Revealing the Chemical Profiles of Airborne Particulate Matter Sources in Lake Baikal Area: A Combination of Three Techniques
Abstract
:1. Introduction
2. Materials and Methods
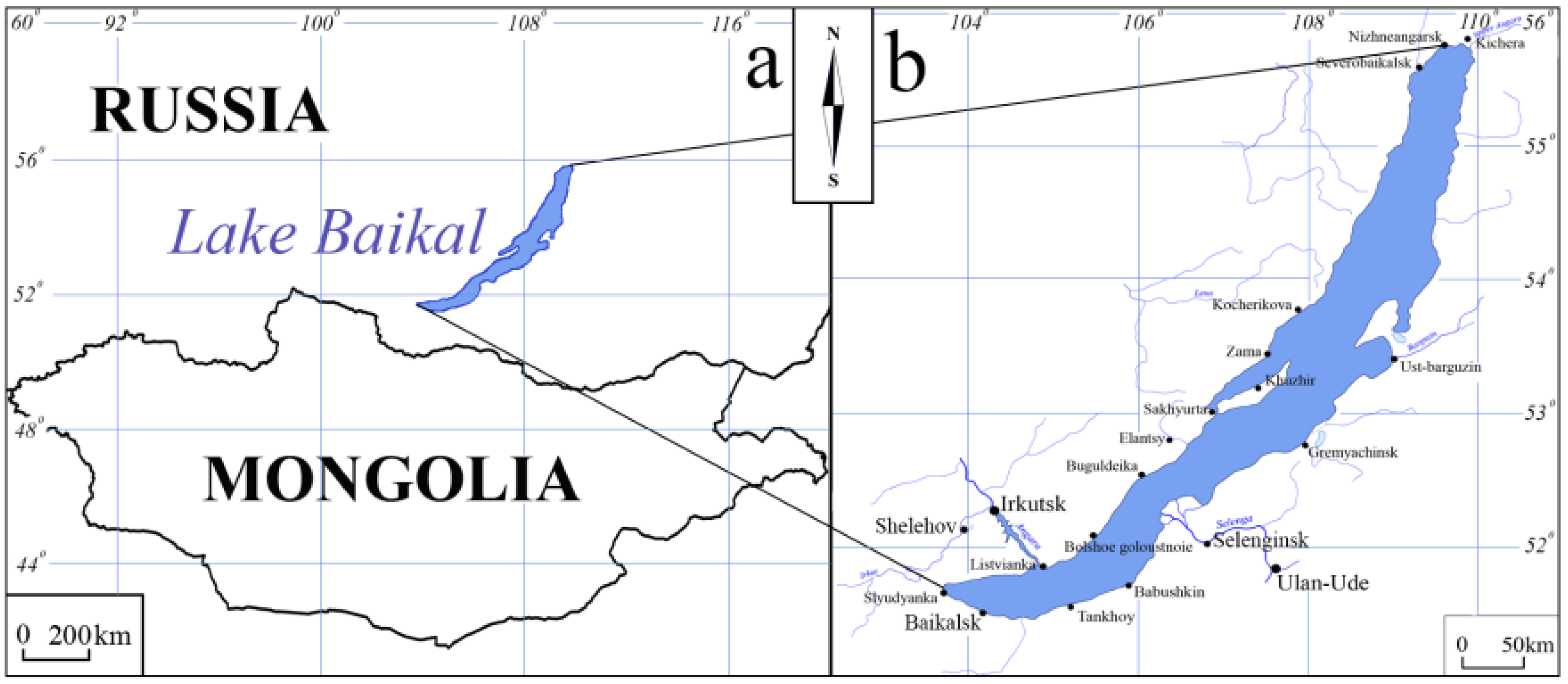
2.1. Study Area and Study Objects
2.2. Sampling Techniques
2.3. Chemical Determinations
2.3.1. Polycyclic Aromatic Hydrocarbons
2.3.2. Inorganic Elements
2.4. Data Processing
2.4.1. Data Analysis Using Positive Matrix Factorization Model
2.4.2. Testing the PMF Results Using Diagnostic Ratios and End-Member Mixing Analysis
3. Results and Discussion
3.1. Chemical Composition of Particulate Matter
3.1.1. PAH Composition of Particulate Matter
3.1.2. Element Composition of Particulate Matter
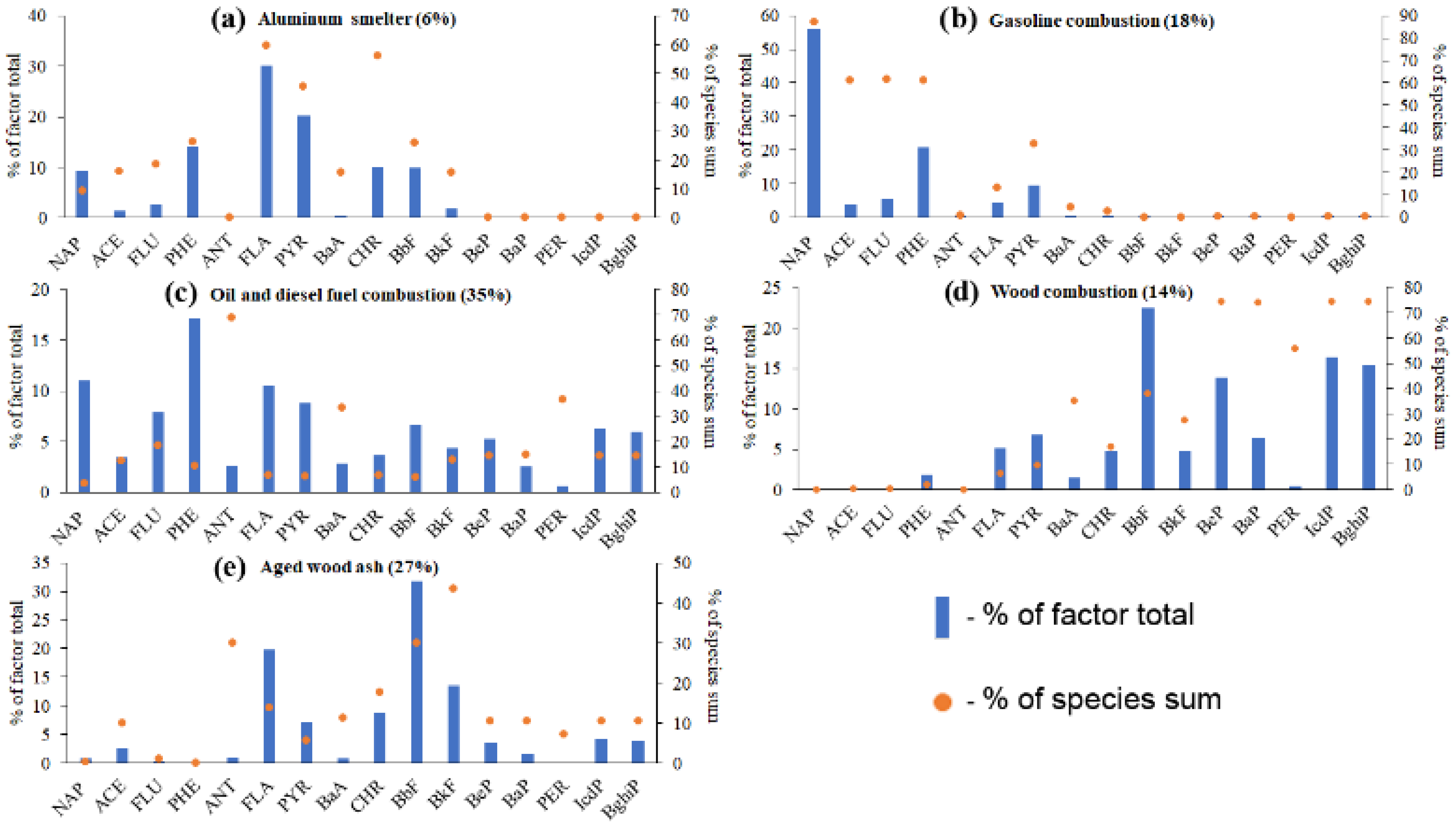
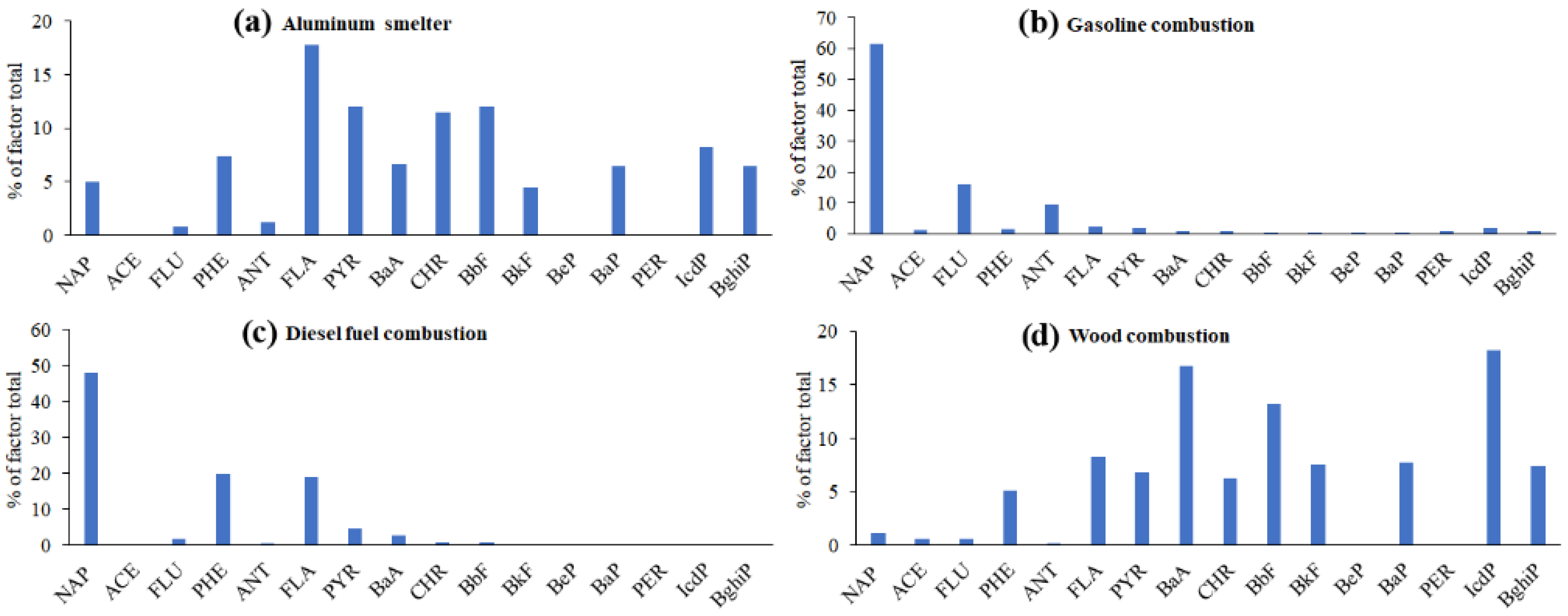
3.2. Resolving the PAH Profiles of PM Sources in the Air above the Lake Baikal Water Using PMF
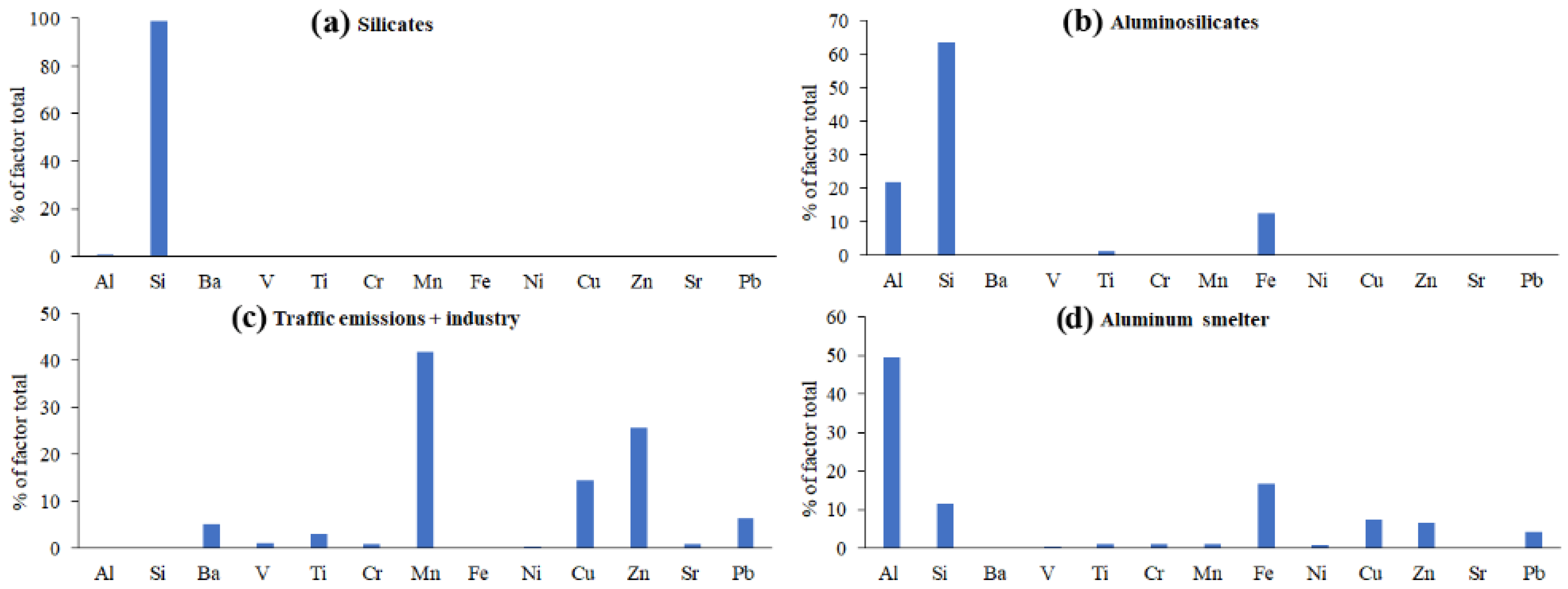
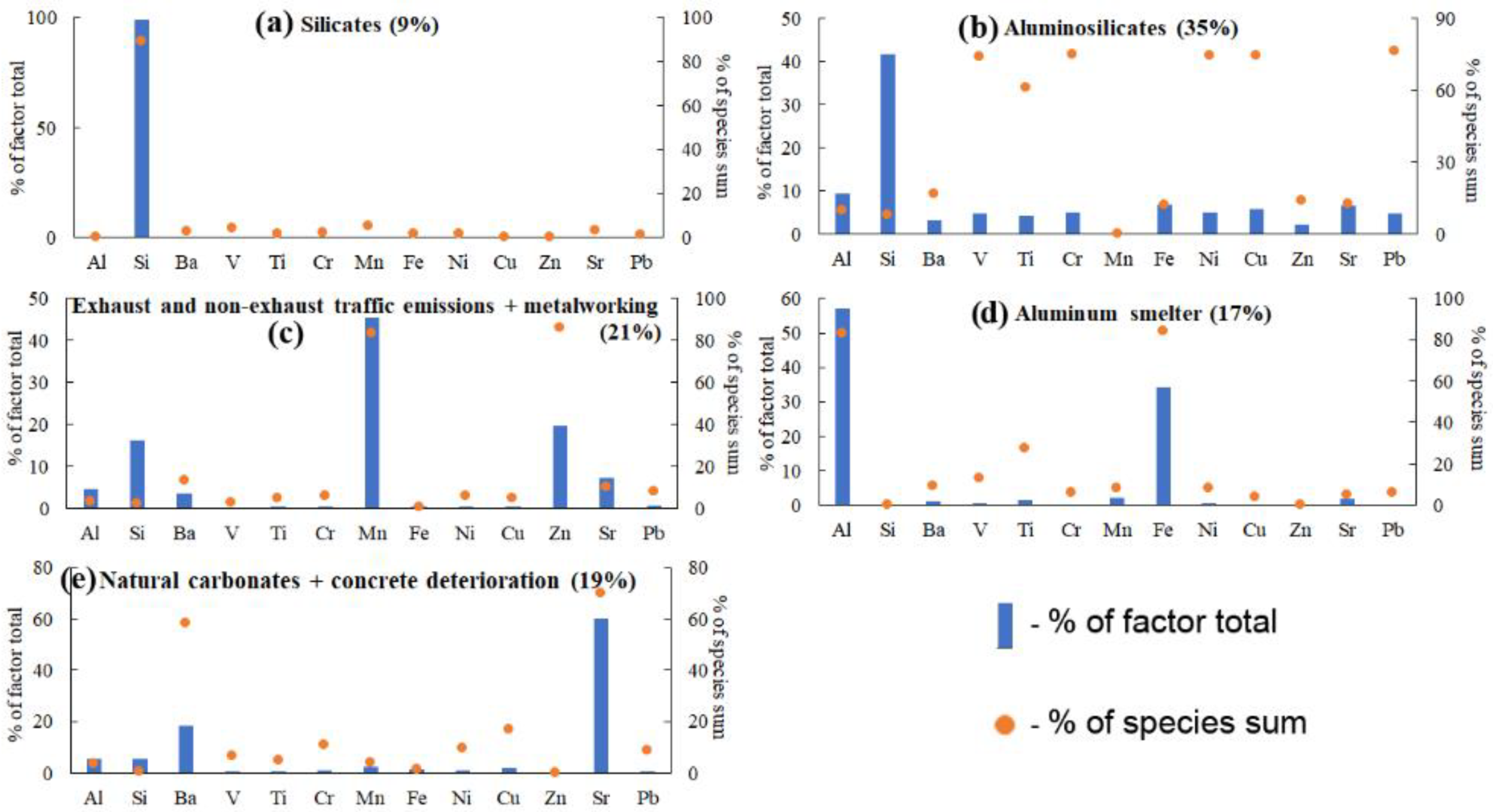
3.3. Resolving the IE Profiles of PM Sources in the Lake Baikal Snowpack Using PMF
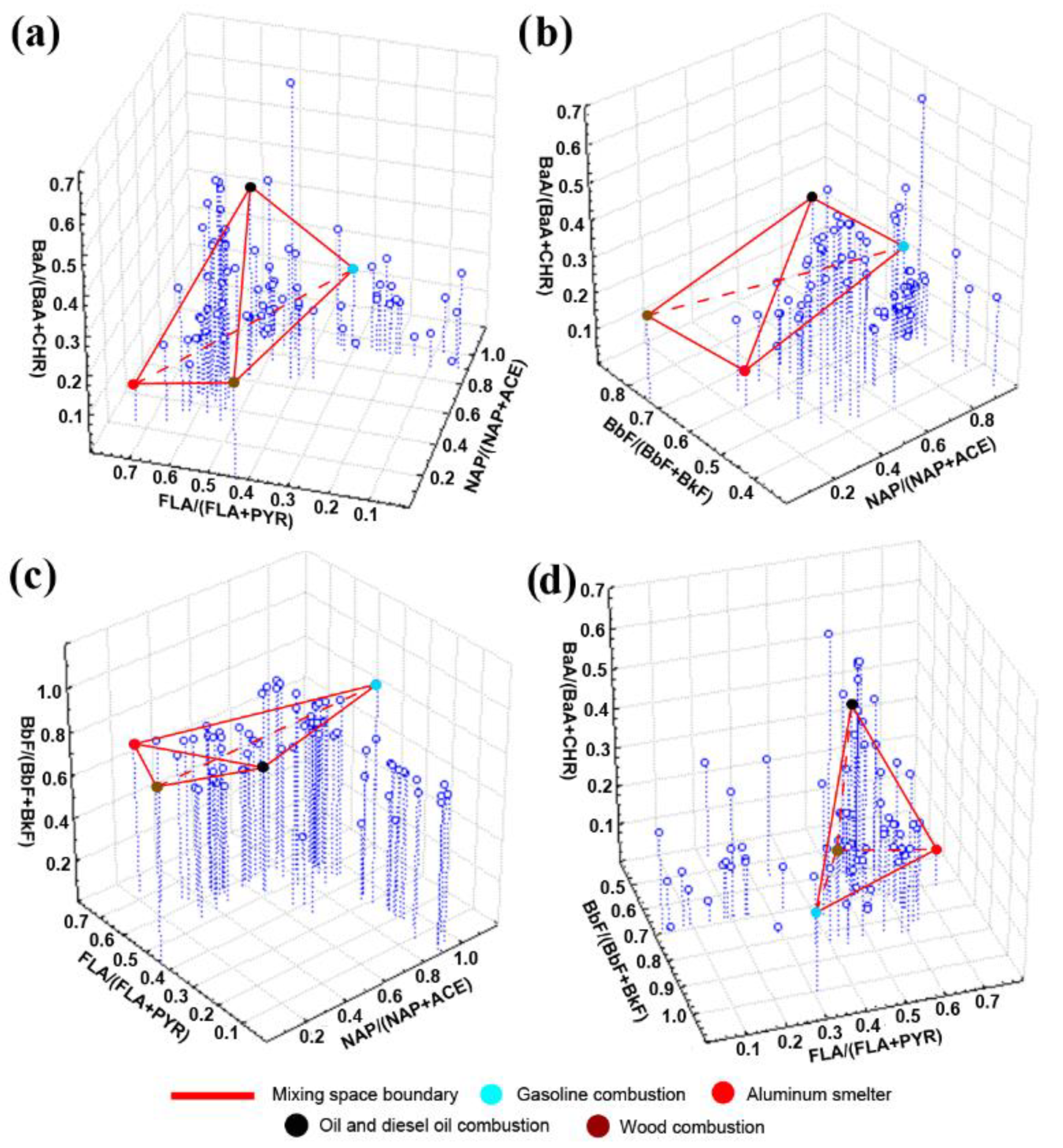
3.4. Testing the PMF-Derived Source Profiles of Airborne PAHs Using EMMA and DR
3.5. Testing the PMF-Derived Source Profiles of Airborne Inorganic PM Using EMMA and DR
4. Conclusions
- Emission profiles of four of the five PAH sources obtained using PMF were quite reliable, because the mixing spaces in EMMA diagrams that used PMF-derived DR ratios as coordinates of PAH source points bounded 25–75% of airborne PM samples. The aged wood ash was recognized as the minor source because its point in any coordinate system matched the position of some other sources (mostly the positions of aluminum smelter and wood combustion).
- The reliability of PMF-derived PAH source profiles was most evident in the mixing diagrams that used PAHs with similar molecular weights as tracers. Similar molecular weights caused similar chemical properties of the tracer PAHs and, consequently, similar degradation rates. Thus, the PAH source fingerprints did not change drastically over time.
- The reliability of the results of EMMA-DR testing of PMF-derived IE source profiles was controversial. On the one hand, the mixing spaces in most of the EMMA diagrams that used PMF-derived DR ratios as coordinates of IE source points did not bound more than one-tenth of the sample points. On the other hand, the mixing space formed by the PMF-derived sources except natural carbonates + concrete deterioration bounded most of the sample points. This probably means that the PMF-derived Ca-Sr-Ba-rich source identified as “natural carbonates + concrete deterioration” was a minor one, whereas the other sources were major ones.
- The identification of continuously operating PM sources (aluminum smelter and traffic emissions) using both organic and inorganic tracers also argues in favor of the reliability of PMF-derived PAH and IE source profiles.
- The PMF-derived PM source profiles whose reliability was not confirmed by EMMA-DR results should be considered unreliable. The contributions of validated PMF-derived sources should be normalized to 100% after removal of the contributions of unvalidated sources.
- To improve the reliability of the PMF results, the concentrations of alkali and alkaline-earth metals like Na, K, Mg and Ca should be included in PMF analysis.
- To increase the capability of EMMA-DR testing of PMF results more chemical species from PMF-derived source profiles should be included in the testing procedure as source tracers. To include more chemical species in EMMA-DR, the relevant Excel-based computational tools should be developed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurston, G.D.; Ito, K.; Lall, R. A source apportionment of U.S. fine particulate matter air pollution. Atmos. Environ. 2011, 45, 3924–3936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Xie, S.; Zhang, Y.; Zeng, L.; Salmon, L.G.; Zheng, M. Source apportionment of PM2.5 in Beijing using principal component analysis/absolute principal component scores and UNMIX. Sci. Total Environ. 2006, 372, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Beddows, D.C.S.; Dall’Osto, M.; Harrison, R.M. Cluster analysis of rural, urban and curbside 916 atmospheric particle size data. Environ. Sci. Technol. 2009, 43, 4694–4700. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, X.; Nie, W.; Wang, J.; Xu, Z.; Wang, T.; Liu, Y.; Shen, Y.; Xu, Z.; Kokkonen, T.V.; et al. Cluster analysis of submicron particle number size distributions at the SORPES station in the Yangtze River Delta of East China. J. Geophys. Res. Atmos. 2021, 126, e2020JD034004. [Google Scholar] [CrossRef]
- Wan, X.; Chen, J.; Tian, F.; Sun, W.; Yang, F.; Saiki, K. Source apportionment of PAHs in atmospheric particulates of Dalian: Factor analysis with nonnegative constraints and emission inventory analysis. Atmos. Environ. 2006, 40, 6666–6675. [Google Scholar] [CrossRef]
- Hu, N.-J.; Huang, P.; Liu, J.-H.; Ma, D.-Y.; Shi, X.-F.; Mao, J.; Liu, Y. Characterization and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in sediments in the Yellow River Estuary, China. Environ. Earth Sci. 2014, 71, 873–883. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef]
- Cai, K.; Li, C. Street dust heavy metal pollution source apportionment and sustainable management in a typical City-Shijiazhuang, China. Int. J. Environ. Res. Public Health 2019, 16, 2625. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, H.; Li, J.; Mao, C.; Ji, J.; Yuan, X.; Li, T.; Ayoko, G.; Frost, R.L.; Feng, Y.-X. Multivariate linear regression model for source apportionment and health risk assessment of heavy metals from different environmental media. Ecotoxicol. Environ. Saf. 2018, 165, 555–563. [Google Scholar] [CrossRef]
- Henry, R.C. Multivariate receptor modeling by N-dimensional edge detection. Chemom. Intell. Lab. Syst. 2003, 65, 179–189. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Marinaite, I.I.; Bashenkhaeva, N.V.; Zhuchenko, N.A.; Khuriganova, O.I.; Molozhnikova, E.V. Polycyclic aromatic hydrocarbons in a small eastern Siberian river: Sources, delivery pathways, and behavior. Environ. Earth Sci. 2016, 75, 954. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Silaev, A.V.; Semenov, Y.M.; Begunova, L.A. Using Si, Al and Fe as tracers for source apportionment of air pollutants in Lake Baikal snowpack. Sustainability 2020, 12, 3392. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.W.A.; Lowenthal, D.H.; Watson, J.G.; Koracin, D.; Kumar, N.; Knipping, E.M.; Wheeler, N.; Craig, K.; Reid, S. Toward effective source apportionment using positive matrix factorization: Experiments with simulated PM 2.5 data. J. Air Waste Manag. Assoc. 2010, 60, 43–54. [Google Scholar] [CrossRef]
- Brown, S.G.; Eberly, S.; Paatero, P.; Norris, G.A. Methods for estimating uncertainty in PMF solutions: Examples with ambient air and water quality data and guidance on reporting PMF results. Sci. Total Env. 2015, 518–519, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Viana, M.; Pandolfi, M.; Minguillón, M.C.; Querol, X.; Alastuey, A.; Monfort, E.; Celades, I. Inter-comparison of receptor models for PM source apportionment: Case study in an industrial area. Atmos. Environ. 2008, 42, 3820–3832. [Google Scholar] [CrossRef]
- Shi, G.L.; Liu, G.R.; Peng, X.; Wang, Y.N.; Tian, Y.Z.; Wang, W.; Feng, Y.C. A comparison of multiple combined models for source apportionment, including the PCA/MLR-CMB, Unmix-CMB and PMF-CMB Models. Aerosol Air Qual. Res. 2014, 14, 2040–2050. [Google Scholar] [CrossRef] [Green Version]
- Salim, I.; Sajjad, R.U.; Paule-Mercado, M.C.; Memon, S.A.; Lee, B.Y.; Sukhbaatar, C.; Lee, C.H. Comparison of two receptor models PCA-MLR and PMF for source identification and apportionment of pollution carried by runoff from catchment and sub-watershed areas with mixed land cover in South Korea. Sci. Total Environ. 2019, 663, 764–775. [Google Scholar] [CrossRef]
- Feng, J.; Song, N.; Yu, Y.; Li, Y. Differential analysis of FA-NNC, PCA-MLR, and PMF methods applied in source apportionment of PAHs in street dust. Environ. Monit. Assess. 2020, 192, 727. [Google Scholar] [CrossRef]
- Zanotti, C.; Rotiroti, M.; Fumagalli, L.; Stefania, G.A.; Canonaco, F.; Stefenelli, G.; Prévôt, A.S.H.; Leoni, B.; Bonomi, T. Groundwater and surface water quality characterization through positive matrix factorization combined with GIS approach. Water Res. 2019, 159, 122–134. [Google Scholar] [CrossRef]
- Souto-Oliveira, C.E.; Kamigauti, L.Y.; Andrade, M.d.F.; Babinski, M. Improving source apportionment of urban aerosol using multi-isotopic fingerprints (MIF) and positive matrix factorization (PMF): Cross-validation and new insights. Front. Environ. Sci. 2021, 9, 623915. [Google Scholar] [CrossRef]
- Wen, Z.; Li, B.; Xiao, H.Y.; Zhe, L.; Zhu, R.G. Combined positive matrix factorization (PMF) and nitrogen isotope signature analysis to provide insights into the source contribution to aerosol free amino acids. Atmos. Environ. 2022, 268, 118799. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, N.; Neal, C.; Hooper, R.P.; Vogt, R.D.; Andersen, S. Modelling streamwater chemistry as a mixture of soilwater end-members—A step towards second-generation acidification models. J. Hydrol. 1990, 116, 307–320. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Onishchuk, N.A.; Netsvetaeva, O.G.; Khodzher, T.V. Source apportionment of particulate matter in urban snowpack using end-member mixing analysis and positive matrix factorization model. Sustainability 2021, 13, 13584. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Marinaite, I.I.; Golobokova, L.P.; Khuriganova, O.I.; Khodzher, T.V.; Semenov, Y.M. Source apportionment of polycyclic aromatic hydrocarbons in Lake Baikal water and adjacent air layer. Chem. Ecol. 2017, 33, 977–990. [Google Scholar] [CrossRef]
- Obolkin, V.A.; Potemkin, V.L.; Makukhin, V.L.; Khodzher, T.V.; Chipanina, E.V. Long-range transport of plumes of atmospheric emissions from regional coal power plants to the South Baikal water basin. Atmos. Ocean. Opt. 2017, 30, 360–365. [Google Scholar] [CrossRef]
- Golobokova, L.; Khodzher, T.; Khuriganova, O.; Marinayte, I.; Onishchuk, N.; Rusanova, P.; Potemkin, V. Variability of chemical properties of the atmospheric aerosol above Lake Baikal during large wildfires in Siberia. Atmosphere 2020, 11, 1230. [Google Scholar] [CrossRef]
- Marinaite, I.I.; Potyomkin, V.L.; Khodzher, T.V. Evaluation of atmospheric pollution with PAHS and PM10 above the water area of Lake Baikal during wildfires in Summer. In Proceedings of the 26th International Symposium on Atmospheric and Ocean Optics, Atmospheric Physics, Moscow, Russia, 6–10 July 2020; Volume 115602. [Google Scholar] [CrossRef]
- Marinaite, I.I.; Potyomkin, V.L.; Molozhnikova, E.V.; Penner, I.E.; Shikhovtsev, M.Y.; Izosimova, O.N.; Khodzher, T.V. Polycyclic aromatic hydrocarbons and PM10 solid particles above the water area of Lake Baikal in the summer of 2020. In Proceedings of the 27th International Symposium on Atmospheric and Ocean Optics, Atmospheric Physics, Moscow, Russia, 5–9 July 2021; Volume 119161. [Google Scholar] [CrossRef]
- Belozertseva, I.A.; Vorobyeva, I.B.; Vlasova, N.V.; Lopatina, D.N.; Yanchuk, M.S. Snow pollution in Lake Baikal water area in nearby land areas. Water Resour. 2017, 44, 471–484. [Google Scholar] [CrossRef]
- Belozertseva, I.A.; Vorobyeva, I.B.; Vlasova, N.V.; Janchuk, M.S.; Lopatina, D.N. Chemical composition of snow water of the water area of the southern hollow of Lake Baikal. Int. J. Appl. Fundam. Res. 2016, 10, 263–272. (In Russian) [Google Scholar]
- Belozertseva, I.A.; Vorobeva, I.B.; Vlasova, N.V.; Yanchuk, M.S.; Lopatina, D.N. Pollution of snow on the water area of the average hollow of Lake Baikal and the adjacent territory. Adv. Curr. Nat. Sci. 2016, 11, 96–105. (In Russian) [Google Scholar]
- Belozertseva, I.A.; Vorobeva, I.B.; Vlasova, N.V.; Yanchuk, M.S.; Lopatina, D.N. Pollution of snow on the water area of the northern hollow of Lake Baikal and the adjacent territory. Adv. Curr. Nat. Sci. 2016, 9, 97–103. (In Russian) [Google Scholar]
- Liang, S.X.; Wu, H.; Sun, H.W. Determination of trace elements in airborne PM10 by inductively coupled plasma mass spectrometry. Int. J. Environ. Sci. Technol. 2015, 12, 1373–1378. [Google Scholar] [CrossRef] [Green Version]
- Lucarelli, F.; Calzolai, G.; Chiari, M.; Giardi, F.; Czelusniak, C.; Nava, S. Hourly elemental composition and source identification by positive matrix factorization (PMF) of fine and coarse particulate matter in the high polluted industrial area of Taranto (Italy). Atmosphere 2020, 11, 419. [Google Scholar] [CrossRef] [Green Version]
- Belis, C.; Favez, O.; Mircea, M.; Diapouli, E.; Manousakas, M.; Vratolis, S.; Gilardoni, S.; Paglione, M.; Decesari, S.; Mocnik, G.; et al. European Guide on Air Pollution Source Apportionment with Receptor Models; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-09001-4. [Google Scholar]
- Javed, W.; Guo, B. Chemical characterization and source apportionment of fine and coarse atmospheric particulate matter in Doha, Qatar. Atmos. Pollut. Res. 2020, 12, 122–136. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhang, T.; Wang, F.; Tao, Y.; Zhang, X.; Wang, F.; Huang, J.; Cheng, T.; Jiang, H.; et al. Chemical nature and predominant sources of PM10 and PM2.5 from multiple sites on the Silk Road, Northwest China. Atmos. Pollut. Res. 2020, 12, 425–436. [Google Scholar] [CrossRef]
- Belykh, L.I.; Malykh, Y.V.; Penzina, E.E.; Smagunova, A.N. Sources of atmosphere pollution by polycyclic aromatic hydrocarbons in industrial Transbaikalia. Atmos. Oceanic Optics 2002, 10, 944–948. (In Russian) [Google Scholar]
- Borgulat, J.; Staszewski, T. Fate of PAHs in the vicinity of aluminum smelter. Environ. Sci. Pollut. Res. Int. 2018, 25, 26103–26113. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.H.; Lee, W.J.; Tsai, P.J.; Chen, C.B. A comparison on the emission of polycyclic aromatic hydrocarbons and their corresponding carcinogenic potencies from a vehicle engine using leaded and lead-free gasoline. Environ. Health Perspect. 2001, 109, 1285–1290. [Google Scholar] [CrossRef]
- Abrantes, R.; Assunção, J.V.; Pesquero, C.R. Emission of polycyclic aromatic hydrocarbons from light-duty diesel vehicles exhaust. Atmos. Environ. 2004, 38, 1631–1640. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Guo, T. Emission of PM2.5-bound polycyclic aromatic hydrocarbons from biomass and coal combustion in China. Atmosphere 2021, 12, 1129. [Google Scholar] [CrossRef]
- Aubin, S.; Farant, J.-P. Benzo[b]fluoranthene. A potential alternative to benzo[a]pyrene as an indicator of exposure to airborne PAHs in the vicinity of Söderberg aluminum smelters. J. Air Waste Manag Assoc. 2000, 50, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Chan, C.; Tosine, H.; Sakuma, T. Relative rates of photochemical and biological oxidation (in vitro) of polynuclear aromatic hydrocarbons. In Polynuclear Aromatic Hydrocarbons Third International Symposium on Chemistry and Biology-Carcinogenesis and Mutagenesis, Battelle Memorial Institute, Columbus, Ohio, USA, 26–29 October 1978; Jones, P., Leber, P., Eds.; Ann Arbor Science: Ann Arbor, MI, USA, 1979; pp. 171–190. [Google Scholar]
- Singh, K.P.; Malik, A.; Kumar, R.; Saxena, P.; Sinha, S. Receptor modeling for source apportionment of polycyclic aromatic hydrocarbons in urban atmosphere. Environ. Monit. Assess. 2008, 136, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Lu, D.; Tan, J.; Yang, X.; Sun, X.; Liu, Q.; Jiang, G. Unraveling the role of silicon in atmospheric aerosol secondary formation: A new conservative tracer for aerosol chemistry. Atmos. Chem. Phys. 2019, 19, 2861–2870. [Google Scholar] [CrossRef] [Green Version]
- Štyriaková, I.; Jablonovská, K.; Mockovčiaková, A. In situ application of bioleaching for improving the quality of quartz sand. Adv. Mat. Res. 2009, 497–500. [Google Scholar] [CrossRef]
- Rokbi, M.; Baali, B. Performance of polymer concrete incorporating waste marble and alfa fibers. Adv. Concr. Constr. 2017, 5, 331–343. [Google Scholar] [CrossRef]
- Bleam, W. Clay Mineralogy and Chemistry. In Soil and Environmental Chemistry, 2nd ed.; Bleam, W., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 3; pp. 87–146. [Google Scholar]
- Van Malderen, H.; Van Grieken, R.; Khodzher, T.; Obolkin, V.; Potemkin, V. Composition of individual aerosol particles above Lake Baikal, Siberia. Atmos. Environ. 1996, 30, 1453–1465. [Google Scholar] [CrossRef]
- Lynam, D.R.; Pfeifer, G.D.; Fort, B.F.; Gelbcke, A.A. Environmental assessment of MMT fuel additive. Sci. Tot. Environ. 1990, 93, 107–114. [Google Scholar] [CrossRef]
- Loranger, S.; Tétrault, M.; Kennedy, G.; Zayed, J. Manganese and other trace elements in urban snow near an expressway. Environ. Pollut. 1996, 92, 203–211. [Google Scholar] [CrossRef]
- Moreno, T.; Pandolfi, M.; Querol, X.; Lavín, J.; Alastuey, A.; Viana, M.; Gibbons, W. Manganese in the urban atmosphere: Identifying anomalous concentrations and sources. Environ. Sci. Pollut. Res. Int. 2011, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Viana, M.; Richard, A.; Furger, M.; Prévôt, A.S.H.; Nava, S.; Lucarelli, F.; Bukowiecki, N.; Alastuey, A.; Reche, C.; et al. Size and time-resolved roadside enrichment of atmospheric particulate pollutants. Atmos. Chem. Phys. 2011, 11, 2917–2931. [Google Scholar] [CrossRef] [Green Version]
- Boullemant, A. PM2.5 emissions from aluminum smelters: Coefficients and environmental impact. J. Air Waste Manag. Assoc. 2011, 61, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Belozertseva, I.A. Monitoring of environmental pollution in a zone of influence of the Irkutsk aluminum factory. Water Chem. Ecol. 2013, 10, 33–38. (In Russian) [Google Scholar]

| NAP | ACE | FLU | PHE | ANT | FLA | PYR | BaA | CHR | BbF | BkF | BeP | BaP | PER | IcdP | BghiP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 0.01 | 0.001 | 0.003 | 0.01 | 0.001 | 0.01 | 0.01 | 3 × 10−4 | 0.003 | 0.01 | 0.001 | 2 × 10−5 | 1 × 10−5 | 1 × 10−5 | 3 × 10−5 | 2 × 10−5 |
| 25th * | 0.02 | 0.01 | 0.01 | 0.04 | 0.002 | 0.03 | 0.02 | 0.001 | 0.01 | 0.02 | 0.01 | 4 × 10−5 | 2 × 10−5 | 1 × 10−5 | 1 × 10−5 | 1 × 10−5 |
| Med | 0.03 | 0.02 | 0.02 | 0.07 | 0.003 | 0.05 | 0.08 | 0.01 | 0.02 | 0.03 | 0.01 | 0.009 | 0.01 | 0.001 | 0.01 | 0.01 |
| 75th * | 0.16 | 0.03 | 0.03 | 0.12 | 0.01 | 0.12 | 0.16 | 0.02 | 0.07 | 0.11 | 0.07 | 0.07 | 0.05 | 0.004 | 0.09 | 0.09 |
| Max | 34.9 | 10.9 | 14 | 36.4 | 0.03 | 7.57 | 10.3 | 1.57 | 6.91 | 14.1 | 5.09 | 0.48 | 0.2 | 0.02 | 0.36 | 0.33 |
| Mean | 0.74 | 0.14 | 0.18 | 0.48 | 0.01 | 0.19 | 0.35 | 0.03 | 0.16 | 0.27 | 0.10 | 0.06 | 0.03 | 0.01 | 0.06 | 0.06 |
| STD ** | 4.19 | 1.12 | 1.43 | 3.73 | 0.01 | 0.78 | 1.33 | 0.16 | 0.77 | 1.45 | 0.52 | 0.11 | 0.05 | 0.01 | 0.09 | 0.08 |
| Parameter | Al | Si | Ba | V | Ti | Cr | Mn | Fe | Ni | Cu | Zn | Sr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 0.77 | 1.97 | 0.45 | 0.25 | 0.41 | 0.56 | 0.56 | 0.88 | 0.34 | 0.42 | 0.62 | 0.82 | 0.20 |
| 25th * | 6.21 | 1.35 | 2.32 | 1.12 | 1.23 | 1.04 | 3.42 | 3.32 | 1.01 | 1.22 | 0.98 | 3.20 | 1.05 |
| Median | 12.2 | 6.06 | 3.21 | 1.44 | 1.05 | 1.13 | 6.23 | 6.45 | 1.31 | 1.97 | 2.43 | 6.22 | 1.14 |
| 75th * | 21.0 | 80.1 | 5.55 | 1.99 | 2.39 | 1.87 | 10.3 | 14.1 | 1.12 | 3.44 | 7.18 | 12.4 | 1.25 |
| Max | 97.6 | 2107 | 62.4 | 7.01 | 10.7 | 26.6 | 54.9 | 70.2 | 8.37 | 9.31 | 26.3 | 98.7 | 10.9 |
| Mean | 18.0 | 88.2 | 4.13 | 1.60 | 1.55 | 1.74 | 8.21 | 11.6 | 1.27 | 2.30 | 4.69 | 10.4 | 1.47 |
| STD ** | 18.4 | 252 | 5.63 | 1.19 | 1.41 | 2.48 | 8.86 | 14.5 | 0.70 | 1.60 | 5.31 | 13.1 | 1.32 |
| NAP/(NAP+ACE) | FLA/(FLA+PYR) | BaA/(BaA+CHR) | BbF/(BbF+BkF) | |
|---|---|---|---|---|
| Gasoline combustion | 0.94 | 0.31 | 0.20 | 1.00 |
| Aluminum smelter | 0.24 | 0.74 | 0.09 | 0.70 |
| Oil combustion | 0.76 | 0.54 | 0.44 | 0.61 |
| Wood combustion | 0.01 | 0.43 | 0.24 | 0.82 |
| Aged wood ash | 0.25 | 0.71 | 0.18 | 0.71 |
| Source/Tracer | NAP/(NAP+ACE) | NAP/(NAP+ACE) | NAP/(NAP+ACE) | FLA/(FLA+PYR) |
|---|---|---|---|---|
| FLA/(FLA+PYR) | BbF/(BbF+BkF) | FLA/(FLA+PYR) | BbF/(BbF+BkF) | |
| BaA/(BaA+CHR) | BaA/(BaA+CHR) | BbF/(BbF+BkF) | BaA/(BaA+CHR) | |
| Gasoline combustion | 32 | 27 | 20 | 23 |
| Aluminum smelter | 39 | 37 | 38 | 49 |
| Oil and diesel fuel combustion | 22 | 32 | 37 | 11 |
| Wood combustion | 6 | 3 | 5 | 17 |
| Al/(Al+Si) | Mn/(Mn+Fe) | Ba/(Ba+Sr) | |
|---|---|---|---|
| Silicates | 0.01 | 0.66 | 0.25 |
| Aluminasilicates | 0.18 | 0.02 | 0.35 |
| Traffic emissions + metalworking | 0.22 | 0.99 | 0.33 |
| Aluminum smelter | 1.00 | 0.06 | 0.42 |
| Natural carbonates + concrete deterioration | 0.50 | 0.63 | 0.24 |
| Average | 0.38 | 0.47 | 0.32 |
| Source | Contribution |
|---|---|
| Silicates | 33 |
| Aluminosilicates | 27 |
| Traffic emissions + metalworking | 13 |
| Aluminum smelter | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenov, M.Y.; Marinaite, I.I.; Golobokova, L.P.; Semenov, Y.M.; Khodzher, T.V. Revealing the Chemical Profiles of Airborne Particulate Matter Sources in Lake Baikal Area: A Combination of Three Techniques. Sustainability 2022, 14, 6170. https://doi.org/10.3390/su14106170
Semenov MY, Marinaite II, Golobokova LP, Semenov YM, Khodzher TV. Revealing the Chemical Profiles of Airborne Particulate Matter Sources in Lake Baikal Area: A Combination of Three Techniques. Sustainability. 2022; 14(10):6170. https://doi.org/10.3390/su14106170
Chicago/Turabian StyleSemenov, Mikhail Y., Irina I. Marinaite, Liudmila P. Golobokova, Yuri M. Semenov, and Tamara V. Khodzher. 2022. "Revealing the Chemical Profiles of Airborne Particulate Matter Sources in Lake Baikal Area: A Combination of Three Techniques" Sustainability 14, no. 10: 6170. https://doi.org/10.3390/su14106170
APA StyleSemenov, M. Y., Marinaite, I. I., Golobokova, L. P., Semenov, Y. M., & Khodzher, T. V. (2022). Revealing the Chemical Profiles of Airborne Particulate Matter Sources in Lake Baikal Area: A Combination of Three Techniques. Sustainability, 14(10), 6170. https://doi.org/10.3390/su14106170







