1. Introduction
Land use, land cover (LULC) changes are defined as variations to the earth’s surface, triggered mainly through anthropogenic activities like agriculture, deforestation, and urbanization; and natural factors like soil erosion, floods, and landslides; and exacerbated by climate change (CC) [
1,
2,
3,
4]. These variations have become significant drivers of change in the terrestrial and aquatic ecosystems since the industrial revolution [
5,
6]. There is a strong connectivity and interdependence between land and water ecosystems through a network of streams and rivers, runoff, or groundwater. This has made the study of the relationship between land and water to find mitigation measures at all levels a current global priority [
2,
7,
8,
9,
10,
11]. Anthropogenic activities in the watersheds, alongside with atmospheric deposition, are the major contributors of nutrients and sediments into the rivers, lakes, and wetlands [
12,
13,
14,
15,
16]. Hassan et al. [
2] has associated LULC changes to the loss of agricultural land, water, and soil quality degradation, global warming, and deforestation. There are several other studies at the global level that have been carried out to show the impacts of different LULC activities in the catchment on water resources, including streams and rivers [
17,
18], reservoirs [
19], wetlands, and lakes [
20,
21]. Intensive anthropogenic activities like mining, deforestation, urbanization, and agriculture have had significant impacts on the normal dynamics and functioning of freshwater ecosystems [
2,
22,
23,
24,
25,
26,
27]. The consequences of the nutrient and sediment loading into water bodies vary, including eutrophication, siltation, and proliferation of invasive water weeds [
28,
29]. LULC activities affect water resources in two major ways. Firstly, through point sources like industrial and domestic effluents [
2,
30]. Secondly, through non-point or diffuse pollution sources like agriculture, through applied agro-chemicals, deforestation, livestock grazing, urbanization, and atmospheric deposition [
15,
30,
31,
32,
33,
34,
35,
36]. Due to the widespread and persistent nature of diffuse pollution, some studies have described it as a ‘wicked problem’ at a global level [
37] and difficult to control.
The significance of research on the impact of LULC changes in the catchments on water resources is based on the importance of good water quality to the environment and human society. Lakes, rivers, and reservoirs provide vital goods, like drinking water, water for irrigation, protein through fisheries, and services like navigation, energy, recreation through tourism, climate moderation, and flood control [
19,
29]. These goods and services are threatened by pollution of water bodies which result in eutrophication [
23,
38,
39,
40]. Although eutrophication of water bodies is a naturally slow process, rapid nutrient enrichment of especially nitrogen and phosphorus [
27] accelerates it from the catchment, making it a global problem [
41,
42]. The studies have shown that LULC activities in the catchment affect water quality and regional climate and alter the hydrological cycle [
43]. Land degradation and fragmentation have resulted in the loss of habitats, degradation of water resources, and loss of biodiversity, all associated with LULC anthropogenic activities at global, regional, and catchment levels [
44,
45]. Therefore, although catchments are very important for the proper functioning of lakes by providing nutrients, excess loading of the nutrients from LULC change activities is detrimental to the aquatic ecosystems.
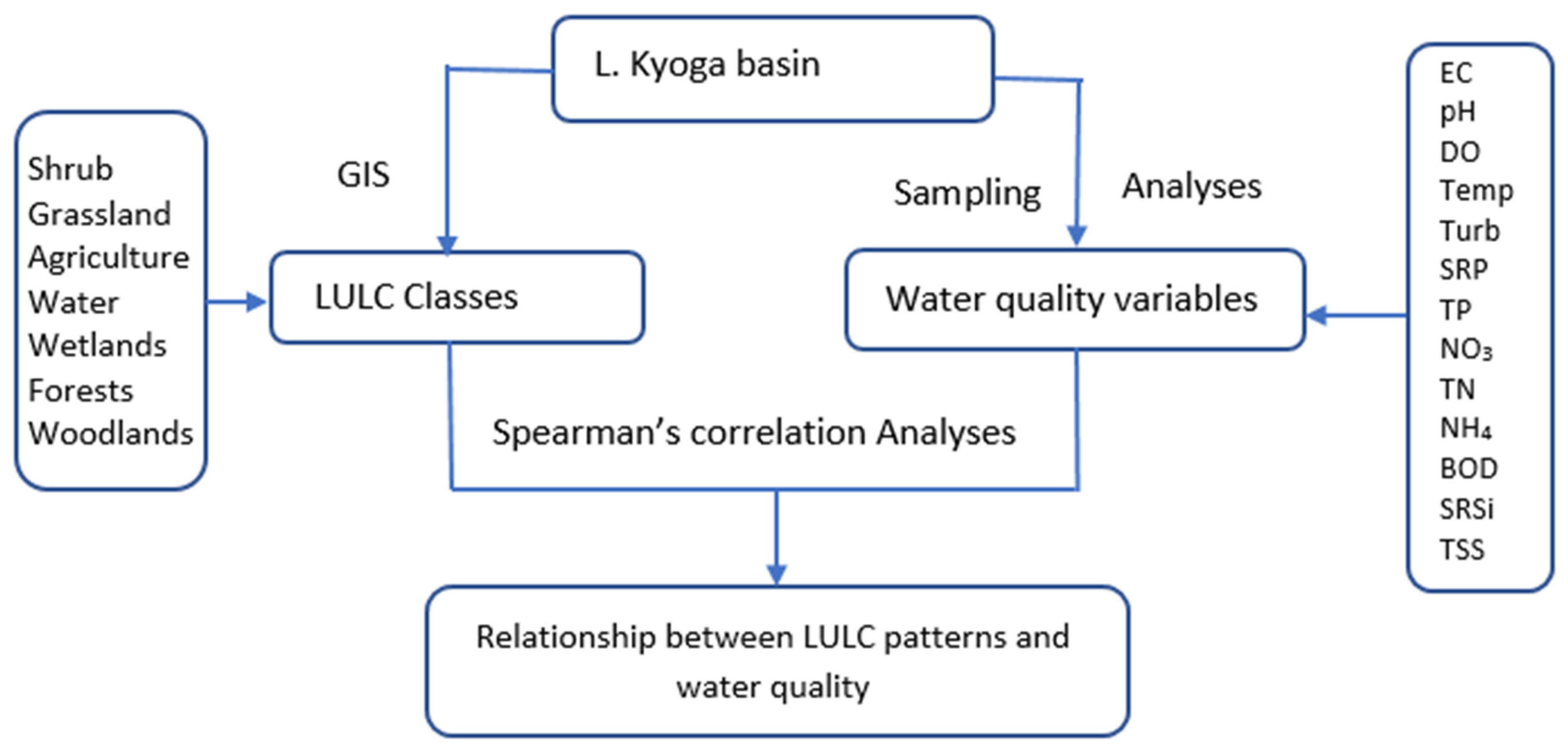
Many approaches have been applied to study the linkage between LULC activities in the catchment with water quality of the receiving water bodies, which started about forty years ago in the 1970s [
46,
47]. One of the current approaches is using remote sensing alongside GIS, to monitor LULC changes and water quality [
17,
48,
49,
50,
51]. This approach has been found to be cheap, comprehensive, consistent, and repetitive, useful for studies at large scales [
52]. Remote sensing and GIS are therefore critical in the assessment of drivers of LULC changes and their relationship with lake and reservoir and river water quality [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55], and for monitoring the causes of eutrophication [
18,
23,
40,
42]. Other approaches include using conservative multivariate practices [
56], like cluster analysis applied to show impacts on arable land on water quality in Germany and Czech Republic [
57]. Principal component analysis (PCA) in combination with cluster analysis has been applied to determine the influence of municipal effluent on the water quality of River Nag in India [
58]. The use of the Pearson coefficient has been applied in many countries, including S. Africa [
59], China [
60], and Zimbabwe [
61].
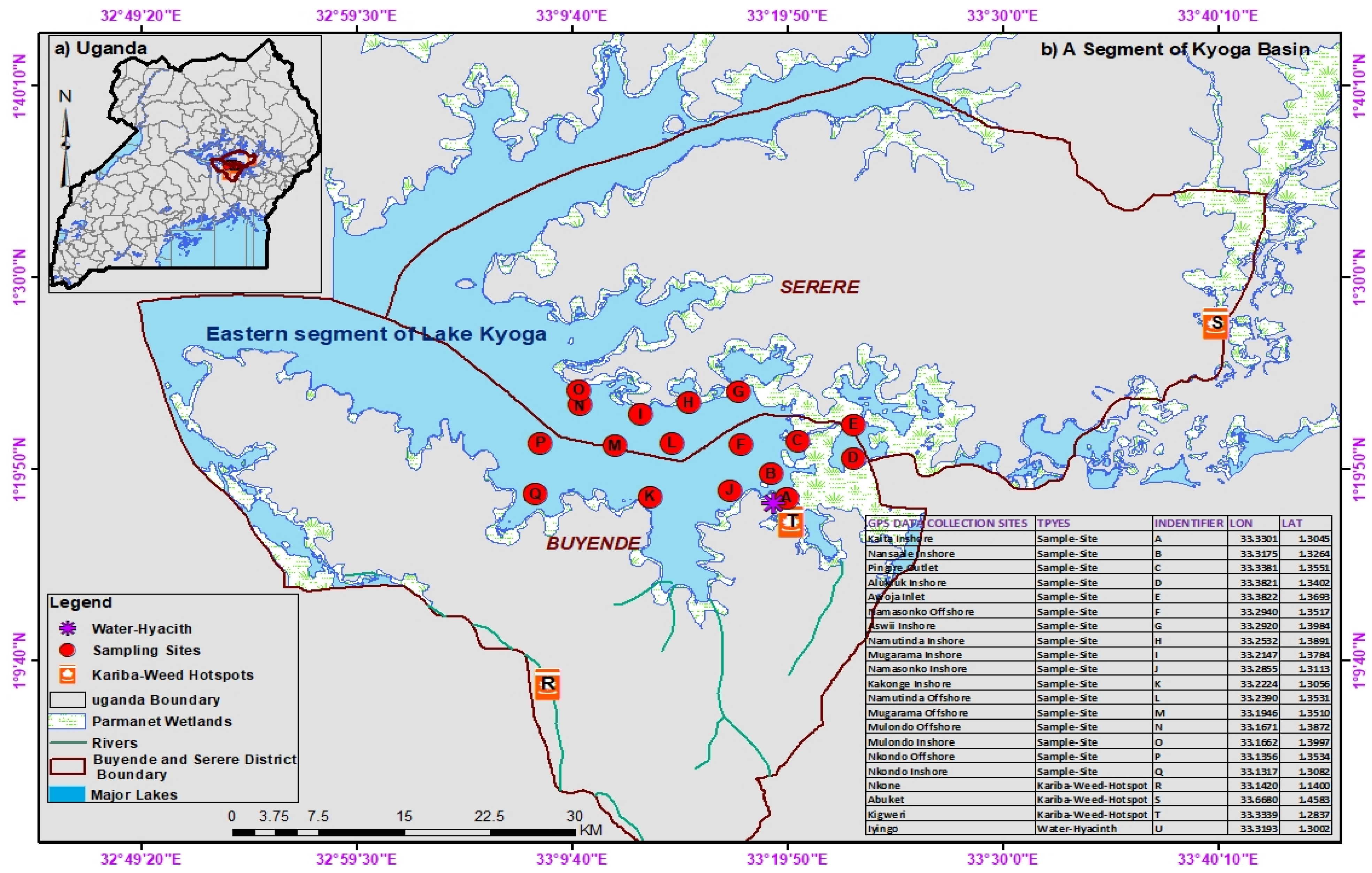
In Africa, for example, many studies have linked LULC changes with water quality deterioration [
29,
49,
60,
62,
63]. In Uganda, the water quality of Lake Kyoga is most likely to be influenced by LULC activities in the catchment, as suggested by Andama et al. [
64]. There may be few industries in the lake’s catchment; hence domestic effluents from urban centers and diffuse pollution from LULC activities and atmospheric deposition could probably be the main sources of nutrient loading into the lake. Further, through increased precipitation, floods, and extreme droughts, climate change exacerbates the influence of LULC on the lake’s water quality by transporting nutrients rapidly to the lake [
34,
35]. Catchment deforestation and wetlands conversion makes the lake more susceptible to the LULC effects. The high annual population growth rate in Uganda, estimated at 3.3% [
65], increased rate of urbanization, industrialization, and the growth of several socio-economic activities are the primary drivers of the anthropogenic impacts on land, environment, and water resources [
22,
45,
66]. These impacts affect shallow lakes more; hence L. Kyoga is facing significant threats which could be responsible for the proliferation of invasive water weeds, such as
Salvinia molesta and
Eichhornia crassipes [
64,
67]. Despite all these threats, detailed studies to link LULC activities in the catchments of L. Kyoga and its water quality have not been carried out. The focus of this study, therefore, is to establish the linkage between LULC activities in the catchments with water quality. The findings will be critical for planning and management of not only the water quality of the lake but also LULC activities in the catchments. Policy and decision-makers like Ministries of Water and Environment (MWE), Agriculture, Animal Industry, and Fisheries (MAAIF), Lands, Housing, and Urban Development (LHUD), and other stakeholders should use the results of this study to inform management actions. Further, studies using the Partial Least Squares (PLS) regression model, especially at the riverine level, are being undertaken to determine the linkage between water quality changes and LULC activities in the catchment [
68,
69]. Such studies are recommended in this study area since they are essential for planning and management purposes by policy makers.
4. Discussion
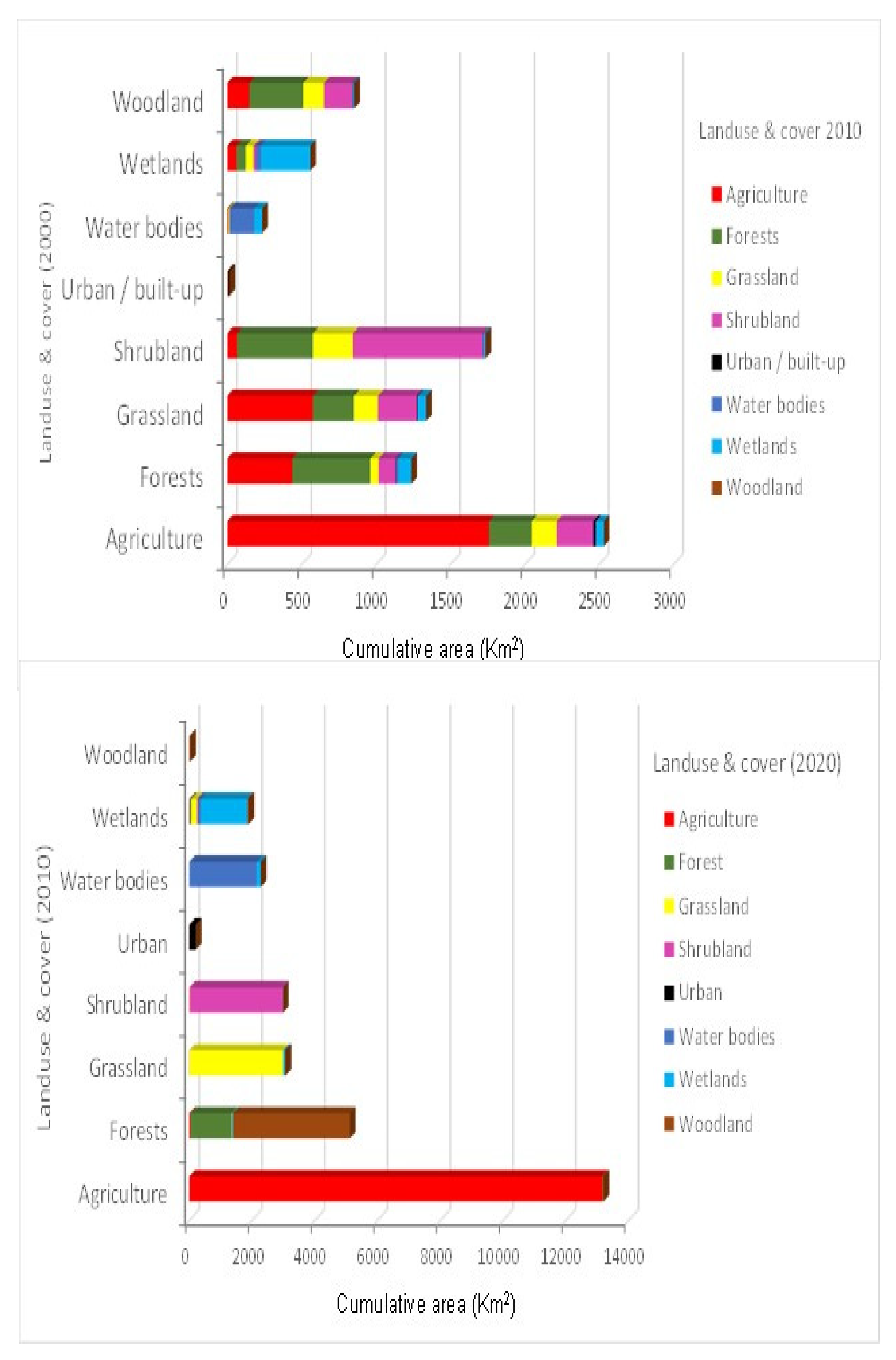
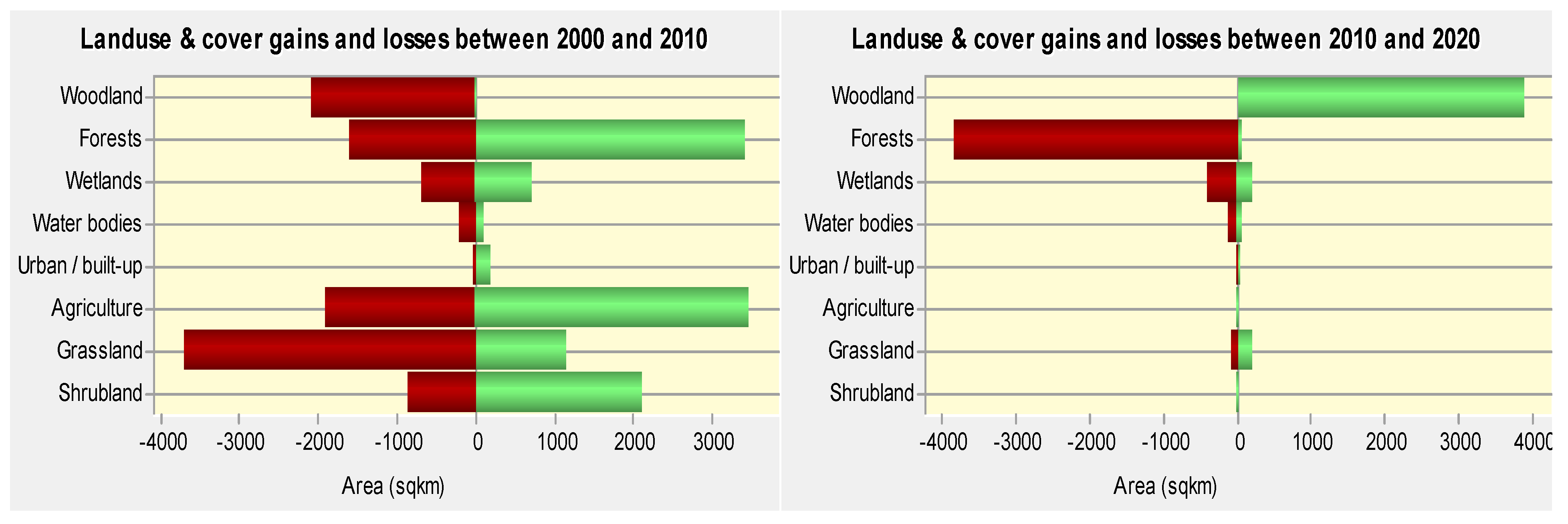
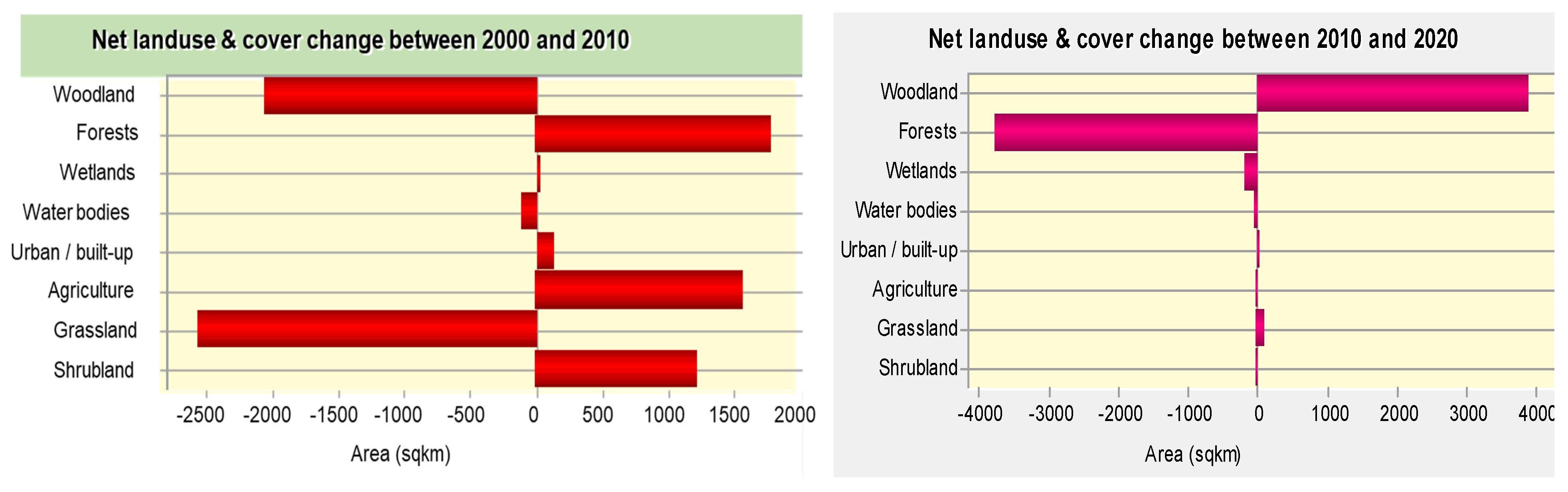
Analysis of LULC changes in the study area showed that non-point sources of pollution were the main driver of water quality changes in the Lake Kyoga basin. Agriculture was the most dominant LU activity in the study area, followed by grasslands, forests and waterbodies. Agriculture was not only dominant in the study area, but also nationally as most people depend on rainfed agriculture as a source of food and income. It had strong correlations with most of the water quality parameters, with significant correlation with nitrate (
Table 6). Its impacts on water quality emanate from different directions, including soil erosion, due to unsuitable tillage approaches, like soil excavation, with poor soil conservation measures, as pointed out by Guzha et al. [
104]; and Fatumah et al. [
105], and cultivation on steep slopes of Mt Elgon. Application of agro-chemicals, like fertilizers, herbicides, and plant growth boosters, which contain phosphorus, nitrogen, and potassium, are also major sources of the nutrients observed. Heavy rains in the area caused by climate change as reported in the related study [
106], exacerbates LULC impacts into the lake as floods help to deliver sediments and nutrients into Lake Kyoga. This is done through a network of rivers and streams including rivers, Awoja, Mpologoma, and Manafwa, with effects on water quality as has been shown elsewhere [
43,
80,
107,
108,
109,
110,
111,
112,
113,
114]. Most of the forests, grasslands, woodlands and shrublands have been destroyed for different reasons, including agriculture and charcoal burning to provide for the ever-growing human demands for energy in the form of charcoal, timber, and livestock grazing, which has impacted water quality, including reduction of essential DO [
115,
116,
117]. The only relatively intact natural resources are those gazeted and guarded by security, like Afromontane Mt. Elgon Forest, Elgon National Park, Pian Upe wildlife reserve and woodlots planted by individuals [
118]. These areas are however, being encroached upon for settlement, and agriculture due to the increasing population pressure.
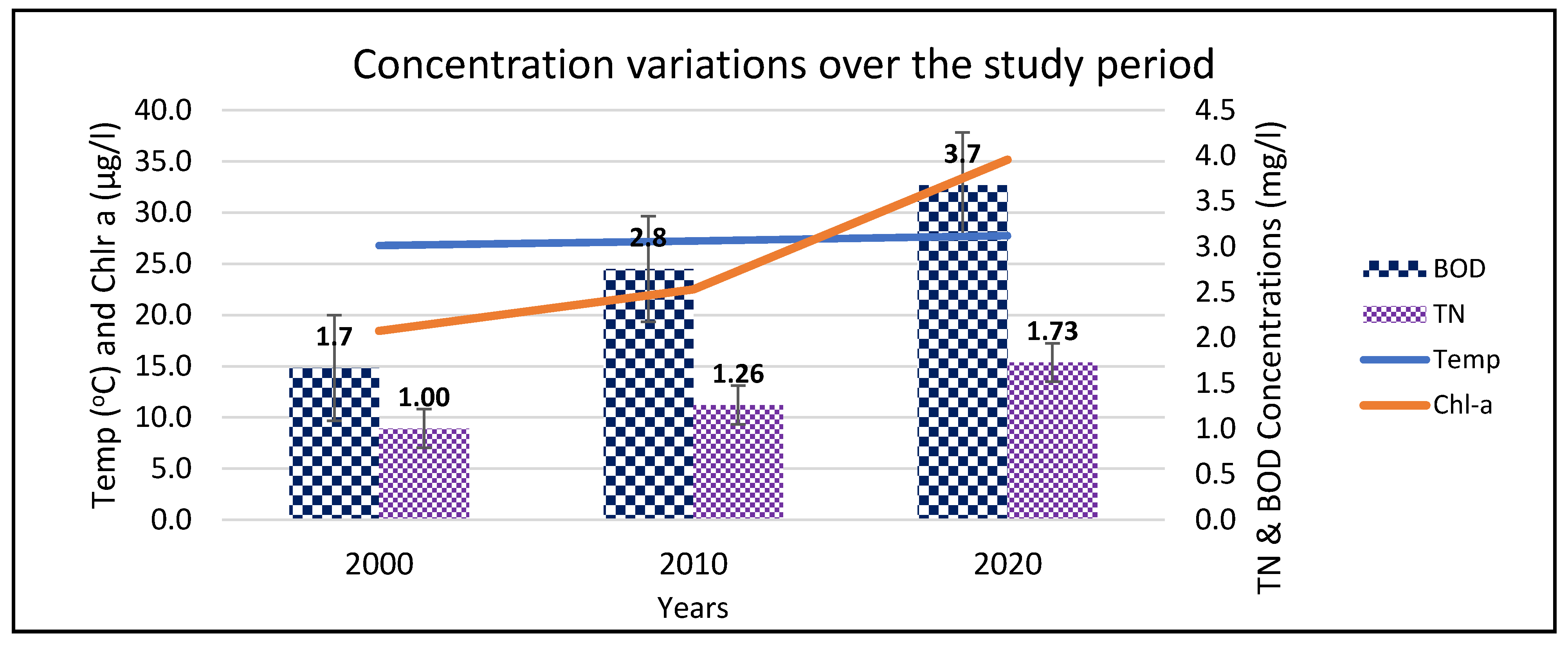
Wetlands play a key role in filtering nutrients and settling sediments from the catchments before the water enters the lake ecosystem; they also act as refugia and nursery areas for fish [
119,
120]. Lake Kyoga and its catchment wetlands, in the 1980s used to have a dense network of natural vegetation with
Miscanthidium spp, papyrus, and
Typha, being the primary vegetation [
71], and the lake had good water quality. Since the increased conversion of wetlands areas into mainly rice fields [
118], an economic activity which cannot sequestrate nutrients like the natural vegetation [
121,
122,
123], loading of nutrients into the lake has continued. This was indicated by the increase in the concentrations of nutrients, sediments and chl-
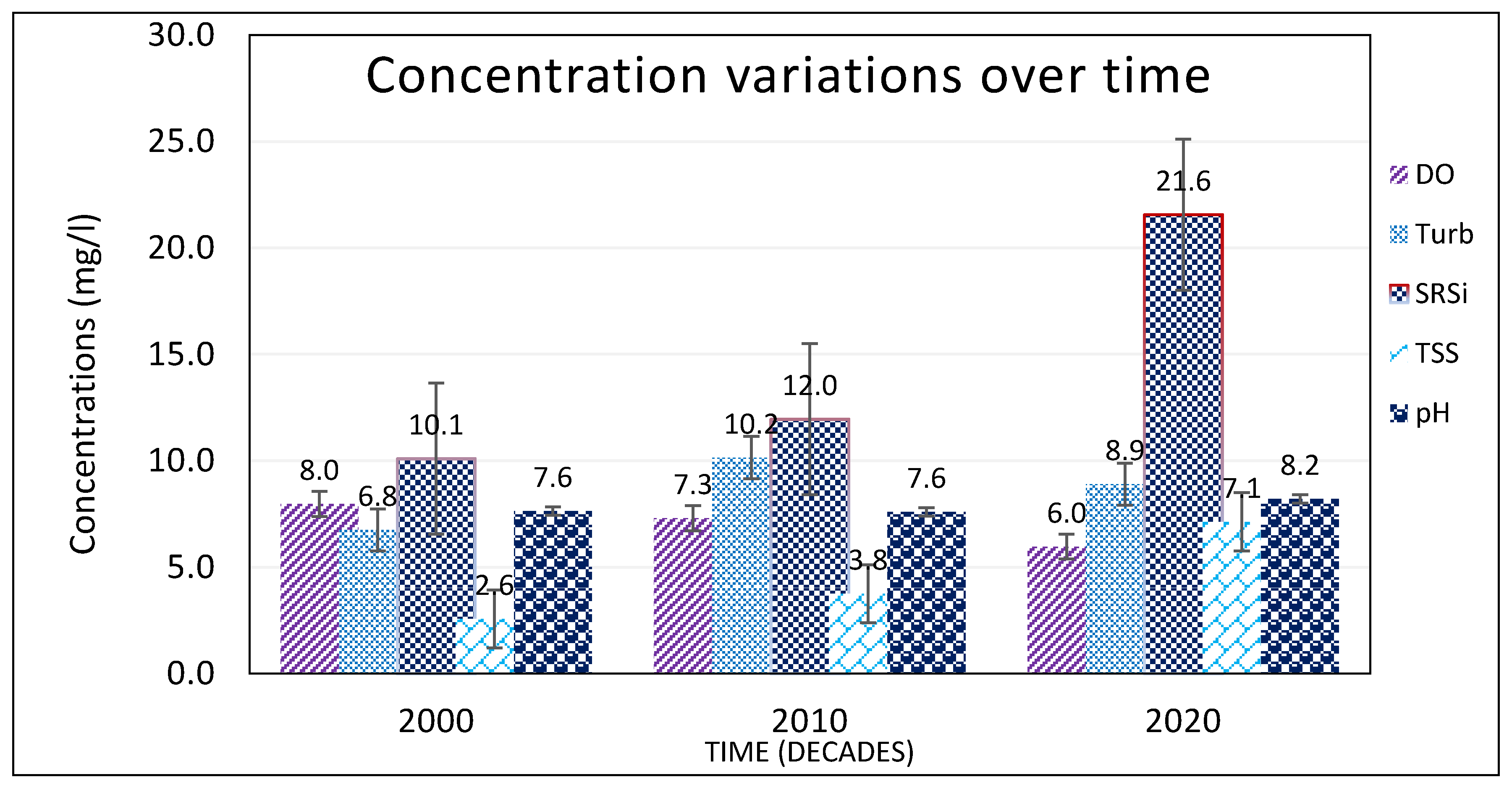
a over the study period (
Figure 4). The increase in urban/built-up areas, especially from 2010, could be associated with population increase and shortage of land for agriculture in the study area. The developing is driven by business purposes as an alternative source of livelihoods. These centers affect not only the water quality through point and non-point sources, but also hydrology of rivers and channels. They are sources of nutrients and sediments into L. Kyoga. The strong correlation between increase in urban centers with physico-chemical and biological parameters, especially DO, temp, turb, SRP, TP, nitrate, TN, BOD, and TSS is evidence of this relationship. Studies from other countries [
14,
59,
124,
125,
126,
127] agree with these findings. Water bodies showed a slightly decreasing trend over the study area; and this could be associated with increased evapotranspiration due to high temperatures and droughts caused by climate change [
106]. This decrease had an impact on all the water quality parameters in L. Kyoga as seen by solid positive and negative correlations. Similar results were obtained by other authors [
29,
30,
128,
129]. The degradation of wetlands, especially for agriculture [
123] was driven by land shortage and the growing demand for food and income from an ever-growing population. Therefore, to protect the lake from further deterioration of water quality, there is need to manage LU activities in the catchment.
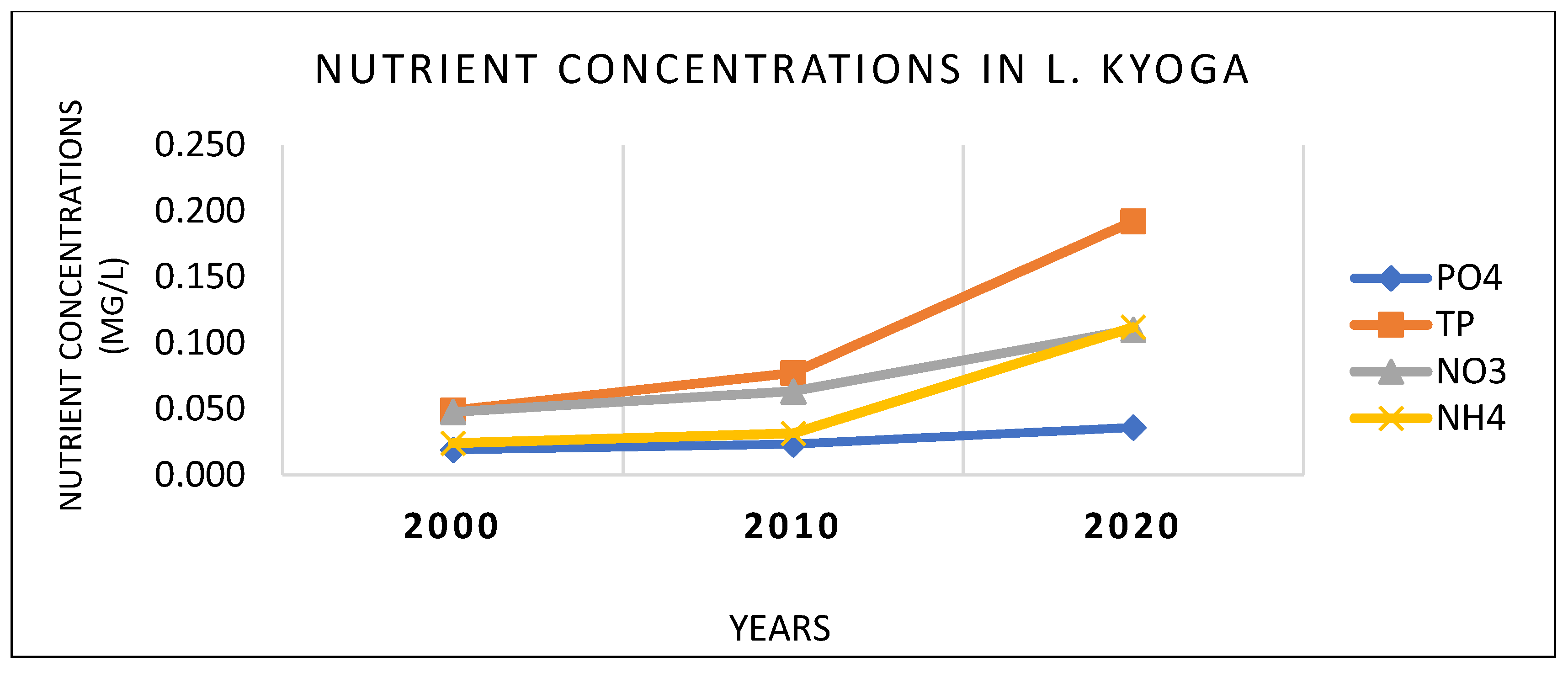
The LULC change activities in Awoja, Mpologoma, and Lwere catchments had impacts on the water quality in the eastern arm of L. Kyoga. This was shown by the steady deterioration over the study period, from year 2000 to 2020. This could be the same situation throughout the lake. The major physical, chemical, and biological parameters showed an increasing trend over the study period (
Figure 4,
Figure 5 and
Figure 6). These nutrients do not work in isolation, but synergize with each other to cause impacts on flora and fauna of the Lake Kyoga ecosystem. They play an essential role in the functioning and productivity of the lake, as pointed out by Wiens [
7] and Miranda et al. [
43]. Phosphorus, for example, is a limiting factor for algal growth and lake productivity in many aquatic ecosystems; hence its input together with nitrogen to L. Kyoga from the catchments had an impact on the trophic status of the lake, which agrees with the findings of Miranda et al. [
43]. Algae and macrophytes, which are primary producers, require these nutrients for their growth, hence forming the basis of the food chain in the lake, thereby supporting fish and other fauna that depend on these nutrients. However, the continued nutrient enrichment of L. Kyoga from agro-chemicals in quantities beyond the systems’ ecological requirements is a threat. It has led to a shift in the lake’s trophic status to a eutrophic state, tending to a hypereutrophic state, characterized by algal blooms, shortage of DO, and increased macrophyte biomass, as has been pointed out by other authors [
130,
131,
132,
133]. The consequences are reduced transparency, and an increase in organic matter [
86], siltation and reduction of the lake depth; deposition and hence storage of nutrients in sediments, unprecedented death of Nile perch in 2020 [
134], as was observed by other earlier studies in L. Victoria basin [
135,
136]. The lake’s shallow nature makes resuspension of nutrients especially phosphorus by wind action, making nutrients available for algae and other flora [
137]. Phosphorus, in particular, is reported to be more in the water column in the shallow lakes than in the sediment due to low retention capacity and frequent sediment resuspension of shallow lakes [
138], since it is a limiting factor for algae, its ever presence leads to algal blooms.
Temperature plays a significant role in controlling the rates of reactions in the water body, especially in shallow lakes, which do not experience stratification. The observed increase in temperature results in an increased algal and macrophyte productivity; together with nutrient enrichment, it has favored the invasion and spread of tropical invasive water weeds, especially
S. molesta (locally known as Nankabirwa weed) and
Eichhornia crassipes [
139,
140]. These invasive weeds have destroyed fish breeding areas, reduced light penetration into the lake, and hence reduced the concentration of DO in the water, affecting fauna and flora in the lake. They have also affected fisheries by destroying fishing gear, affected water supply, watering of domestic animals, and navigation, as has been reported by other authors [
64,
67]. Further, an increase in temperatures results in increased evapotranspiration rates, leading to the lake’s net water loss, as has been pointed out by other studies [
28,
141,
142]. The reduction in water bodies, together with wetland degradation, and forest and woodland reduction impacted chl-
a, SRSi, and pH (
Table 4), which agrees with other findings [
85,
143,
144]. The increased algal biomass, indicated by increased chl-
a, impacts fish yields; by supporting secondary and tertiary producers, and also increases DO levels in the lake. But the increased nutrient enrichment from the catchment has led to increased poor water quality, with negative impacts in the L. Kyoga ecosystem, as has been observed in other studies [
24,
145]. Although SRSi is vital for the growth of invertebrates like snails and diatoms, its increased enrichment is a cause of concern as it could increase algal blooms and proliferation of invertebrates. The pH of a water body influences the release of SRP from sediments [
146], and the growth of macrophytes [
147], it also buffers water and helps in dissolution of metals in water [
85]. Its variation over the study period, therefore, had impacts on the functioning, food chain, and food web interactions, and EC of L. Kyoga ecosystem. There was an observed increase in EC over the study period, a finding which agrees with results from other studies [
117,
148], and is likely to affect the mineral balance of the lake. The increase in BOD
5 showed raised loading of organic matter, which is biodegraded by aerobic microorganisms, leading to the reduction of DO, and release of nutrients as has been reported by other authors [
149,
150,
151]. Further, studies in Poland, Brazil, and Malawi [
85,
152,
153] also showed the same correlation between DO and LULC changes.
The impacts of LULC change do not only affect water quality, but also economic activities and livelihoods. For example, fishing in L. Kyoga for Nile perch, Nile tilapia, and silverfish locally called ‘Mukene’ [
67], is one of the major economic activities that provides food/protein and generate revenue for the central and local governments, in addition to the local communities. The lake’s pollution from catchment LULC change activities is threatening not only the fisheries but also other ecosystem functions and services provided by lake Kyoga. For example, the lake ecosystem has a huge potential for tourism, water supply to the riparian communities and upcoming cities like Mbale and Soroti, irrigation, and agriculture, which are under threat. Therefore, restoration of forests, woodlands, grasslands, shrublands, and wetlands, which are great sinks for nutrients and sediments from the catchments and management of urban centers should be prioritized as pointed out by the findings of MWE in the Awoja catchment plan report [
75]. Proper management of built-up areas will lead to reduction in the concentrations of physico-chemical and biological parameters and an increase in DO. The impacts of LULC change have also been observed in Chaohu Lake Basin China, USA, and Japan [
124,
154,
155,
156].
5. Conclusions
This study has proved the linkage between land and water resources as observed in different parts of the world. It has further shown that LULC change activities in the catchment affect the water quality of the adjacent water bodies, with climate change being the catalyst of the linkage. Over the study period, the water quality of L. Kyoga has shown a deteriorating trend associated with LULC change activities in the catchments, and these trends will continue getting worse if no action is taken to restore good water quality. The deterioration of water quality has been rapid, especially from 2010 to 2020, a worrying trend that requires urgent mitigation measures. The trends showed degraded water quality, as indicated by physico-chemical and biological parameters that are getting degraded. This study has proved that Lake Kyoga is in a eutrophic state, tending to a hypereutrophic state according to the Florida physico-chemical trophic state indices. The consequences of this eutrophic state include invasion of the lake by invasive water weeds like water hyacinth and Nankabirwa and death of Nile Perch in the lake due to poor water quality, especially reduction in the concentration of DO. The suitability of this water for drinking, irrigation, biodiversity conservation, fisheries, and recreation are also threatened. Agriculture was a dominant land use form in the lake’s catchment, alongside land, the cover was characterized by forests, wetlands, woodlands, grasslands, shrublands, and urban centers. These LULC changes impact the water quality of L. Kyoga negatively in most cases.
Any efforts to improve the water quality of the Lake Kyoga ecosystem from the current degradation trend should prioritize management of LULC changes in the catchments. The responsible stakeholders, like local government officials and the line ministries, need to enforce statutory laws and regulations on protecting water bodies. Like river bank protection, protecting and preserving of 100–200 m of lake banks, prohibition of wetland degradation. These findings are, therefore valuable for all relevant stakeholders, including decision and policy-makers responsible for the management of water resources including L. Kyoga. These include MWE, MAAIF, and regional bodies like Nile Basin Initiative (NBI) Countries, CSOs, and NGOs. Drivers and pressures of these LULC changes that include population increase, poverty, shortage of land, need for energy in the form of charcoal, and shortage of better alternative ways of livelihoods should be studied, pointed out, and addressed. This will help reduce the pressure of the LULC changes and water quality eventually. Management activities, like restoration of wetlands, re-afforestation, and forestation, improvement of agricultural practices like the use of terraces of the steep slopes of Mt. Elgon, and provision of cheaper alternative sources of power like solar and biogas should be prioritized.
Conservation and restoration efforts are being made in the study area by MWE, through the wetlands department and regional offices under KWMZ, together with local governments, non-government organizations (NGOs), civil society organizations (CSOs), and the local community. The continued degradation trend shows that more work remains to be done to stop and reverse the situation. This kind of research should be extended to other water bodies in Uganda and the region. Therefore, strategic measures for sustainable utilization of the lake’s resources, management, and protection of its catchment from further degradation to stem further water quality deterioration should be prioritized. This study focused on the physico-chemical and biological aspects of the lake’s water quality. There is a need to study the impact of LULC activities on other aspects of L. Kyoga ecosystem, including sediment deposition and quality; bathymetry studies should be helpful in this area. Other limnological aspects like zooplankton, phytoplankton, macroinvertebrates, macrophytes dynamics with LULC changes should be done. The other potential source of nutrients load into the lake includes atmospheric deposition, and it is recommended that this is done to facilitate a wholistic mitigation approach. Monitoring of pollution status of aquatic ecosystems like lakes, reservoirs, streams and rivers, and wetlands is vital for management purposes. It is therefore recommended that Uganda, East Africa, and Africa as a whole should develop water quality trophic status indices to guide management options by policy and decision-makers.