density Context. Ph.D. Thesis, Univeristy of California, Berkeley, CA, USA, 2008. [Google Scholar]
- Goode, M.L. Effects of Thermal Acclimation on the Critical Thermal Maxima of the Tropical Cockroaches: Blaptica dubia, Eublaberus Posticus and Blaberus discoidalis (Blaberidae). Master’s Thesis, Eastern Kentucky University, Richmond, KY, USA, 2013. Available online: https://encompass.eku.edu/etd/171/ (accessed on 6 May 2022).
- Yen, A.L. Insects as food and feed in the Asia Pacific region: Current perspectives and future directions. J. Insects Food Feed 2015, 1, 33–55. [Google Scholar] [CrossRef]
- Xu, H.; Ding, H.; Li, M.; Qiang, S.; Guo, J.; Han, Z.; Huang, Z.; Sun, H.; He, S.; Wu, H.; et al. The distribution and economic losses of alien species invasion to China. Biol. Invasions 2006, 8, 1495–1500. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-Legged Livestock: Edible Insect Farming, Collecting and Marketing in Thailand; RAP Publication 3: Bangkok, Thailand, 2013; Available online: https://www.fao.org/publications/card/en/c/76e0a383-3ca0-5a7c-8d5a-b3a4262b857f/ (accessed on 6 May 2022).
- EPPO Rhynchophorus ferrugineus. EPPO Datasheets on Pests Recommended for Regulation. Available online: https://gd.eppo.int (accessed on 22 February 2021).
- Abraham, V.A.; Shuaibi, M.A.A.; Faleiro, J.R.; Abozuhairah, R.A.; Vidyasagar, P.S.P.V. An Integrated Management Approach for Red Palm Weevil Rhynchophorus ferrugineus Oliv. a Key Pest of Date Palm in the Middle East. J. Agric. Mar. Sci. JAMS 1998, 3, 77–83. Available online: https://journals.squ.edu.om/index.php/jams/article/view/522/525 (accessed on 6 May 2022). [CrossRef]
- Leatemia, J.A.; Patty, J.A.; Masauna, E.D.; Noya, S.H.; Hasinu, J.V. Utilization of sago grub (Rhynchophorus ferrugineus Olivier) (Coleoptera: Curculionidae) as an alternative source of protein. IOP Conf. Ser. Earth Environ. Sci. 2021, 800, 012028. [Google Scholar] [CrossRef]
- Barber, E.J.W. Prehistoric Textiles: The Development of Cloth in the Neolithic and Bronze Ages with Special Reference to the Aegean; Princeton University Press: Princeton, NJ, USA, 1992; ISBN 9780691002248. [Google Scholar]
- Wei, Z.J.; Liao, A.M.; Zhang, H.X.; Liu, J.; Jiang, S.T. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresour. Technol. 2009, 100, 4214–4219. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.U. Chemical Composition and Nutritional Evaluation of Spent Silk Worm Pupae. J. Agric. Food Chem. 1994, 42, 2201–2203. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.A.; Liang, Y.; Wang, M. Process optimization for the enrichment of α-linolenic acid from silkworm pupal oil using response surface methodology. Afr. J. Biotechnol. 2010, 9, 2956–2964. [Google Scholar] [CrossRef]
- Popescu, A. Trends in world silk cocoons and silk production and trade, 2007–2010. Anim. Sci. Biotechnol. 2013, 46, 418–423. [Google Scholar]
- File, O.S. Teleogryllus emma (Ohmachi & Matsuura, 1951) in Cigliano M M (2019). Available online: https://www.gbif.org/species/100538918 (accessed on 11 September 2021).
- Mazaki, S. Adaptation to local climatic conditions in the Emma field cricket (Orthoptera: Gryllidae). Entomol. Soc. Jpn. 1963, 31, 249–260. [Google Scholar]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia. Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Kim, N.-J.; Hong, S.-J.; Seol, K.-Y.; Kim, S.-H.; Ahn, N.-H.; Kim, M.-A. Effect of Temperature on Development and Reproduction of the Emma Field Cricket, Teleogryllus emma (Orthoptera: Gryllidae). Int. J. Ind. Entomol. 2007, 15, 69–73. Available online: https://www.koreascience.or.kr/article/JAKO200735822452285.page (accessed on 6 May 2022).
- Lee, E.; Ohseok, K. The Effect of Invasive Cricket Species, Gryllus bimaculatus on the Survival of Korean Cricket Species, Teleogryllus emma. Korean J. Ecol. Environ. 2013, 46, 67–74. [Google Scholar] [CrossRef]
- IBGE PAM 2020: Valor da Produção Agrícola Nacional Cresce 30,4% e Chega a R$ 470,5 Bilhões, Recorde da Série. Available online: https://agenciadenoticias.ibge.gov.br/agencia-sala-de-imprensa/2013-agencia-de-noticias/releases/31672-pam-2020-valor-da-producao-agricola-nacional-cresce-30-4-e-chega-a-r-470-5-bilhoes-recorde-da-serie (accessed on 23 March 2022).
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Chua, T.H.; Chandrapal, R. The influence of restricted food supplies on the development of larvae and on the fecundity of Palembus dermestoides fairn. (Tenebrionidae). J. Stored Prod. Res. 1978, 14, 81–86. [Google Scholar] [CrossRef]
- Rehn, J.A.G. Man’s Uninvited Fellow Traveler-The Cockroach. Sci. Mon. 1945, 61, 265–276. Available online: https://www.jstor.org/stable/18346 (accessed on 6 May 2022).
- Schimpf, N.G.; Matthews, P.G.D.; White, C.R. Discontinuous gas exchange exhibition is a heritable trait in speckled cockroaches Nauphoeta cinerea. J. Evol. Biol. 2013, 26, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, E.J.; Bywater, C.L.; White, C.R. The effect of ambient oxygen on the thermal performance of a cockroach, Nauphoeta cinerea. J. Exp. Biol. 2020, 223, jeb208306. [Google Scholar] [CrossRef] [PubMed]
- Greven, H.; Herrmann, J.; Kiwitt, G. Supplementary Observations on the Reproduction of the Viviparous Cockroach Oxyhaloa deusta (Thunberg, 1784) with Some Notes on its Locomotory Activity (Blaberidae: Oxyhaloinae). Entomol. Heute 2019, 31, 1–24. [Google Scholar]
- Wormblog. Red Head Roach Care Sheet. Available online: https://wormman.blog/red-head-roach-care-sheet/ (accessed on 24 March 2022).
- Ozymandias Feeder Roaches: Care and Breeding. Available online: https://www.roachforum.com/topic/3029-feeder-roaches-care-and-breeding/ (accessed on 3 May 2022).
- Blattodea313. Oxyhaloa Deusta Housing. Available online: https://www.roachforum.com/topic/4632-oxyhaloa-deusta-housing/ (accessed on 3 May 2022).
- Fenilli, R. Life Cycle, Morphology and Effects of Gamma Radiation (60 Co) on Adults of Palembus dermestoides (Fairmaire, 1893) (Coleoptera, Tenebrionidae). Ph.D. Thesis, USP, São Paulo, Brazil, 1982. [Google Scholar]
- Stumpf, I.V.K.; Luz, E.; Tonin, V.R. Infecção experimental de Palembus dermestoides por larvas de Macracanthorhynchus hirudinaceus. Acta Biológica Parana. 1990, 19, 45–49. [Google Scholar] [CrossRef]
- Adler, A.I.; Brancato, F.P. Human furuncular myiasis caused by Hermetia illucens (Diptera: Stratiomyidae). J. Med. Entomol. 1995, 32, 745–746. [Google Scholar] [CrossRef]
- Harwood, P.D.; Meleney, H.E. Human Intestinal Myiasis Due to the Larvae of the Soldier Fly, Hermetia illucens Linné (Diptera, Stratiomyidae). Am. J. Trop. Med. Hyg. 1935, 1, 45–49. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Magaña, C.; Saloña, M.; Rojo, S. First record of Hermetia illucens (Diptera: Stratiomyidae) on human corpses in Iberian Peninsula. Forensic Sci. Int. 2011, 206, 2010–2012. [Google Scholar] [CrossRef]
- Banga, K.S.; Kumar, S.; Kotwaliwale, N.; Mohapatra, D. Major insects of stored food grains. Int. J. Chem. Stud. 2020, 8, 2380–2384. [Google Scholar] [CrossRef]
- Mackauer, M. Genetic aspects of insect production. Entomophaga 1972, 17, 27–48. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Bell, J.G.; Ghioni, C.; Sargen, J.R. Fatty acid composition of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar): A comparison with commercial diets. Aquaculture 1994, 128, 301–313. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia. Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Prado e Castro, C.; Ameixa, O.M.C.C. Blow flies (Diptera: Calliphoridae) promising candidates as animal feed ingredients. J. Insects Food Feed 2021, 7, 1065–1076. [Google Scholar] [CrossRef]
- Sing, K.W.; Kamarudin, M.S.; Wilson, J.J.; Sofian-Azirun, M. Evaluation of blowfly (Chrysomya megacephala) maggot meal as an effective, sustainable replacement for fishmeal in the diet of farmed juvenile red tilapia (Oreochromis sp.). Pak. Vet. J. 2014, 34, 288–292. [Google Scholar]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Uyttenbroeck, R.; Malik, P.; Filocco, S.; Richel, A.; Heuskin, S.; Alabi, T.; Megido, R.C.; Franck, T.; et al. Nutritional composition and rearing potential of the meadow grasshopper (Chorthippus parallelus Zetterstedt). J. Asia. Pac. Entomol. 2016, 19, 1111–1116. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Lucilia Sericata (Meigen, 1826) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/5063973 (accessed on 13 September 2021).
- Hall, M.; Wall, R. Myiasis of Humans and Domestic Animals. Adv. Parasitol. 1995, 35, 257–334. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Megu, K.; Jung, C.; Meyer-Rochow, V.B. Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymenoptera: Formicidae) and Odontotermes sp. (Isoptera: Termitidae): Two preferred edible insects of Arunachal Pradesh, India. J. Asia. Pac. Entomol. 2016, 19, 711–720. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos-Bueno, R.P.; González-Fernandez, M.J.; Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G. Insects as Food: Fatty Acid Profiles, Lipid Classes, and sn-2 Fatty Acid Distribution of Lepidoptera Larvae. Eur. J. Lipid Sci. Technol. 2018, 120, 1700391. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Aphodius Rufipes (Linnaeus, 1758) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/8321792 (accessed on 13 September 2021).
- Oriolowo, O.B.; John, O.J.; Mohammed, U.B.; Joshua, D. Amino acids profile of catfish, crayfish and larva of edible dung beetle. Ife J. Sci. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Cladomorphus phyllinus G.R.Gray, 1835 in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/1413211 (accessed on 13 September 2021).
- Botton, V.; Chiarello, L.M.; Klunk, G.A.; Marin, D.; Curbani, L.; Gonçalves, M.J.; Vitorino, M.D. Evaluation of nutritional composition and ecotoxicity of the stick insect Cladomorphus phyllinum. Eur. Food Res. Technol. 2020, 247, 605–611. [Google Scholar] [CrossRef]
- File, O.S. Acrida cinerea (Thunberg, 1815) in Cigliano M M (2019). Available online: https://www.gbif.org/species/5099473 (accessed on 13 September 2021).
- Wang, D.; Zhai, S.W.; Zhang, C.X.; Zhang, Q.; Chen, H. Nutrition value of the Chinese grasshopper Acrida cinerea (Thunberg) for broilers. Anim. Feed Sci. Technol. 2007, 135, 66–74. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File Version 5.0/5.0. Available online: http://orthoptera.speciesfile.org/HomePage/Orthoptera/HomePage.aspx (accessed on 6 May 2022).
- Finke, M.D.; Sunde, M.L.; DeFoliart, G.R. An Evaluation of the Protein Quality of Mormon Crickets (Anabrus Simplex Haldeman) When Used as a High Protein Feedstuff for Poultry. Poult. Sci. 1985, 64, 708–712. [Google Scholar] [CrossRef]
- Wang, D.; Shao, W.Z.; Chuan, X.Z.; Yao, Y.B.; Shi, H.A.; Ying, N.X. Evaluation on nutritional value of field crickets as a poultry feedstuff. Asian-Australas. J. Anim. Sci. 2005, 18, 667–670. [Google Scholar] [CrossRef]
- Taxonomy, G.B. Zonocerus variegatus (Linnaeus, 1758) in GBIF Secretariat (2021). Available online: https://www.gbif.org/species/1727818 (accessed on 13 September 2021).
- Alegbeleye, W.O.; Obasa, S.O.; Olude, O.O.; Otubu, K.; Jimoh, W. Preliminary evaluation of the nutritive value of the variegated grasshopper (Zonocerus variegatus L.) for African catfish Clarias gariepinus (Burchell. 1822) fingerlings. Aquac. Res. 2012, 43, 412–420. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Recorpinc Butterworms. Available online: http://www.recorpinc.com/butterworms.php (accessed on 7 May 2022).
- Topflightdubia Butterworms. Available online: https://www.topflightdubia.com/butterworms (accessed on 7 May 2022).
- Iriarte, J.A.; Feinsinger, P.; Jaksic, F.M. Trends in wildilife use and trade in Chile. Biol. Conserv. 1997, 81, 9–20. [Google Scholar] [CrossRef]
- Ureta, E. Revisión de la familia Cossidae en Chile. Bol. Mus. Nac. Hist. Nat. 1957, 27, 129–153. Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/7999 (accessed on 6 May 2022).
- Duarte, P.M.; Maciel, E.; Pinho, M.; Domingues, M.R.; Calado, R.; Lillebø, A.I.; Ameixa, O.M.C.C. Omega-3 on the fly: Long-legged fly Machaerium maritimae as a potential source of eicosapentaenoic acid for aquafeeds. J. Insects Food Feed 2021, 7, 1089–1100. [Google Scholar] [CrossRef]
- Committee, E.S. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 60. [Google Scholar] [CrossRef]
- Montanari, F.; Pinto de Moura, A.; Cunha, L.M. Production and Commercialization of Insects as Food and Feed; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–39. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO Commission on Genetic Resources for Food and Agriculture Assessments; Scherf, B.D., Pilling, D., Eds.; FAO: Rome, Italy, 2015. [Google Scholar]
- Premrov Bajuk, B.; Zrimšek, P.; Kotnik, T.; Leonardi, A.; Križaj, I.; Jakovac Strajn, B. Insect protein-based diet as potential risk of allergy in dogs. Animals 2021, 11, 1942. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1–12. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Šipoš, J.; Burda, M.; Soares, A.M.V.M.; Soares, A.O. Factors influencing the introduction and spread of Harmonia axyridis in the Iberian Peninsula. Biol. Invasions 2019, 21, 323–331. [Google Scholar] [CrossRef]
- Hill, M.P.; Clusella-Trullas, S.; Terblanche, J.S.; Richardson, D.M. Drivers, impacts, mechanisms and adaptation in insect invasions. Biol. Invasions 2016, 18, 883–891. [Google Scholar] [CrossRef]
- Peden, D. Hermetia illucens. Public Domain. Available online: https://www.inaturalist.org/photos/194457135 (accessed on 5 May 2022).
- Lucas, B. Musca domestica. CC-BY-NC. Available online: https://www.inaturalist.org/observations/27665746 (accessed on 24 March 2022).
- Rorabaugh, J. Drosophila melanogaster. CC-BY-NC. Available online: https://inaturalist.ca/photos/14360123 (accessed on 24 March 2022).
- Bailey, J. Drosophila hydei. CC-BY-NC. Available online: https://www.inaturalist.org/observations/8441616 (accessed on 24 March 2022).
- Pavlou, V. Gryllus bimaculatus. CC-BY-NC. Available online: https://www.inaturalist.org/observations/109283434 (accessed on 24 March 2022).
- Fortnash, J. Gryllodes sigillatus. Public Domain. Available online: https://www.inaturalist.org/photos/186351772 (accessed on 24 March 2022).
- Hospers, A. Gryllus assimilis. CC-BY. Available online: https://www.inaturalist.org/photos/23333074 (accessed on 24 March 2022).
- Mallory, C. Acheta domesticus. CC-BY-NC. Available online: https://www.inaturalist.org/observations/5277240 (accessed on 24 March 2022).
- Oikonen, V. Tenebrio molitor. Public Domain. Available online: https://www.inaturalist.org/observations/84739305 (accessed on 24 March 2022).
- Gratwicke, B. Zophobas morio. CC-BY-2.0. Available online: https://www.flickr.com/photos/briangratwicke/5414327015/in/photolist-9frQMM-9frR5e (accessed on 25 March 2022).
- Zafeiriou, S. Callosobruchus maculatus. CC-BY-NC. Available online: https://www.inaturalist.org/observations/68570028 (accessed on 25 March 2022).
- Schulting, S. Pachnoda marginata. CC-BY-NC. Available online: https://inaturalist.ca/photos/54808258 (accessed on 25 March 2022).
- Legrand, M. Lou Alphitobius diaperinus. CC-BY-NC. Available online: https://inaturalist.ca/observations/49949068 (accessed on 25 March 2022).
- Baquero, E. Locusta migratoria. CC-BY-NC. Available online: https://www.inaturalist.org/observations/4314983 (accessed on 25 March 2022).
- Habib, A. Schistocerca gregaria. CC-BY-NC. Available online: https://www.inaturalist.org/observations/20990673 (accessed on 25 March 2022).
- Sanctuary, E. Galleria mellonella. CC-BY-NC-ND. Available online: https://www.inaturalist.org/observations/46916897 (accessed on 25 March 2022).
- White, D. Shelfordella lateralis. CC-BY-NC. Available online: https://www.inaturalist.org/observations/51307601 (accessed on 25 March 2022).
- Lago, M.C. Blaptica dubia. CC-BY-NC. Available online: https://inaturalist.ca/observations/62266049 (accessed on 25 March 2022).
- Schwartz, B. Blaberus craniifer. CC-BY-NC. Available online: https://www.inaturalist.org/observations/2584268 (accessed on 25 March 2022).
- Fraire, M. Gromphadorhina portentosa. CC-BY-NC. Available online: https://www.inaturalist.org/observations/36924580 (accessed on 25 March 2022).
- Skrentny, J. Manduca sexta. CC-BY-NC. Available online: https://www.inaturalist.org/observations/93210706 (accessed on 25 March 2022).
- Méndez, L. Blaberus discoidalis. CC-BY-NC. Available online: https://www.inaturalist.org/photos/127331827 (accessed on 25 March 2022).
- Nadal, M. Rhynchophorus ferrugineus. CC-BY-NC. Available online: https://www.inaturalist.org/observations/102432275 (accessed on 25 March 2022).
- Roland, L. Bombyx mori. CC-BY-NC. Available online: https://www.inaturalist.org/observations/69171140 (accessed on 25 March 2022).
- Borzée, A. Teleogryllus emma. CC-BY-NC. Available online: https://www.inaturalist.org/observations/94811230 (accessed on 25 March 2022).
- Taylor, R. Nauphoeta cinerea. CC-BY. Available online: https://uk.inaturalist.org/observations/21385592 (accessed on 25 March 2022).
- Booysen, R. Oxyhaloa deusta. CC-BY-NC. Available online: https://www.inaturalist.org/observations/79272523 (accessed on 25 March 2022).
- Biological Museum, L.U. Ulomoides dermestoides. CC-BY-NC. Available online: https://www.flickr.com/photos/127240649@N08/45560037744/in/photolist-ACJiHE-AoqqLw-zHZT4U-AF2yvP-2cpZ6W9-2c8gdZ8/ (accessed on 25 March 2022).
- Govaerts, C. Apis mellifera. CC-BY-NC. Available online: https://www.inaturalist.org/observations/19301657 (accessed on 25 March 2022).
- Cash, L. Chrysomya megacephala. CC-BY-NC. Available online: https://inaturalist.ca/observations/85549044 (accessed on 25 March 2022).
- Wahlberg, N. Anacridium aegyptium. Public Domain. Available online: https://www.inaturalist.org/photos/188061647 (accessed on 25 March 2022).
- Vakarė, R. Pseudochorthippus parallelus. CC-BY-NC. Available online: https://inaturalist.ca/observations/102162754 (accessed on 25 March 2022).
- Rorabaugh, J. Lucilia Sericata. Public Domain. Available online: https://inaturalist.ca/observations/12062145 (accessed on 25 March 2022).
- Rosli, R. Oechophylla smaragdina. CC-BY-NC. Available online: https://www.inaturalist.org/observations/76430415 (accessed on 25 March 2022).
- Fang, X. Odontotermes formosanus. CC-BY-NC. Available online: https://www.inaturalist.org/photos/196990438 (accessed on 25 March 2022).
- Méndez, L. Caligo memnon. CC-BY-NC. Available online: https://www.inaturalist.org/photos/197834596 (accessed on 25 March 2022).
- Smirnova, L. Acrossus rufipes. CC-BY-NC. Available online: https://www.inaturalist.org/observations/100110967 (accessed on 25 March 2022).
- Bernardes, L. Cladomorphus Phyllinum. CC-BY-NC. Available online: https://www.inaturalist.org/observations/90645896 (accessed on 25 March 2022).
- Macfie, R. Acrida cinerea. CC-BY-NC. Available online: https://www.inaturalist.org/observations/93819021 (accessed on 25 March 2022).
- Lauer, M. Anabrus Simplex. CC-BY-NC. Available online: https://inaturalist.nz/observations/6684282 (accessed on 25 March 2022).
- Chiu, C. Teleogryllus mitratus. CC-BY-NC. Available online: https://www.inaturalist.org/observations/104687913 (accessed on 25 March 2022).
- Caswell, I. Zonocerus variegatus. Public Domain. Available online: https://www.inaturalist.org/observations/37759544 (accessed on 25 March 2022).
- Yáñez, R. Chilecomadia moorei. CC-BY-NC. Available online: https://www.inaturalist.org/observations/37670097 (accessed on 25 March 2022).
- Associação Vita Nativa Machaerium maritimae. CC-BY-NC. Available online: https://inaturalist.ca/observations/92608804 (accessed on 25 March 2022).
| Species | Native Range | Current World Distribution in the Wild | Known or Potential Impacts | Main Uses | Invasive Potential/Impacts | Invasive Potential (References to Studies) |
|---|---|---|---|---|---|---|
| Diptera | ||||||
| Drosophila hydei | Cosmopolitan | Worldwide except Antarctica | Exotic pet’s food | NO | ||
| Drosophila melanogaster | Sub-Saharan Africa | Worldwide except Antarctica | Exotic pet’s food | NO | ||
| Hermetia illucens | Neotropical | Worldwide except Antarctica | Enteric myasis | Livestock Feed, Pets feed | YES | [32,33,34,35,36,37,39,178,179,180] |
| Musca domestica | Central Asia | Worldwide except Antarctica | Carries human/livestock pathogens | Livestock Feed, Pets feed | YES | [46] |
| Orthoptera | ||||||
| Acheta Domesticus | Southwestern Asia | Worldwide except Antarctica | Livestock Feed, Pets feed | NO | ||
| Gryllodes sigillatus | Southeast Asia | Worldwide except Antarctica | Livestock Feed, Pets feed | NO | [59] | |
| Gryllus assimilis | Carribean islands and South Texas | North America, South America and Europe | Livestock Feed, Pets feed | YES | [65,66] | |
| Gryllus bimaculatus | Mediterranean | Europe, Asia and Africa | Exotic pet’s food | NO | ||
| Locusta migratoria | Africa | Africa, Asia, Europe and Oceania | Crop pest | Exotic pet’s food | YES | [108] |
| Schistocerca gregaria | American continent | Europe, Africa, Southeast Asia, Central America | Crop pest | Exotic pet’s food | YES | [109] |
| Teleogryllus emma | Japan | Korea and Japan | Exotic pet’s food | NO | ||
| Coleoptera | ||||||
| Alphitobius diaperinus | Sub-Saharan Africa | Worldwide except Antartica | Poultry litter pest | Livestock Feed, Pets feed | YES | [140] |
| Callosobruchus maculatus | West Africa | Worldwide except Antartica | Stored legumes pest | Exotic pet’s food | YES | [85] |
| Pachnoda marginata | Equatorial Africa | Equatorial Africa | Exotic pet’s food | NO | ||
| Rhynchophorus ferrugineus | South and Southeast Asia | Europe, Asia and Oceania | Palm tree pest | Exotic pet’s food | YES | [153] |
| Tenebrio molitor | Europe | Worldwide except Antarctica and South America | Stored good’s pest | Livestock Feed, Pets feed | NO | [181] |
| Ulomoides dermestoides | Asia | Asia | Stored cereals pest | Exotic pet’s food | YES | [177] |
| Zophobas morio | Central America | Central America | Stored good’s pest | Livestock Feed, Pets feed | YES | [79] |
| Lepidoptera | ||||||
| Bombyx mori | China | Worldwide except Antartica | Livestock Feed, Pets feed | NO | ||
| Galleria mellonella | Asia | Worldwide except Antarctica | Honey-bee nest pest | Exotic pet’s food | YES | [117] |
| Manduca sexta | North America | American continent | Solanaceous pest | Exotic pet’s food | YES | [145] |
| Blattodea | ||||||
| Blaberus craniifer | North and Central America | North and Central America | Exotic pet’s food | NO | ||
| Blaberus discoidales | Central America and Florida (USA) | Central America and Florida (USA) | Exotic pet’s food | NO | ||
| Blaptica dubia | Neotropical | American Continent, Europe | Exotic pet’s food | YES | [126] | |
| Gromphadorhina Portentosa | Madagascar | North America, Madagascar | Exotic pet’s food | NO | ||
| Nauphoeta cinerea | Northeastern Africa | North America, Africa and Australia | Exotic pet’s food | NO | [169] | |
| Oxyhaloa deusta | Africa | Africa | Exotic pet’s food | NO | ||
| Shelfordella lateralis | Middle East | North America, Europe and Middle East | Indoor pest | Exotic pet’s food | YES | [124,141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, F.; Calado, R.; Medina, I.; Ameixa, O.M.C.C. The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review. Sustainability 2022, 14, 6361. https://doi.org/10.3390/su14106361
Lourenço F, Calado R, Medina I, Ameixa OMCC. The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review. Sustainability. 2022; 14(10):6361. https://doi.org/10.3390/su14106361
Chicago/Turabian StyleLourenço, Felipe, Ricardo Calado, Isabel Medina, and Olga M. C. C. Ameixa. 2022. "The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review" Sustainability 14, no. 10: 6361. https://doi.org/10.3390/su14106361
APA StyleLourenço, F., Calado, R., Medina, I., & Ameixa, O. M. C. C. (2022). The Potential Impacts by the Invasion of Insects Reared to Feed Livestock and Pet Animals in Europe and Other Regions: A Critical Review. Sustainability, 14(10), 6361. https://doi.org/10.3390/su14106361








