Abstract
In recent years, people’s environmental awareness has increased. The high density of the urban population has caused a considerable increase in the demand for car washing services, which has created large quantities of car wash wastewater. The main pollutants in car wash wastewater are detergents, dirt, oil, and grease. Untreated wastewater released into rainwater sewer systems or other water bodies may pollute the water and generate excessive bubble foams, which negatively affects urban appearance. Car washes are divided into mechanical car washes and manual or self-service car washes. In general, car washes have a small operation and scale, occupy limited land, and cannot afford wastewater treatment costs. Therefore, most car washes are not equipped with wastewater treatment facilities. Consequently, the discharge of wastewater from car washes negatively affects the water quality in the surrounding environment and results in wasteful use of water resources. This study reviewed 68 research papers on the quality, treatment techniques, treatment costs, and treatment effectiveness of car wash wastewater to provide a reference for car wash operators to contribute to the preservation of water resources. We found that there is a higher chance of recycling car wash wastewater when combing two different techniques for car wash wastewater treatment.
1. Introduction
The rapid growth in human population has resulted in increased car use, which has increased the demand for car wash services and thereby generated large amounts of car wash wastewater. In metropolitan areas, the foam in the wastewater produced during car washing overflows and spoils the appearance of the city. However, compared with industrial wastewater it is relatively easy to treat car wash wastewater and improve its water quality. In Taiwan, the conditions of the narrow and densely populated area and the small scale of the industry make low-cost and low-space car wash wastewater treatment technology an urgent need, and it is believed that this demand is applicable to other metropolitan areas in Asia as well. The goals of car wash wastewater treatment are to prevent environmental pollution and to reuse water resources. According to one estimation, the world had 1.5 billion cars in 2020 []. If each car was washed monthly and each wash consumed 100 L of water [], the amount of water used for car washing would be 1.8 billion tons/year. At a price of one US dollar per ton of water, the annual total cost of car washing worldwide would be USD 1.8 billion per year []. Considering that each person consumes approximately 150 L of water per day, the amount of water used for car washing annually is equal to that used by 33 million people annually []. This consumption approximately represents the amount of water used annually by the entire population of Malaysia (33 million), Venezuela (32 million), the Republic of Ghana (30 million), Oceania (including Australia (25 million) and New Zealand (5 million)) or the combined population of Denmark (5.8 million), Norway (5.4 million), Switzerland (10 million), Finland (5.5 million), and Iceland (360,000). The 2030 Agenda established by the United Nations proposes 17 sustainable development goals as the core objectives for sustainable development among governments and corporations. In particular, goal six is aimed at ensuring access to water and sanitation for all. Access to water is a basic right; thus, the value of water exceeds the price of water. Consequently, the circulation and reuse of water resources is essential. This study reviewed 68 research papers and obtained data on the car wash wastewater produced in 38 cities in 21 countries. These data mainly contained information on the suspended solid (SS, mg/L) concentration, turbidity (Nephelometric Turbidity Unit, NTU), chemical oxygen demand (COD, mg O2/L), and oil and grease (O&G, mg /L) concentration of car wastewater, as well as on the anionic surfactants (AS, mg /L) used in car wash wastewater treatment. The aforementioned data and the corresponding removal techniques of these pollutants are comprehensively discussed in the following sections.
2. Car Wash Wastewater Quality
Car wash wastewater generally contains suspended particles that originate from the dirt on vehicles, the oil on vehicle exteriors, the oil and grease generated from car wax, and the anionic surfactants caused by detergent use [,]. This wastewater has a high COD. Table 1 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] presents data on the car wash wastewater quality of each region investigated in the literature review. The SS concentration, turbidity, COD, O&G, and AS values in the collected data ranged from 68 to 1990 mg/L, 60 to 1000 NTU, 85 to 1295 mg O2/L, 12 to 325 mg/L, and 3 to 68 mg/L, respectively. The median values of the aforementioned parameters were 186 mg/L, 187 NTU, 418 mg O2/L, 28 mg/L, and 13 mg/L, respectively. The car wash wastewater data of different countries (Table 1) exhibited no significant correlations. In particular, the extreme values of the SS concentration, turbidity, and COD values were 2929 mg/L, 3649 NTU, and 14133 mg O2/L, respectively []. Moreover, the turbidity and COD ranged from 559~733 NTU and from 2640~4160 mg O2/L, respectively [,]. Because the collected data include data on wastewater created when washing garbage trucks, the different water quality parameters were relatively higher in value. If extreme values such as those for the wastewater from washing garbage trucks are eliminated, it is believed that the normal SS, turbidity, COD, O&G and AS values of car wash wastewater would be around level 200 mg/L, 200 NTU, 450 mg O2/L, 30 mg/L, and 30 mg/L, respectively. The most direct intention of car washing is to remove dust; therefore, Figure 1 shows the NTU data as surveyed from the literature. From Figure 1, it can be seen that the NTU of car washing wastewater is not directly related to the desertification of the urban environment.

Table 1.
Car wash wastewater characteristics from various literature sources.
Figure 1.
NTU data of carwash wastewater surveyed from Table 1.
3. Water Quantities Required to Wash a Car
The quantity required for washing a single car has been measured by different studies as approximately 45~60 L, 130~350 L, 45~60 L, 189~379 L, 400 L, and 151~227 L of water, respectively [,,,,,]. These results indicate that varying amounts of water are required for car washing in different countries. A reasonable amount of water for washing a car is 100~200 L. Several studies have collected data on car wash wastewater for unique vehicles. The car wash water consumption for heavy vehicles and waste container washing vehicles was recorded as approximately 350~900 L and 5000 L, respectively [,]. Monney et al. [] reported that the car wash water consumption of multiple vehicles (e.g., saloon cars, sport utility vehicles or pick-ups, buses or vans, heavy articulators, and graders or loaders) ranged between 105 and 1381 L. The car wash water consumption for washing heavy vehicles, trucks, and trailers ranged between 250 and 1200 L []. Germany and Austria have stipulated regulations mandating the recycling of 80% of car wash wastewater. Alternatively, the Netherlands and Scandinavian countries impose restrictions on water consumption for each car wash of 60~70 L []. There is a scarce record on car wash water consumption based on what kinds of cars are washed in the literature reviewed, however, it is natural to assume that larger cars require a larger amount of water in a car wash. The amount of water used in a car wash is not highly correlated with the region where the car is washed or the type of car; rather, it is more likely to be related to the culture of water usage. However, there is no significant evidence to support this supposition.
4. Car Wash Wastewater Treatment Technique
Currently, various car wash wastewater treatment techniques are available, as reported in reviewed literatures [,,,,,]. The scope of this study includes discussion of such techniques as electrocoagulation (EC) [,,], flocculation flotation (FF) [,], filtration (F) [], coagulation–flocculation (CF) [,], biological treatment (Bio) [,], adsorption (AD) [], electro-oxidation (EO) [,], and other less-known technologies such as photo-Fenton application [,]. In general, the combination of at least two wastewater treatment techniques can enable high treatment efficiency of car wash wastewater [,,,].
4.1. Electrocoagulation (EC)
Figure 2 depicts the general mechanism of the electrocoagulation process. EC uses metal hydroxides produced by electrolysis to remove pollutants in wastewater. During the electrolysis reaction, a sacrificial anode undergoes an oxidation reaction to release metal ions, while the cathode undergoes a reduction reaction to reduce the metal ions to metal and generate hydrogen. Commonly used metal anodes include aluminum and iron. The EC process has a turbidity removal rate of approximately 90% [,,]. When coupled with adsorption treatment or electro-oxidation treatment, the turbidity removal rate of the EC process can be increased. Moreover, the EC process has a COD removal rate of approximately 80%. When combined with other treatments, the COD removal rate of the EC process can be increased (Table 2). Obviously, it is not efficiently to remove SS by EC.
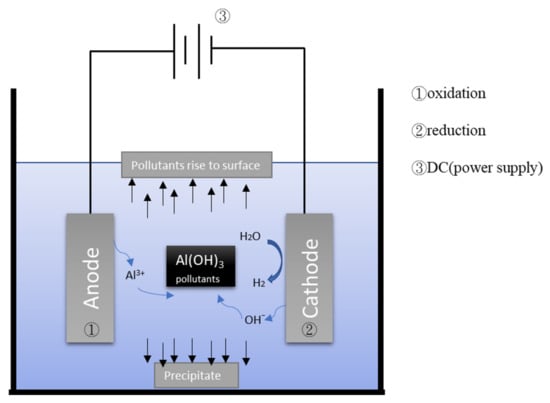
Figure 2.
Schematic illustration of electrocoagulation/flotation.

Table 2.
Removal rate of various water qualities by EC method.
4.2. Flocculation–Flotation (FF)
Figure 3 presents the mechanism and process of flocculation–flotation (FF). FF combines polymer flocculant addition and air bubble flotation to separate pollutants in carwash wastewater. The SS and turbidity removal rates of the FF process are approximately 85% and 90%, respectively [,]. When coupled with other treatments, the SS and turbidity removal rates of this process can reach as high as 96% [,]. The FF process has a COD removal rate of approximately 70~80%, which can be increased when this process is coupled with other treatments. Thus, the FF process exhibits a turbidity and COD removal performance comparable to that of the EC process (Table 3).
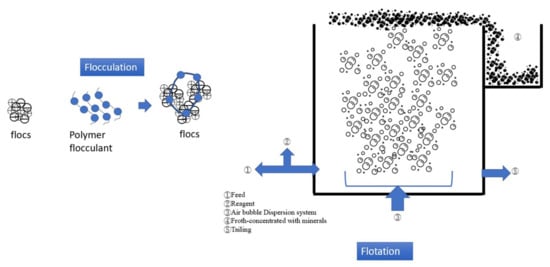
Figure 3.
Schematic illustration of flocculation–flotation.

Table 3.
Removal rate of various water qualities by flocculation–flotation method.
4.3. Filtration (F)
In recent years, filtration has become an excellent method for solid–liquid separation [], and membrane filtration has especially been used in many fields, for example mineral processing [], removing surfactants [], suspension filtration [], and more. Figure 4 illustrates the mechanism of filtration.
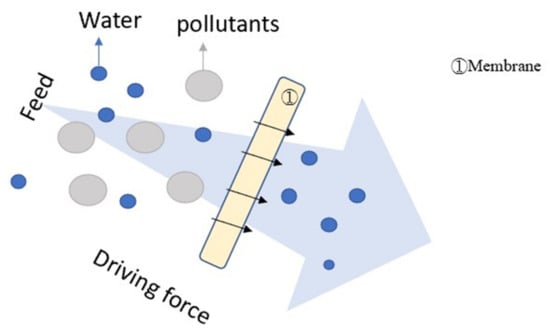
Figure 4.
Illustration of filtration.
When the filter element has sufficient selectivity, the flocculation–filtration process can achieve SS and turbidity removal rates over 99%, as presented in [,]. However, the filtrate flux of flocculation–ultrafiltration and flocculation–nanofiltration are only approximately 50 and 10 LMH (L/m2-h), respectively. To provide wastewater treatment for the medium-scale car wash factory discussed in [], an ultrafiltration plant with a size of approximately 100 m2 would be required. Such a plant would occupy a large space, and would thus be unsuitable for highly developed urban areas. Despite being able to remove partial COD, the general COD removal rate of the flocculation–filtration process is approximately 60% (Table 3).
The coagulation–filtration process has turbidity and COD removal rates of approximately 90% and 60%, respectively (Table 4).

Table 4.
Removal rate of various water qualities by filtration.
When coupled with filtration technology, the biological treatment process achieves turbidity and COD removal rates of approximately 99% and 95%, respectively [,].
4.4. Coagulation–Flocculation (CF)
Figure 5 depicts the processes of coagulation–flocculation (CF), which is a two-stage reaction system. In coagulation, a coagulant such as polyaluminum chloride (PAC) or ferric chloride is added to the wastewater to modify the surface charge of the particle pollutants, thereby eliminating the electrostatic repulsion between the particles was. The flocculant (i.e., polymer) is then added to the wastewater to aggregate the near-neutral electrostatic particles and form flocs for easier pollutant removal. Generally speaking, the turbidity removal rate of CF with car wash wastewater is good, generally over 90%; however, the removal rates of COD, O&G, and AS are not as good [,]. In addition, CF needs to add a suitable flocculant, which can easily cause cost increases and secondary pollution. Table 5 lists the effects of using CF and its combinations on car wash wastewater treatment as found in the literature.
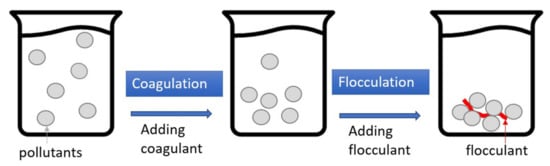
Figure 5.
Schematic illustration of coagulation–flocculation (CF).

Table 5.
Removal rate of various water qualities by coagulation–flocculation.
4.5. Bio-Treatment
Figure 6 illustrates the mechanism of bio-treatment. Aerobic microorganisms in the wastewater degrade the organics into H2O and CO2, while the dead biomass of microorganisms forms a sludge in the wastewater. Table 6 lists the effects of biological treatment combined with other technologies on car wash wastewater treatment. For biological treatment followed by filtration treatment, the removal rate of turbidity, COD, and AS can reach more than 95% [,,,].
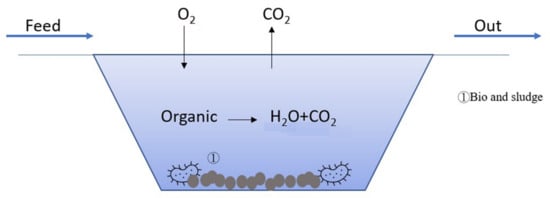
Figure 6.
Illustration of bio-treatment.

Table 6.
Removal rate of various water qualities by bio-treatment.
4.6. Other Methods
A few other single treatment methods, such as the Photo-Fenton’s process [], adsorption [], electro-oxidation [], etc., are listed in the table below (Table 7). Except for electro-oxidation, these single-unit processing technologies have a removal rate of less than 90%.

Table 7.
Removal rate of various water qualities by other single unit treatment techniques.
Treated water with low turbidity can be obtained by coagulation and flocculation, however, the added chemicals increase the amount of sludge. Treated water with relatively low turbidity can be obtained by ultrafiltration and nanofiltration, as well; however, the filter material is expensive, and a large filtration area is required to obtain a large amount of recycled water. While the electrocoagulation treatment method has a good treatment effect on AS and O&G, it is less effective than filtration in turbidity treatment. Meanwhile, the sacrificial electrode causes additional sludge. Bio-treatment has an excellent effect on COD, although the biological treatment method is relatively slow and unstable. The combination of at least two wastewater treatment techniques can enable the achievement of high treatment efficiency of car wash wastewater treatment.
5. Energy Consumption
The energy consumption rates of the wastewater treatment methods used by EC have been reported by Pinto et al. [], Kara [], and Nguegang et al. [] as 0.14, 1.5, and 2.7 kWh/m3, respectively. The energy consumption rate based on COD reduction was 66 kWh/kg after 6 h operation []. In [], the energy consumption rate was 10 kWh/m3; in [], the energy consumption rate ranged from 1.5 to 2.7 kWh/m3. In [], car wash wastewater was subjected to electrocoagulation using a new cell design featuring a horizontal spiral anode placed above a horizontal disk cathode. Excellent treatment results were achieved through electrocoagulation. El-Ashtoukhy et al. [] used a new EC cell with a spiral tube anode placed above a flat plate cathode resting on the cell bottom; the energy consumption based on COD reduction ranged from 2.3 to 15.1 kWh/kg. In [], the energy consumption for the treatment of transport container washing wastewater ranged from 3.1 to 46.5 kWh/m3. The aforementioned electrochemical techniques can have varying flow capacities and involve the use of different types of electrodes. In general, an electricity consumption rate between 0.5 and 2 kWh/m3 is considered reasonable.
6. Operating Cost
The operating cost depends on the techniques processes used in carwash wastewater treatment, including materials, chemicals, energy consumption, sludge disposal, labor, etc. Table 8 summarizes the operating cost and payback duration for the various techniques. Among the EC methods, the case using a titanium electrode costs substantially more than using an Al and Fe electrode [,,]. In [], the treatment of transport container washing wastewater is discussed. Because transport container washing wastewater has high COD and turbidity (specifically 8200 mg/L and 420 NTU, respectively), the sludge production rate was 12 kg/m3. The payback duration was reported as 6 and 15 months for the bio-membrane and electrocoagulation methods when combined with flotation (ECF), respectively [,].

Table 8.
Comparison of operating costs.
The detailed items of operating cost for FF technology have been previously determined; the respective cost for chemicals, sludge disposal, and electricity consumption was USD 0.43/m3, USD 0.07/m3, and USD 0.423/m3 []. The market value of a flocculation-column flotation system with a capacity of 1.0 L/h was USD 8687.50 in Brazil. The wastewater treatment system discussed in [] had a fixed operating cost of USD 2677 and an electricity consumption cost of USD 258.4 kWh/year in filtration, equivalent to USD 51.6/year.
7. Discharge Standards
Table 9 presents the car wash wastewater treatment regulations of various countries. Most countries have imposed the following rigorous regulations on car wash wastewater treatment: SS concentration < 40 mg/L, turbidity < 5 NTU, COD < 50 mg/L, O&G concentration < 5 mg/L, and AS value < 2 mg/L.

Table 9.
Discharge standards of different countries.
The regulations on suspended solids in China and Australia require less than 5 mg/L, which is stricter. In terms of nephelometric turbidity units, most of the specifications listed in the table are NTU < 5. As the particulate pollution of car wash wastewater is comprehensively reflected in SS and NTU, the regulation of SS < 5 m/L and NTU < 5 is relatively reasonable.
8. Conclusions
This study reviewed the literature on wastewater quality, wastewater treatment technology, the electricity consumption and operating costs of wastewater treatment, and wastewater treatment-related regulations. In summary, car wash wastewater treatment facilities are worth investing in for the reasons described in the text. First, the required filtering, electrochemical, and bioprocessing technologies are relatively mature and able to remove pollutants, i.e., SS, COD, O&G and AS, at rates above ~85%. The operating procedures of the treatment facilities are not complicated, and the operating costs are within a reasonable range, from USD/m3 0.3–0.92. Second, by selecting and coupling various types of wastewater treatment technologies, operators can treat wastewater in a way that meets government regulations. Third, regions with abundant water resources may experience short- or medium-term droughts under the effects of climate change; thus, water resources are highly valuable. However, whether recycling wastewater yields profit is a valid commercial concern.
Water resources are relatively scarce in the face of frequent droughts and floods in extreme weather. From the second half of 2020 to the first half of 2021, Taiwan has experienced nearly a year of drought (Taiwan is a country with an average annual rainfall of 2500 mm). In Taichung City, water is only available four days a week. While effective and active treatment of car wash wastewater may not be economical, it is extremely important for the sustainability of precious water resources.
Author Contributions
Data curation, C.-Y.H. and L.-W.K.; Investigation, W.-H.K.; Methodology, C.-Y.H.; Supervision, W.-H.K.; Writing—original draft, J.-M.W.; Writing—review & editing, J.-M.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Environmental Protection Administration of the China, Taiwan, for financially supporting this research under Contract No. EPA 109-A339.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Environmental Protection Administration of the Republic of China, Taiwan, for financially supporting this research under Contract No. EPA 109-A339.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| AD | adsorption |
| AS | anionic surfactant |
| Bio | biological treatment |
| COD | chemical oxygen demand |
| C | chemical coagulation |
| CF | coagulation-flocculation |
| DAF | dissolved air flotation |
| EC | electrocoagulation |
| EO | electro-oxidation |
| F | filtration |
| FF | flocculation flotation |
| M | membrane filtration |
| MBR | membrane bio-reactor |
| MF | microfiltration |
| NF | nanofiltration |
| NJDEP | New Jersey Department of Environmental Protection |
| NTU | nephelometric turbidity units |
| O&G | oil and grease |
| O | ozonation |
| RBC | rotating biological contactor |
| RO | reverse osmosis |
| SC | sand filtration and chlorination |
| SED | sedimentation |
| SF | sand filtration |
| SS | suspended solids |
| UF | ultrafiltration |
References
- Available online: https://www.statista.com/statistics/200002/international-car-sales-since-1990/ (accessed on 18 April 2022).
- Bakacs, M.E.; Yergeau, S.E.; Obropt, C.C.; ASCE, P.E.M. Assessment of car wash runoff treatment using bioretention mesocosms. J. Environ. Eng. 2013, 139, 1132–1136. [Google Scholar] [CrossRef]
- Available online: https://www.slideshare.net/guest7527d21f/ss-2773696 (accessed on 18 April 2022).
- Available online: https://kknews.cc/zh-tw/agriculture/xmm99mg.html (accessed on 18 April 2022).
- Torkashvand, J.; Farzadkia, M.; Younesi, S.; Gholami, M. A systematic review on membrane technology for carwash wastewater treatment: Efficiency and limitations. Desalin. Water Treat. 2021, 210, 81–90. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Valeo, C.; Gupta, R.; Constabel, C.P. Exploring the Potential in LID Technologies for Remediating Heavy Metals in Carwash Wastewater. Sustainability 2021, 13, 8727. [Google Scholar] [CrossRef]
- Gomes, A.J.; Das, K.K.; Jame, S.A.; Cocke, D.L. Treatment of truck wash water using electrocoagulation. Desalin. Water Treat. 2016, 57, 25991–26002. [Google Scholar] [CrossRef]
- Rubi-Juarez, H.; Barrera-Diaz, C.; Uena-Nunez, F. Adsorption-assisted electrocoagulation of real car wash wastewater with equilibrium and kinetic studies. Pollut. Res. 2017, 36, 175–184. [Google Scholar]
- Rubi-Juarez, H.; Barrera-Diaz, C.; Linares-Hernandez, I.; Fall, C.; Bilyeu, B. A combined electrocoagulation-electrooxidation process for carwash wastewater reclamation. Int. J. Electrochem. Sci. 2015, 10, 6754–6767. [Google Scholar]
- Zaneti, R.; Etchepare, R.; Rubio, J. More environmentally friendly vehicle washes: Water reclamation. J. Clean. Prod. 2012, 37, 115–124. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. Car wash wastewater reclamation. Full-scale application and upcoming features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Subtil, E.L.; Rodrigues, R.; Hespanhol, I.; Mierzwa, J.C. Water reuse potential at heavy-duty vehicles washing facilities—The mass balance approach for conservative contaminants. J. Clean. Prod. 2017, 166, 1226–1234. [Google Scholar] [CrossRef]
- Rubio, J.; Zaneti, R.N. Treatment of washrack wastewater with water recycling by advanced flocculation–column flotation. Desalin. Water Treat. 2009, 8, 146–153. [Google Scholar] [CrossRef]
- Zaneti, R.N.; Etchepare, R.; Rubio, J. Car wash wastewater treatment and water reuse—A case study. Water Sci. Technol. 2013, 67, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; dos Santos, E.V.; de Araújo Costa, E.C.T.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.S.; de Barros Grossi, L.; de Melo, R.A.C.; de Assis, T.M.; Ribeiro, V.M.; Amaral, M.C.S.; de Souza Figueiredo, K.C. Carwash wastewater treatment by micro and ultrafiltration membranes: Effects of geometry, pore size, pressure difference and feed flow rate in transport properties. J. Water Process Eng. 2017, 17, 143–148. [Google Scholar] [CrossRef]
- Etchepare, R.; Zaneti, R.; Azevedo, A.; Rubio, J. Application of flocculation–flotation followed by ozonation in vehicle wash wastewater treatment/disinfection and water reclamation. Desalin. Water Treat. 2015, 56, 1728–1736. [Google Scholar] [CrossRef]
- Boussu, K.; Kindts, C.; Vandecasteele, C.; van der Bruggen, B. Applicability of nanofiltration in the carwash industry. Sep. Purif. Technol. 2007, 54, 139–146. [Google Scholar] [CrossRef]
- Paxéus, N. Vehicle washing as a source of organic pollutants in municipal wastewater. Water Sci. Technol. 1996, 33, 1–8. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Applicability of electrochemical methods to carwash wastewaters for reuse. Part 1: Anodic oxidation with diamond and lead dioxide anodes. J. Electroanal. Chem. 2010, 638, 28–32. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Applicability of electrochemical methods to carwash wastewaters for reuse. Part 2: Electrocoagulation and anodic oxidation integrated process. J. Electroanal. Chem. 2010, 638, 236–240. [Google Scholar] [CrossRef]
- Vaccari, M.; Gialdini, F.; Collivignarelli, C. Study of the reuse of treated wastewater on waste container washing vehicles. Waste Manag. 2013, 33, 262–267. [Google Scholar] [CrossRef]
- Sablayrolles, C.; Vialle, C.; Vignoles, C.; Montrejaud-Vignoles, M. Impact of carwash discharge on stormwater quality (Toulouse, France). Water Sci. Technol. 2010, 62, 2737–2746. [Google Scholar] [CrossRef][Green Version]
- Tony, M.A.; Bedri, Z. Experimental design of photo-Fenton reactions for the treatment of car wash wastewater effluents by response surface methodological analysis. Adv. Environ. Chem. 2014, 2014, 958134. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Amin, N.K.; Fouad, Y.O. Treatment of real wastewater produced from Mobil car wash station using electrocoagulation technique. Environ. Monit. Assess. 2015, 187, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Barakat, N.A.M.; Moaz, A. Treatment of wastewater contaminated with detergents and mineral oils using effective and scalable technology. Water Sci. Technol. 2013, 68, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Monney, I.; Donkor, E.A.; Buamah, R. Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef]
- Tekere, M.; Sibanda, T.; Maphangwa, K.W. An assessment of the physicochemical properties and toxicity potential of carwash effluents from professional carwash outlets in Gauteng Province, South Africa. Environ. Sci. Pollut. Res. 2016, 22, 12816–12828. [Google Scholar] [CrossRef] [PubMed]
- Nguegang, B.; Sibanda, T.; Tekere, M. Cultivable bacterial diversity, physicochemical profiles, and toxicity determination of car wash effluents. Environ. Monit. Assess. 2019, 191, 478. [Google Scholar] [CrossRef]
- Baddor, I.M.; Farhoud, N.; Abdel-Magid, I.M.; Alshami, S.; Hassan Ahmad, F.; Olabi, E.A. Study of car wash wastewater treatment by adsorption. In Proceedings of the International Conference of Engineering, Information Technology, and Science, Kuala Lumpur, Malaysia, 1 May 2014; pp. 2–22. [Google Scholar]
- Uçar, D. Membrane processes for the reuse of car washing wastewater. J. Water Reuse Desalin. 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Hashim, N.H.; Ibrahim, M.S.S.; Awang, Z. Adsorption of anionic surfactant presence in synthetic car wash wastewater by limestone. J. Appl. Chem. Nat. Resour. 2019, 1, 1–5. [Google Scholar]
- Kara, S. Treatment of transport container washing wastewater by electrocoagulation. Environ. Prog. Sustain. Energy 2013, 32, 249–256. [Google Scholar] [CrossRef]
- Gonder, Z.B.; Balcioglu, G.; Vergili, I.; Kaya, Y. An integrated electrocoagulation-nanofiltration process for carwash wastewater reuse. Chemosphere 2020, 253, 126713. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapoor, F.K.; Soori, M.M.; Mahvi, A.H. Application of combined chemical coagulation and electrocoagulation process to carwash wastewater treatment. Fresenius Environ. Bull. 2012, 21, 2694–2701. [Google Scholar]
- Mirshahghassemi, S.; Aminzadeh, B.; Torabian, A.; Afshinnia, K. Optimizing electrocoagulation and electro-Fenton process for treating car wash wastewater. Environ. Health Eng. Manag. J. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Mohammadi, M.J.; Takdastan, A.; Jorfi, S.; Neisi, A.; Farhadi, M.; Yari, A.R.; Dobaradaran, S.; Khaniabadi, Y.O. Electrocoagulation process to Chemical and Biological Oxygen Demand treatment from carwash grey water in Ahvaz megacity, Iran. Data Brief 2017, 11, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.J.; Salari, J.; Takdastan, A.; Farhadi, M.; Javanmardi, P.; Yari, A.R.; Dobaradaran, S.; Almasi, H.; Rahimi, S. Removal of turbidity and organic matter from car wash wastewater by electrocoagulation process. Desalin. Water Treat. 2017, 68, 122–128. [Google Scholar] [CrossRef]
- Do, K.U.; Kim, J.H.; Chu, X.Q. Sludge characteristics and performance of a membrane bioreactor for treating oily wastewater from a car wash service station. Desalin. Water Treat. 2018, 120, 166–172. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Firdaus, S. Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep. Purif. Technol. 2013, 104, 26–31. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Mohamed, R.M.S.R.; Rahman, M.A.A.; Johari, M.R.; Kassim, A.H.M. Treatment of wastewater from car washes using natural coagulation and filtration system. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012046. [Google Scholar] [CrossRef]
- Radin Mohamed, R.M.S.; Abdul Rahman, N.; Mohd Kassim, A.H. Moringa Oleifera and Strychnos Potatorum seeds as natural coagulant compared with synthetic common coagulants in treating car wash wastewater: Case study 1. Asian J. Appl. Sci. 2014, 2, 693–700. [Google Scholar]
- Mohamed, R.M.S.R.; Saphira, R.M.; Kutty, A.I.; Mariam, N.; Kassim, M.; Hashim, A. Efficiency of using commercial and natural coagulants in treating car wash wastewater treatment. Aust. J. Basic Appl. Sci. 2014, 8, 227–234. [Google Scholar]
- Zhang, J.K.; Yang, Y.B.; Wang, H.Y.; Dong, Z.B. CFU combined process for the treatment of oily car washing wastewater. Appl. Mech. Mater. 2013, 253–255, 999–1004. [Google Scholar] [CrossRef]
- Tan, X.; Tang, L. Application of enhanced coagulation aided by UF membrane for car wash wastewater treatment. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 3653–3656. [Google Scholar] [CrossRef]
- Chu, J.Y.; Li, Y.R.; Li, N.; Huang, W.H. Treatment of Car-washing Wastewater by Electrocoagulation-Ultrasound Technique for Reuse. Adv. Mater. Res. 2012, 433–440, 227–232. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef] [PubMed]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Moazzem, S.; Ravishankar, H.; Fan, L.; Roddick, F.; Jegatheesan, V. Application of enhanced membrane bioreactor (eMBR) for the reuse of carwash wastewater. J. Environ. Manag. 2020, 254, 109780. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Mahmood, Q.; Raja, I.A.; Malik, A.H.; Khan, M.S.; Wu, D. Chemical oxidation of carwash industry wastewater as an effort to decrease water pollution. Phys. Chem. Earth Parts A/B/C 2011, 36, 465–469. [Google Scholar] [CrossRef]
- Syed, N.H.; Ahmad, J.; Khan, N.A.; Khan, N.; Shafiq, M.A. A low-cost wastewater treatment unit for reducing the usage of fresh water at car wash stations in Pakistan. Pak. J. Sci. Ind. Res. A Phys. Sci. 2019, 62A, 57–66. [Google Scholar] [CrossRef]
- Bhatti, S.; Siddiqui, Z.; Memon, S.; Kandhir, I.; Memon, M.A.; Mahesar, A.W. Analysis and treatment wash off water from vehicular service station in Hyderabad. Sindh Univ. Res. J. SURJ (Sci. Ser.) 2017, 49, 473–478. [Google Scholar] [CrossRef]
- Hsu, S.K.; Chen, C.H.; Chang, W.K. Reclamation of car washing wastewater by a hybrid system combining bio-carriers and non-woven membranes filtration. Desalin. Water Treat. 2011, 34, 349–353. [Google Scholar] [CrossRef]
- Tu, W.K.; Chang, C.C.; Chang, C.Y.; Ji, D.R.; Tseng, J.Y.; Chiu, C.Y.; Chen, Y.H.; Chang, C.F.; Yu, Y.H. Treatment of car wash wastewater via novel technologies for recycling and reutilization. J. Environ. Eng. Manag. 2009, 19, 49–57. [Google Scholar]
- Istirokhatun, T.; Destianti, P.; Hargianintya, A.; Oktiawan, W.; Susanto, H. Treatment of car wash wastewater by UF membranes. In Proceedings of the International Conference of Chemical and Material Engineering, Kyoto, Japan, 29 December 2015; p. 060025. [Google Scholar]
- Asha, M.N.; Chandan, K.S.; Harish, H.P.; NikhileswarReddy, S.; Sharath, K.S.; Liza, G.M. Recycling of waste water collected from automobile service station. Procedia. Environ. Sci. 2016, 35, 289–297. [Google Scholar] [CrossRef]
- Alam, J.; Farooqi, I.H. Management of grey water of an automobile workshop—A case study. In Proceedings of the International Workshop on Civil Engineering and Architecture, Istanbul, Turkey, 8–9 August 2014; pp. 133–138. [Google Scholar]
- Kiran, S.A.; Arthanareeswaran, G.; Thuyavan, Y.L.; Ismail, A.F. Influence of bentonite in polymer membranes for effective treatment of car wash effluent to protect the ecosystem. Ecotoxicol. Environmen. Saf. 2015, 121, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Al-Odwani, A.; Ahmed, M.; Bou-Hamad, S. Carwash water reclamation in Kuwait. Desalination 2007, 206, 17–28. [Google Scholar] [CrossRef]
- Torkashvand, J.; Pasalari, H.; Gholami, M.; Younesi, S.; Oskoei, V.; Farzadkia, M. On-site carwash wastewater treatment and reuse: A systematic review. Int. J. Environ. Anal. Chem. 2020, 100, 1–15. [Google Scholar] [CrossRef]
- Nadzirah, Z.; Nor Haslina, H.; Rafidah, H. Removal of important parameter from car wash wastewater—A review. Appl. Mech. Mater. 2015, 773–774, 1153–1157. [Google Scholar] [CrossRef]
- Genuino, H.C.; Opembe, N.N.; Njagi, E.C.; McClain, S.; Suib, S.L. A review of hydrofluoric acid and its use in the car wash industry. J. Ind. Eng. Chem. 2012, 18, 1529–1539. [Google Scholar] [CrossRef]
- Kumar, N.S.; Chauhan, M.S. Water Quality Management; Treatment of car washing unit wastewater—A review; Springer International Publishing: Singapore, 2018; pp. 247–255. [Google Scholar]
- Sarmadi, M.; Foroughi, M.; Saleh, H.N.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient technologies for carwash wastewater treatment: A systematic review. Environ. Sci. Pollut. Res. 2020, 28, 34823–34839. [Google Scholar] [CrossRef]
- Hassani, A.; Malhotra, M.; Karim, A.V.; Krishnan, S.; Nidheesh, P.V. Recent progress on ultrasound-assisted electrochemical processes: A review on mechanism, reactor strategies, and applications for wastewater treatment. Environ. Res. 2022, 205, 112463. [Google Scholar] [CrossRef]
- Boussu, K.; van Baelen, G.; Colen, W.; Eelen, D.; Vanassche, S.; Vandecasteele, C.; van der Bruggen, B. Technical and economical evaluation of water recycling in the carwash industry with membrane processes. Water Sci. Technol. 2008, 57, 1131–1135. [Google Scholar] [CrossRef]
- Gonder, Z.B.; Balcioglu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Gonder, Z.B.; Balcıoğlu, G.; Kaya, Y.; Vergili, I. Treatment of carwash wastewater by electrocoagulation using Ti electrode: Optimization of the operating parameters. Int. J. Environ. Sci. Technol. 2019, 16, 8041–8052. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Olfati, D.; Gohari, F.; Hassani, A. TiO2 nanoparticles removal by electrocoagulation using iron electrodes: Catalytic activity of electrochemical sludge for the degradation of emerging pollutant. J. Mol. Liq. 2020, 310, 113217. [Google Scholar] [CrossRef]
- Enoh, B.S.; Christopher, W. Adsorption of metal ions from carwash wastewater by phosphoric acid modified clay: Kinetics and thermodynamic studies. Chem. Mater. Res. 2015, 7, 278026770. [Google Scholar]
- Ghanbari, F.; Wang, Q.; Hassani, A.; Wacławek, S.; Rodríguez-Chueca, J.; Lin, K.Y.A. Electrochemical activation of peroxides for treatment of contaminated water with landfill leachate: Efficacy, toxicity and biodegradability evaluation. Chemosphere 2021, 279, 130610. [Google Scholar] [CrossRef]
- Ahmadt, A.; Zarei, M.; Hassani, A.; Ebratkhahan, M.; Olad, A. Facile synthesis of iron(II) doped carbonaceous aerogel as a three-dimensional cathode and its excellent performance in electro-Fenton degradation of ceftazidime from water solution. Sep. Purif. Technol. 2021, 278, 119559. [Google Scholar] [CrossRef]
- Zapién Serrano, L.Z.; Ortiz Lara, N.O.; Ríos Vera, R.R.; Cholico-González, D. Removal of Fe(III), Cd(II), and Zn(II) as Hydroxides by Precipitation–Flotation System. Sustainability 2021, 13, 11913. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Y.S.; Ji, S.W. Investigation of Mineral-Processing Wastewater Recycling Processes: A Pilot Study. Sustainability 2018, 10, 3069. [Google Scholar] [CrossRef]
- Siddig, O.; Al-Afnan, S.; Elkatatny, S.; Bahgat, M. Novel Cake Washer for Removing Oil-Based Calcium Carbonate Filter Cake in Horizontal Wells. Sustainability 2020, 12, 3427. [Google Scholar] [CrossRef]
- Mohamed, A.; Basfar, S.; Elkatatny, S.; Al-Majed, A. Prevention of Barite Sag in Oil-Based Drilling Fluids Using a Mixture of Barite and Ilmenite as Weighting Material. Sustainability 2019, 11, 5617. [Google Scholar] [CrossRef]
- Jönsson, C.; Jönsson, A.S. The influence of degreasing agents used at car washes on the performance of ultrafiltration membranes. Desalination 1995, 100, 115–123. [Google Scholar] [CrossRef]
- Hamada, T.; Miyazaki, Y. Reuse of carwash water with a cellulose acetate ultrafiltration membrane aided by flocculation and activated carbon treatments. Desalination 2004, 169, 257–267. [Google Scholar] [CrossRef]
- Tang, L.; Tan, X.J.; Cui, F.Y.; Zhou, Q.; Yin, J. Reuse of carwash wastewater with hollow fiber membrane aided by enhanced coagulation and activated carbon treatments. Water Sci. Technol. 2007, 56, 111–118. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).