Effects of Increasing Concentrations of Enrofloxacin on Co-Digestion of Pig Manure and Corn Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Design
2.3. Analytical Methods
2.3.1. Water-Quality Indices
2.3.2. Biogas Measurement
2.3.3. Microbial Community Analysis
2.4. Statistical Analysis
3. Results
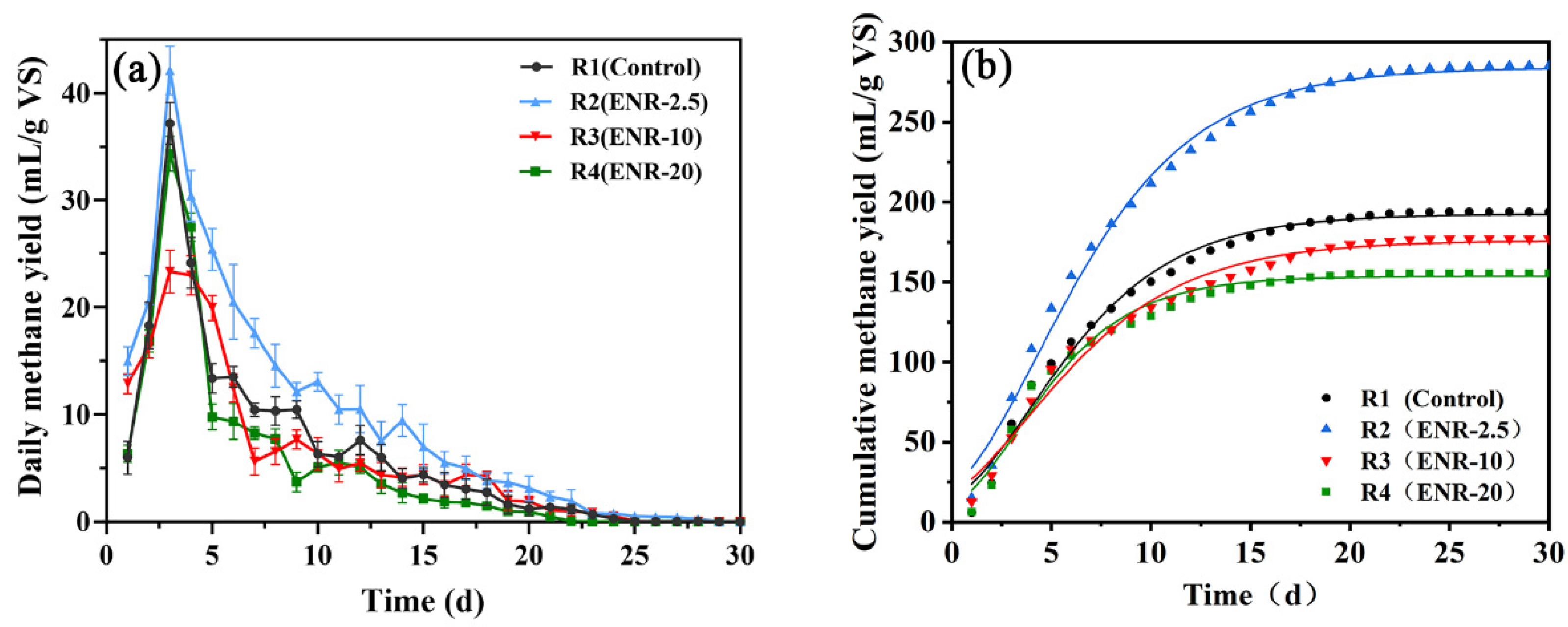
3.1. Methane Production
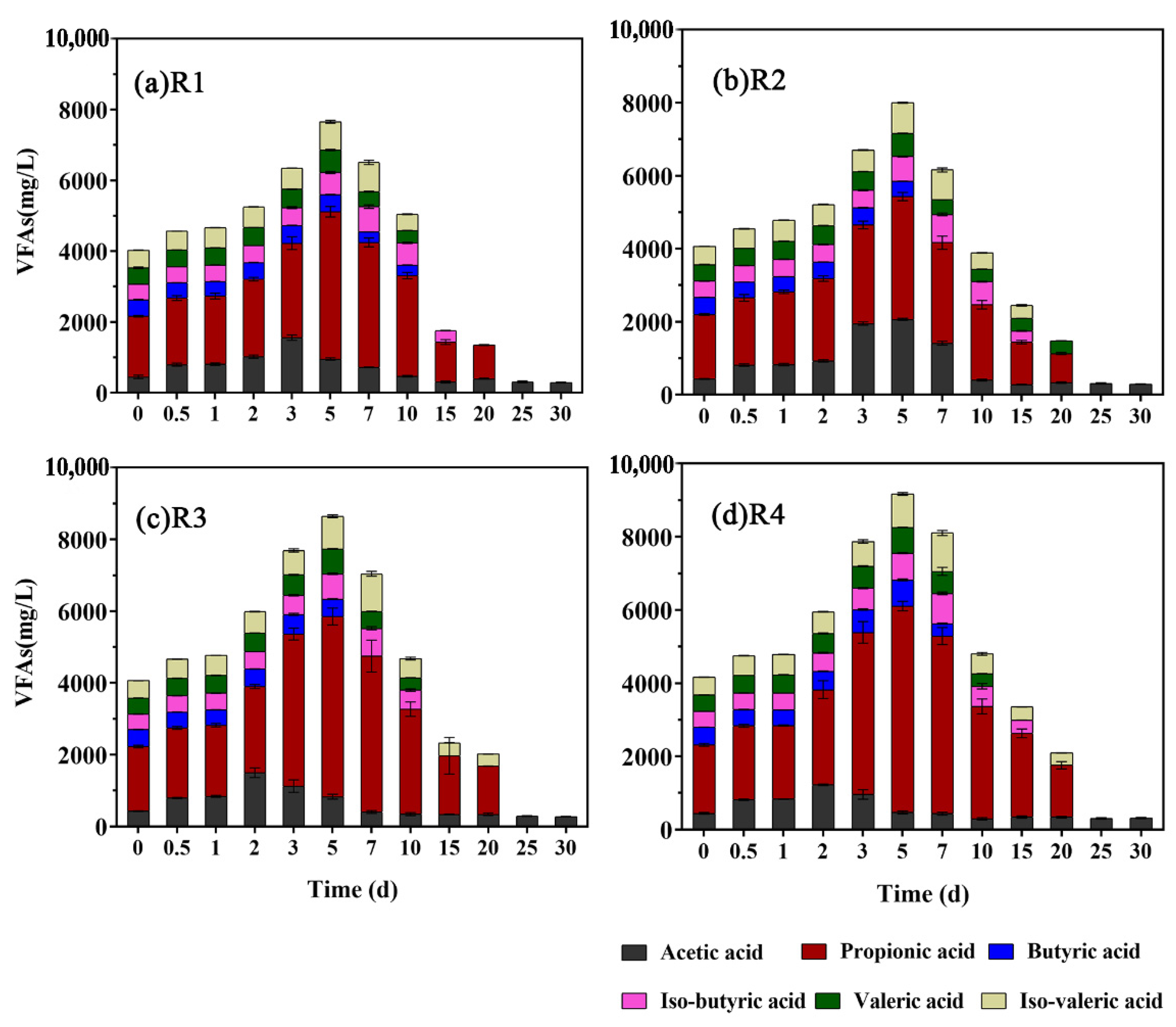
3.2. Variation in VFAs
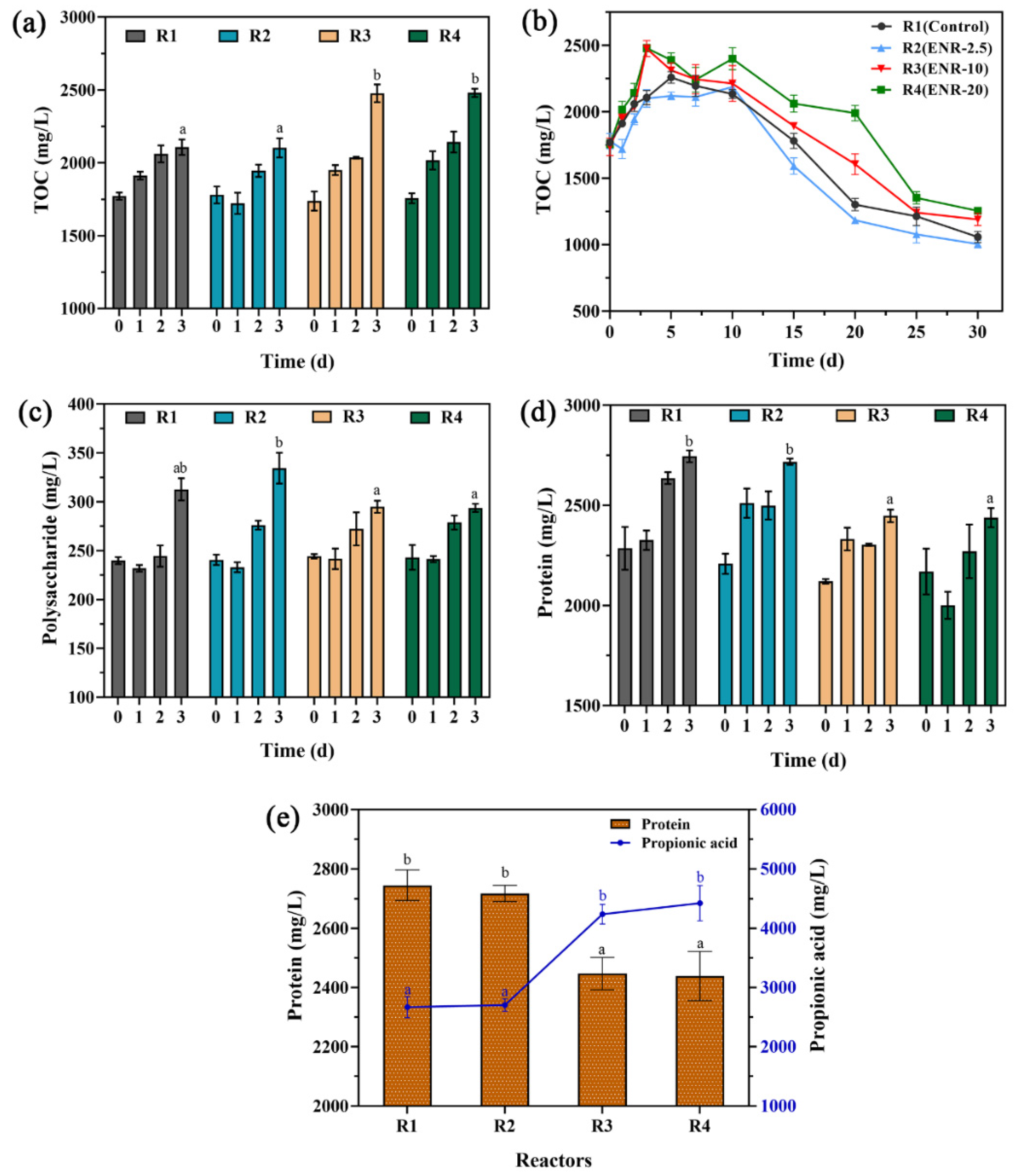
3.3. Variation in TOC and EPS Levels
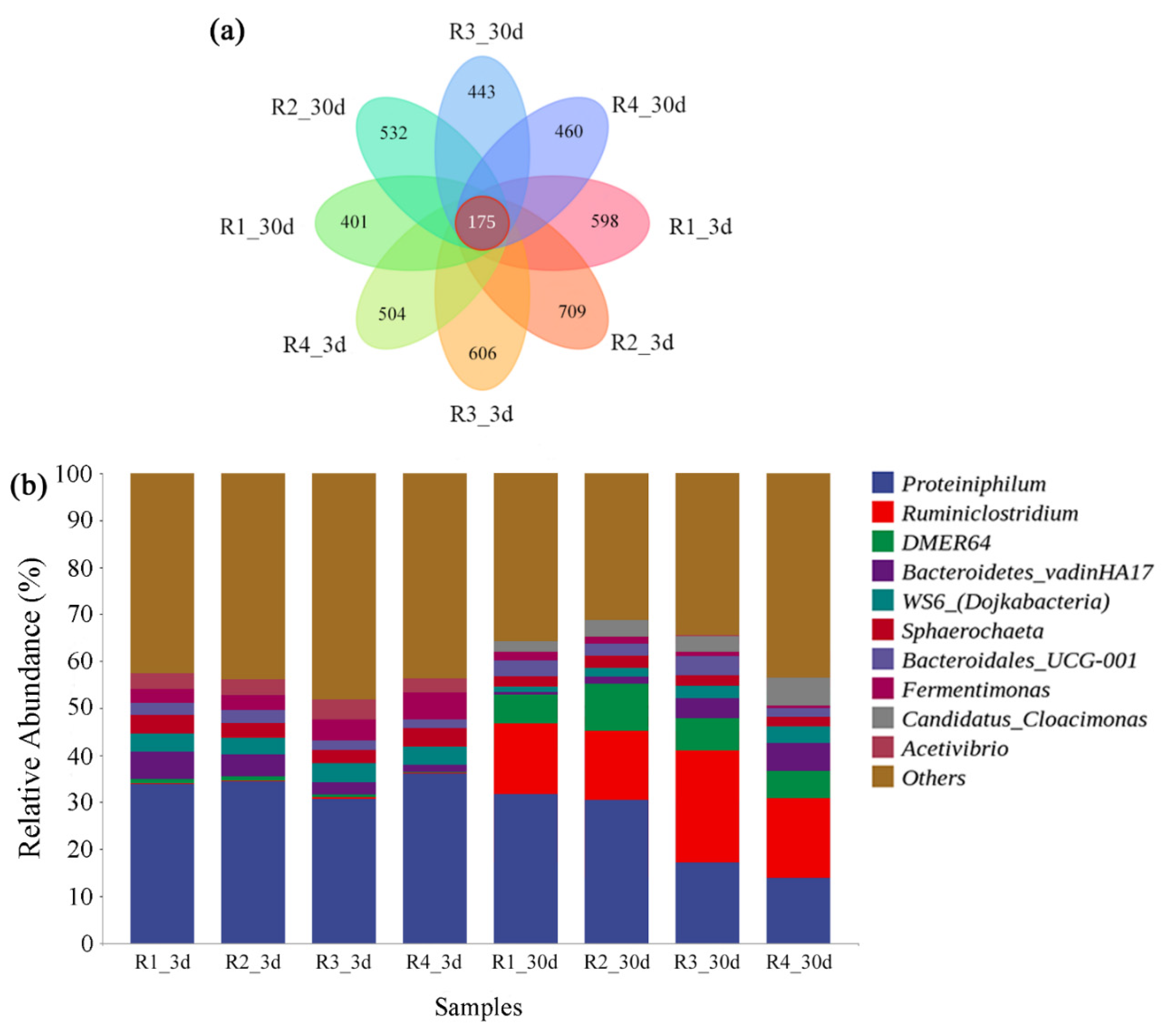
3.4. Microbial Diversity Analysis
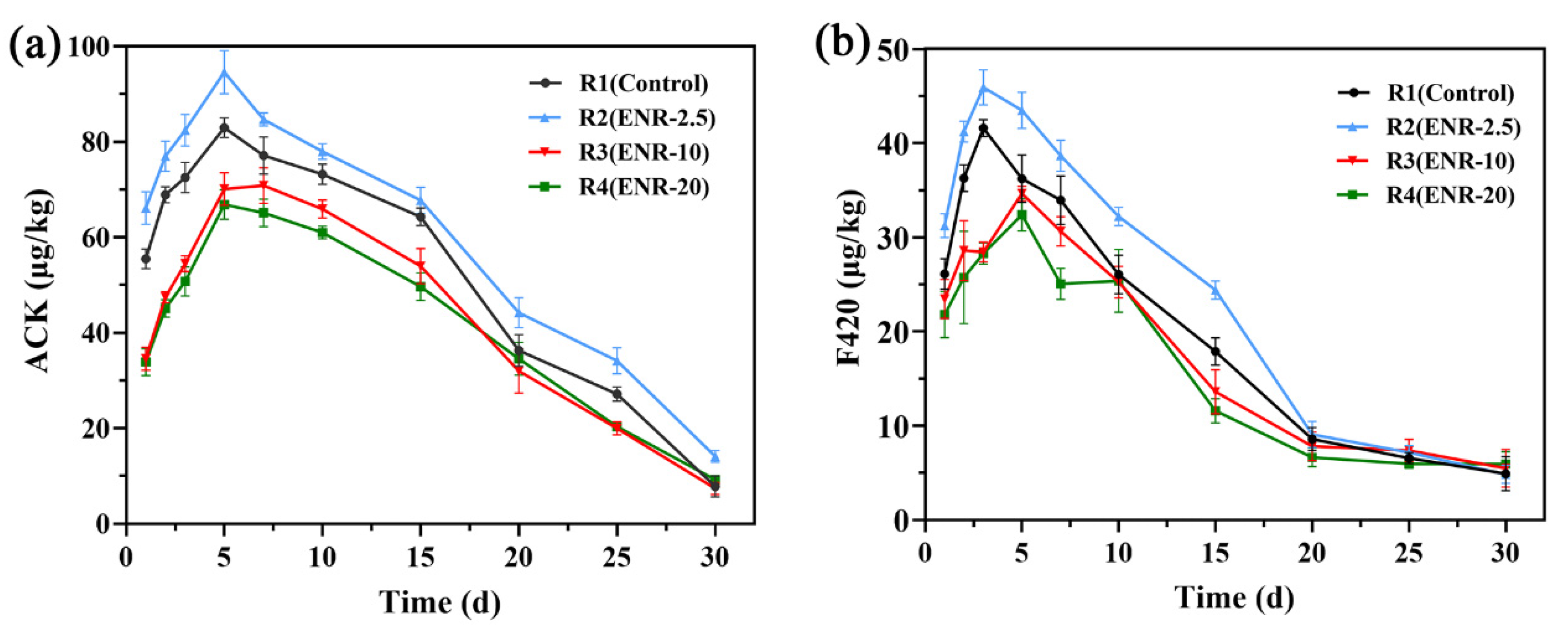
3.5. Enzyme Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.L.; Feng, Y.Z.; Wang, X.J.; Ren, G.X. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Hao, X.D.; Liu, R.B.; Huang, X. Evaluation of the potential for operating carbon neutral WWTPs in China. Water Res. 2015, 87, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.X.; Song, W.P.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Sui, Q.W.; Zhang, J.Y.; Tong, J.; Chen, M.X.; Wei, Y.S. Seasonal variation and removal efficiency of antibiotic resistance genes during wastewater treatment of swine farms. Environ. Sci. Pollut. Res. 2017, 24, 9048–9057. [Google Scholar] [CrossRef]
- Yin, F.B.; Dong, H.M.; Zhang, W.Q.; Zhu, Z.P.; Shang, B. Antibiotic degradation and microbial community structures during acidification and methanogenesis of swine manure containing chlortetracycline or oxytetracycline. Bioresour. Technol. 2018, 250, 247–255. [Google Scholar] [CrossRef]
- Tao, C.W.; Hsu, B.M.; Ji, W.T.; Hsu, T.K.; Kao, P.M.; Hsu, C.P.; Shen, S.M.; Shen, T.Y.; Wan, T.J.; Huang, Y.L. Evaluation of five antibiotic resistance genes in wastewater treatment systems of swine farms by real-time PCR. Sci. Total Environ. 2014, 496, 116–121. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Z.Y.; Liu, R.; Lei, Z.F. Removal of veterinary antibiotics from anaerobically digested swine wastewater using an intermittently aerated sequencing batch reactor. J. Environ. Sci. 2018, 65, 8–17. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Ince, O. Assessment of anaerobic bacterial diversity and its effects on anaerobic system stability and the occurrence of antibiotic resistance genes. Bioresour. Technol. 2016, 207, 332–338. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, Z.Y.; Wang, Y.W.; Zhong, H.; Sui, Q.W.; Zhang, C.P.; Wei, Y.S. Effects of graphene oxide on the performance, microbial community dynamics and antibiotic resistance genes reduction during anaerobic digestion of swine manure. Bioresour. Technol. 2017, 245, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Rodriguez, N.; Amils, R. The action of antibiotics on the anaerobic digestion process. Appl. Microbiol. Biotechnol. 1996, 46, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Lizasoain, J.; Nettmann, E.; Bergmann, I.; Mundt, K.; Klocke, M.; Rincon, M.; Amon, T.; Piringer, G. Effects of the antibiotics chlortetracycline and enrofloxacin on the anaerobic digestion in continuous experiments. Bioenergy Res. 2014, 7, 1244–1252. [Google Scholar] [CrossRef]
- Xie, W.Y.; Shen, Q.; Zhao, F.J. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Burboa-Charis, V.A.; Alvarez, L.H. Methane production from antibiotic bearing swine wastewater using carbon-based materials as electrons’ conduits during anaerobic digestion. Int. J. Energy Res. 2020, 44, 10996–11005. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Harnisz, M.; Korzeniewska, E.; Czatzkowska, M.; Jastrzebski, J.P.; Paukszto, L.; Bajkacz, S.; Felis, E.; Rusanowska, P. The effect of antibiotics on mesophilic anaerobic digestion process of cattle manure. Energies 2021, 14, 1125. [Google Scholar] [CrossRef]
- Madariaga, S.T.; Marin, S.L. Sanitary and environmental conditions of aquaculture sludge. Aquac. Res. 2017, 48, 1744–1750. [Google Scholar] [CrossRef]
- Beneragama, N.; Lateef, S.A.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. The combined effect of cefazolin and oxytertracycline on biogas production from thermophilic anaerobic digestion of dairy manure. Bioresour. Technol. 2013, 133, 23–30. [Google Scholar] [CrossRef]
- Zhou, M.M.; Yan, B.H.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barcelo, D.; Ince, O.; Orhon, D. Biodegradation and reversible inhibitory impact of sulfamethoxazole on the utilization of volatile fatty acids during anaerobic treatment of pharmaceutical industry wastewater. Sci. Total Environ. 2015, 536, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Wang, H.H.; Li, J.; Sun, Z.Y.; Nie, Y.; Nobu, M.K.; Tang, Y.Q. Different inhibitory mechanisms of chlortetracycline and enrofloxacin on mesophilic anaerobic degradation of propionate. Environ. Sci. Pollut. Res. 2020, 27, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.M.; Mott, H.V.; Lupo, C.D.; Oswald, A.S.; Clay, S.A.; Stone, J.J. Effects of chlortetracycline amended feed on anaerobic sequencing batch reactor performance of swine manure digestion. Bioresour. Technol. 2012, 125, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.D.; Xu, Q.X.; Wu, Y.X.; Wang, D.B.; Yang, Q.; Chen, F.; Wu, Y.; Pi, Z.J.; Chen, Z.; Li, X.M.; et al. Effect of clarithromycin on the production of volatile fatty acids from waste activated sludge anaerobic fermentation. Bioresour. Technol. 2019, 288, 121598. [Google Scholar] [CrossRef]
- Loftin, K.A.; Henny, C.; Adams, C.D.; Surampali, R.; Mormile, M.R. Inhibition of microbial metabolism in anaerobic lagoons by selected sulfonamides, tetracyclines, lincomycin, and tylosin tartrate. Environ. Toxicol. Chem. 2005, 24, 782–788. [Google Scholar] [CrossRef]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Venkiteshwaran, K.; Bocher, B.; Maki, J.; Zitomer, D. Relating anaerobic digestion microbial community and process function. Microbiol. Insights 2015, 8, 37–44. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association (APHA): Washington DC, USA, 2012. 22nd Edition. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1982598 (accessed on 1 March 2022).
- Li, X.Y.; Yang, S.F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- Yu, H.R.; Qu, F.S.; Sun, L.P.; Liang, H.; Han, Z.S.; Chang, H.Q.; Shao, S.L.; Li, G.B. Relationship between soluble microbial products (SMP) and effluent organic matter (EfOM): Characterized by fluorescence excitation emission matrix coupled with parallel factor analysis. Chemosphere 2015, 121, 101–109. [Google Scholar] [CrossRef]
- Frolund, B.; Griebe, T.; Nielsen, P.H. Enzymatic-Activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- Bashiri, G.; Rehan, A.M.; Greenwood, D.R.; Dickson, J.M.; Baker, E.N. Metabolic engineering of cofactor F420 production in Mycobacterium smegmatis. PLoS ONE 2010, 5, e15803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.Y.; Chen, Y.G.; Feng, L.Y. Polycyclic Aromatic Hydrocarbon Affects Acetic Acid Production during Anaerobic Fermentation of Waste Activated Sludge by Altering Activity and Viability of Acetogen. Environ. Sci. Technol. 2016, 50, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Linton, R.H.; Carter, W.H.; Pierson, M.D.; Hackney, C.R. Use of a modified Gompertz Equation to model nonlinear survival curves for Listeria Monocytogenes Scott A. J. Food Prot. 1995, 58, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Mau, M.; George, U.; Zahn, J.A.; Raskin, L. Effect of the presence of the antimicrobial tylosin in swine waste on anaerobic treatment. Water Res. 2008, 42, 2377–2384. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Boe, K.; Batstone, D.J.; Steyer, J.P.; Angelidaki, I. State indicators for monitoring the anaerobic digestion process. Water Res. 2010, 44, 5973–5980. [Google Scholar] [CrossRef]
- Lu, M.Q.; Niu, X.J.; Liu, W.; Zhang, J.; Wang, J.; Yang, J.; Wang, W.Q.; Yang, Z.Q. Biogas generation in anaerobic wastewater treatment under tetracycline antibiotic pressure. Sci. Rep. 2016, 6, 28336. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xie, L.; Luo, G.; Zhou, Q.; Lu, Q. Optimization of biohydrogen and methane recovery within a cassava ethanol wastewater/waste integrated management system. Bioresour. Technol. 2012, 120, 165–172. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Sousa, D.Z.; Kleerebezem, R.; Plugge, C.M. Role of syntrophic microbial communities in high-rate methanogenic bioreactors. Water Sci. Technol. 2012, 66, 352–362. [Google Scholar] [CrossRef]
- Stone, J.J.; Clay, S.A.; Zhu, Z.W.; Wong, K.L.; Porath, L.R.; Spellman, G.M. Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion. Water Res. 2009, 43, 4740–4750. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chou, J.; Lee, Y.S. Heavy metal-affected degradation of butyric acid in anaerobic digestion. Bioresour. Technol. 1998, 65, 159–161. [Google Scholar] [CrossRef]
- Lins, P.; Reitschuler, C.; Illmer, P. Impact of several antibiotics and 2-bromoethanesulfonate on the volatile fatty acid degradation, methanogenesis and community structure during thermophilic anaerobic digestion. Bioresour. Technol. 2015, 190, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Y.; Xing, Y.J.; Ma, Z.T.; Zhang, M.; Zheng, P. Acute toxicity of pharmaceutical wastewaters containing antibiotics to anaerobic digestion treatment. Chemosphere 2013, 91, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Steyer, J.P.; Bouvier, J.C.; Conte, T.; Gras, P.; Harmand, J.; Delgenes, J.P. On-line measurements of COD, TOC, VFA, total and partial alkalinity in anaerobic digestion processes using infra-red spectrometry. Water Sci. Technol. 2002, 45, 133–138. [Google Scholar] [CrossRef]
- Luo, J.Y.; Zhang, Q.; Zha, J.N.; Wu, Y.; Wu, L.J.; Li, H.; Tang, M.; Sun, Y.Q.; Guo, W.; Feng, Q.; et al. Potential influences of exogenous pollutants occurred in waste activated sludge on anaerobic digestion: A review. J. Hazard. Mater. 2020, 383, 121176. [Google Scholar] [CrossRef]
- Li, X.; Dai, X.; Takahashi, J.; Li, N.; Jin, J.; Dai, L.; Dong, B. New insight into chemical changes of dissolved organic matter during anaerobic digestion of dewatered sewage sludge using EEM-PARAFAC and two-dimensional FTIR correlation spectroscopy. Bioresour. Technol. 2014, 159, 412–420. [Google Scholar] [CrossRef]
- Xu, Q.X.; Liu, X.R.; Zhao, J.W.; Wang, D.B.; Wang, Q.L.; Li, X.M.; Yang, Q.; Zeng, G.M. Feasibility of enhancing short-chain fatty acids production from sludge anaerobic fermentation at free nitrous acid pretreatment: Role and significance of Tea saponin. Bioresour. Technol. 2018, 254, 194–202. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Gu, J.; Liu, Y. Evaluation of anaerobic digestion of food waste and waste activated sludge: Soluble COD versus its chemical composition. Sci. Total Environ. 2018, 643, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, D.B.; Liu, Y.W.; Wang, Q.L.; Chen, F.; Yang, Q.; Li, X.M.; Zeng, G.M.; Li, H.L. Triclocarban enhances short-chain fatty acids production from anaerobic fermentation of waste activated sludge. Water Res. 2017, 127, 150–161. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Jia, Y.Y.; Khanal, S.K.; Lu, H.; Fang, H.T.; Zhao, Q. Understanding the role of extracellular polymeric substances on ciprofloxacin adsorption in aerobic sludge, anaerobic sludge, and sulfate-reducing bacteria sludge systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.E.; Brady, A.L.; Sharp, G.H.; Grasby, S.E.; Stott, M.B.; Dunfield, P.F. Humboldt’s spa: Microbial diversity is controlled by temperature in geothermal environments. ISME J. 2014, 8, 1166–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.Z.; Song, Q.X.; Li, Q.; Zhang, H.; Luo, X.Z.; Zheng, Z. Damage of heavy metals to Vallisneria natans (V. natans) and characterization of microbial community in biofilm. Aquat. Toxicol. 2020, 225, 105515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Hu, X.; Sun, Z.R. Microbial community distribution and dominant bacterial species analysis in the bio-electrochemical system treating low concentration cefuroxime. Chem. Eng. J. 2016, 303, 137–144. [Google Scholar] [CrossRef]
- Ariesyady, H.D.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Huang, Z.X.; Ruan, W.Q.; Miao, H.F.; Shi, W.S.; Zhao, M.X. Enriching ruminal polysaccharide-degrading consortia via co-inoculation with methanogenic sludge and microbial mechanisms of acidification across lignocellulose loading gradients. Appl. Microbiol. Biotechnol. 2018, 102, 3819–3830. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Whiteley, C.G.; Enongene, G.; Pletschke, B.I.; Rose, P.; Whittington-Jones, K. Co-digestion of primary sewage sludge and industrial wastewater under anaerobic sulphate reducing conditions: Enzymatic profiles in a recycling sludge bed reactor. Water Sci. Technol. 2003, 48, 129–138. [Google Scholar] [CrossRef]
- Miao, H.F.; Lu, M.F.; Zhao, M.X.; Huang, Z.X.; Ren, H.Y.; Yan, Q.; Ruan, W.Q. Enhancement of Taihu blue algae anaerobic digestion efficiency by natural storage. Bioresour. Technol. 2013, 149, 359–366. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y.G.; Xiao, N.D. Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion. Bioresour. Technol. 2011, 102, 10305–10311. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Z.J.; Hu, Z.D.; Luo, X.Z. Enhanced anaerobic digestion with the addition of chelator-nickel complexes to improve nickel bioavailability. Sci. Total Environ. 2021, 759, 143458. [Google Scholar] [CrossRef] [PubMed]

| Reactor | Inoculum and Substrates | ENR (mg/L) | Abbreviation |
|---|---|---|---|
| R1 | + | − | Control |
| R2 | + | 2.5 | ENR-2.5 |
| R3 | + | 10 | ENR-10 |
| R4 | + | 20 | ENR-20 |
| Reactor | Gmax (mL CH4/g VS) | Rmax (mL CH4/g VS/d) | λ (d) | R2 |
|---|---|---|---|---|
| R1 (Control) | 193.88 ± 1.32 | 17.91 ± 0.87 | 0.21 ± 0.08 | 0.9850 |
| R2 (ENR-2.5) | 284.55 ± 2.11 | 23.87 ± 0.88 | 0.35 ± 0.14 | 0.9912 |
| R3 (ENR-10) | 176.57 ± 1.88 | 14.74 ± 0.72 | 0.12 ± 0.04 | 0.9841 |
| R4 (ENR-20) | 153.84 ± 1.63 | 18.02 ± 1.15 | 0.42 ± 0.26 | 0.9882 |
| Reactor | Chao | Shannon | Simpson | Good’s Coverage |
|---|---|---|---|---|
| R1_3d | 1701.11 | 6.930 | 0.9788 | 0.9955 |
| R2_3d | 1891.87 | 7.382 | 0.9854 | 0.9952 |
| R3_3d | 1720.22 | 6.983 | 0.9836 | 0.9957 |
| R4_3d | 1619.77 | 6.874 | 0.9826 | 0.9953 |
| R1_30d | 1464.68 | 6.410 | 0.9428 | 0.9968 |
| R2_30d | 1526.69 | 6.858 | 0.9435 | 0.9954 |
| R3_30d | 1289.75 | 6.466 | 0.9338 | 0.9955 |
| R4_30d | 1209.17 | 6.403 | 0.9312 | 0.9955 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Q.; Cheng, H.; Chen, X.; Wang, J.; Du, Z.; Hong, J.; Zheng, Z.; Luo, X. Effects of Increasing Concentrations of Enrofloxacin on Co-Digestion of Pig Manure and Corn Straw. Sustainability 2022, 14, 5894. https://doi.org/10.3390/su14105894
Shu Q, Cheng H, Chen X, Wang J, Du Z, Hong J, Zheng Z, Luo X. Effects of Increasing Concentrations of Enrofloxacin on Co-Digestion of Pig Manure and Corn Straw. Sustainability. 2022; 14(10):5894. https://doi.org/10.3390/su14105894
Chicago/Turabian StyleShu, Qihang, Hongkuan Cheng, Xiaxia Chen, Jie Wang, Zunqing Du, Jun Hong, Zheng Zheng, and Xingzhang Luo. 2022. "Effects of Increasing Concentrations of Enrofloxacin on Co-Digestion of Pig Manure and Corn Straw" Sustainability 14, no. 10: 5894. https://doi.org/10.3390/su14105894
APA StyleShu, Q., Cheng, H., Chen, X., Wang, J., Du, Z., Hong, J., Zheng, Z., & Luo, X. (2022). Effects of Increasing Concentrations of Enrofloxacin on Co-Digestion of Pig Manure and Corn Straw. Sustainability, 14(10), 5894. https://doi.org/10.3390/su14105894






